Identification of Climate-Smart Bread Wheat Germplasm Lines with Enhanced Adaptation to Global Warming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Site, Design and Weather Conditions
2.3. Field Phenotyping and Data Recording
2.4. Scanning Electron Microscopy
2.5. Statistical Analysis
3. Results
3.1. Weather Conditions during Crop Seasons
3.2. Crop Growth and Genetic Variability
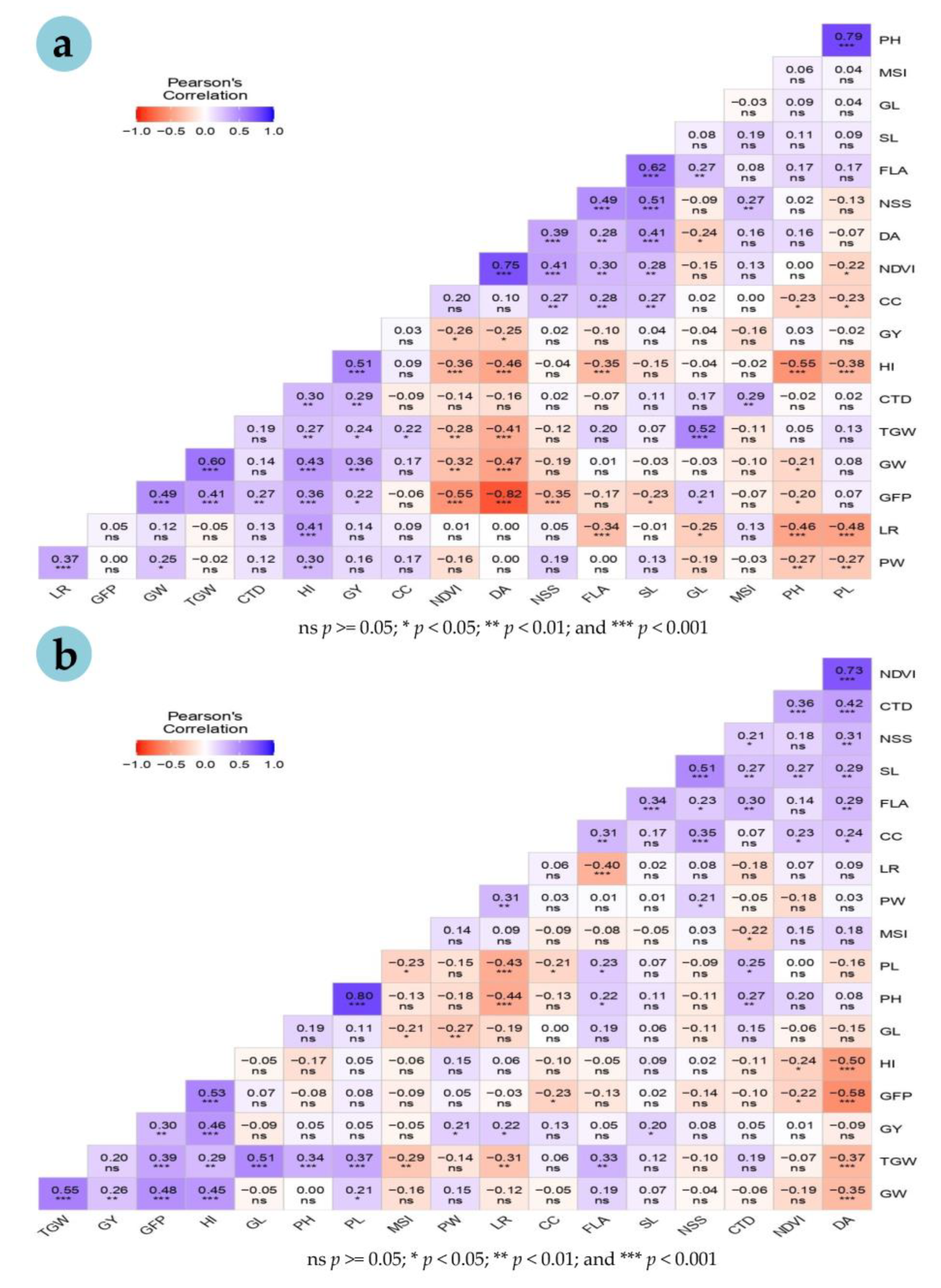
3.3. Genetic Relationship in Wheat Accessions
3.4. Correlations with Grain Yield
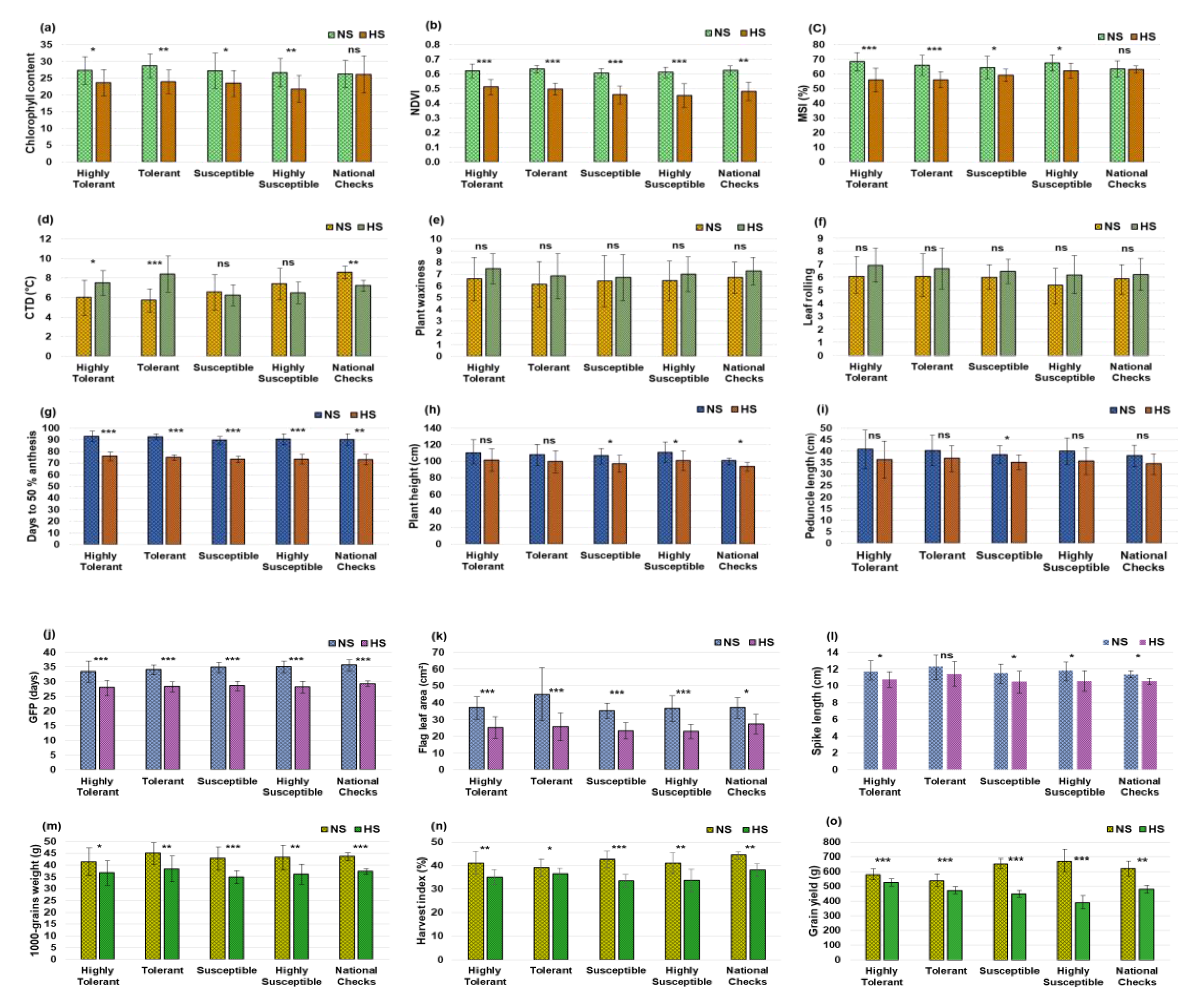
3.5. Impact of HeatStress on Yield and Morpho-Physiological Traits
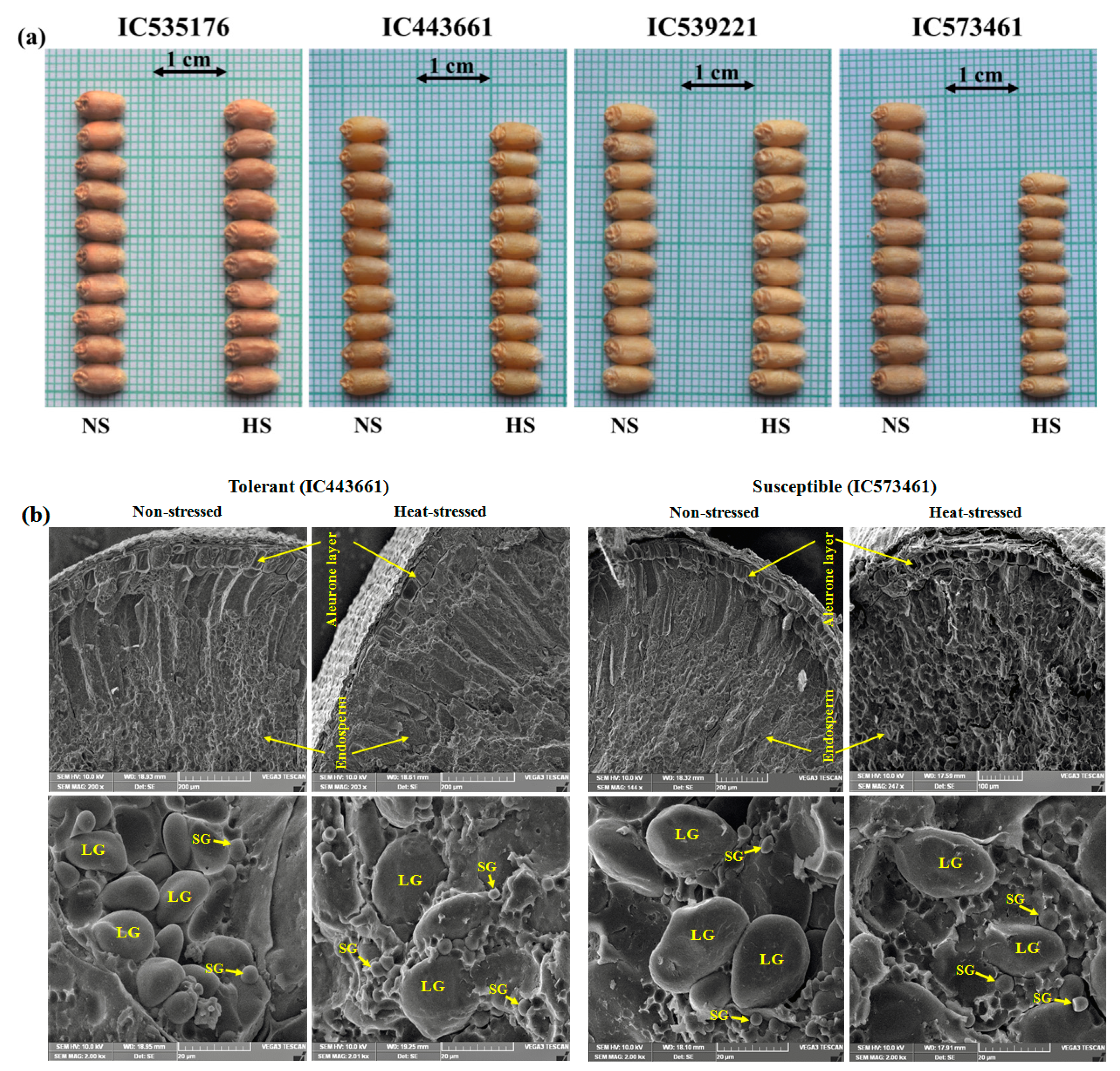
3.6. Impact of HeatStress on Wheat Grains
3.7. Selection of Heat-Stress-Adapted Germplasm
4. Discussion
4.1. Trait Variability and Impact of HeatStress
4.2. Association of Grain Yield with Other Traits
4.3. Grain Development under Heat Stress
4.4. Yield Stability and Selection of Heat-Adapted Accessions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acevedo, M.; Zurn, J.D.; Molero, G.; Singh, P.; He, X.; Aoun, M.; McCandless, L. The role of wheat in global food security. In Agricultural Development and Sustainable Intensification: Technology and Policy Challenges in the Face of Climate Change, 1st ed.; Routledge: New York, NY, USA, 2018; pp. 81–110. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes andfuture challenges to the role played by wheat in global food security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Food and Agriculture–Statistical Yearbook; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- CIMMYT. FFAR Grant Develops Climate-Resilient Wheat. CIMMYT Press Release 11 January 2021. Available online: https://www.cimmyt.org/news/ffar-grant-develops-climate-resilient-wheat/ (accessed on 25 January 2021).
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, T.; von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture–Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; p. 151. [Google Scholar]
- Wang, P.; Deng, X.; Jiang, S. Global warming, grain production and its efficiency: Case study of major grain production region. Ecol. Indic. 2018, 105, 563–570. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Change 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- McCouch, S.R.; Navabi, Z.K.; Abberton, M.; Anglin, N.L.; Barbieri, R.L.; Baum, M.; Bett, K.; Booker, H.; Brown, G.L.; Bryan, G.J.; et al. Mobilizing crop biodiversity. Mol. Plant 2020, 13, 1341–1344. [Google Scholar] [CrossRef]
- Reynolds, M.; Tattaris, M.; Cossani, C.M.; Ellis, M.; Yamaguchi-Shinozaki, K.; Pierre, C.S. Exploring genetic resources to increase adaptation of wheat to climate change. In Advances in Wheat Genetics: From Genome to Field; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 355–368. [Google Scholar] [CrossRef] [Green Version]
- Mujeeb-Kazi, A.; Kazi, A.G.; Dundas, I.; Rasheed, A.; Ogbonnaya, F.; Kishii, M.; Bonnett, D.; Wang, R.R.C.; Xu, S.; Chen, P.; et al. Genetic diversity for wheat improvement as a conduit to food security. Adv. Agron. 2013, 122, 179–257. [Google Scholar] [CrossRef]
- Matsuoka, Y. Evolution of polyploid Triticum wheats under cultivation: The role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol. 2011, 52, 750–764. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic gains in wheat breeding and its role in feeding the world. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Vikram, P.; Sehgal, D.; Burgueño, J.; Sharma, A.; Singh, S.K.; Sansaloni, C.P.; Joynson, R.; Brabbs, T.; Ortiz, C.; et al. Harnessing genetic potential of wheat germplasm banks through impact-oriented-prebreeding for future food and nutritional security. Sci. Rep. 2018, 8, 12527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.V.; Ban, T.; Hodson, D.; Dixon, J.M.; Ortiz-Monasterio, J.I.; Reynolds, M. Climate change: Can wheat beat the heat? Agric. Ecosyst. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Rane, J.; Pannu, R.K.; Sohu, V.S.; Saini, R.S.; Mishra, B.; Shoran, J.; Crossa, J.; Vargas, M.; Joshi, A.K. Performance of yield and stability of advanced wheat genotypes under heat stress environments of the Indo-Gangetic plains. Crop Sci. 2007, 47, 1561–1573. [Google Scholar] [CrossRef]
- Kumar, S.N.; Aggarwal, P.K.; Rani, D.N.S.; Saxena, R.; Chauhan, N.; Jain, S. Vulnerability of wheat production to climate change in India. Clim. Res. 2014, 59, 173–187. [Google Scholar] [CrossRef]
- Joshi, A.K.; Mishra, B.; Chatrath, R.; Ortiz -Ferrara, G.; Singh, R.P. Wheat improvement in India: Present status, emerging challenges and future prospects. Euphytica 2007, 157, 431–446. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Crossa, J.; Huerta-Espino, J.; Sharma, I.; Chatrath, R.; Singh, G.P.; Sohu, V.S.; Mavi, G.S.; Sukuru, V.S.P.; et al. Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in South Asia. Field Crops Res. 2013, 151, 19–26. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Ortiz-Monasterio, J.I.; McNab, A. Application of Physiology in Wheat Breeding; D.F. CIMMYT: Texococo, Mexico, 2001. [Google Scholar]
- Huggins, T.D.; Mohammed, S.; Sengodon, P.; Ibrahim, A.M.H.; Tilley, M.; Hays, D.B. Changes in leaf epicuticular wax load and its effect on leaf temperature and physiological traits in wheat cultivars (Triticum aestivum L.) exposed to high temperatures during anthesis. J. Agro. Crop Sci. 2018, 204, 49–61. [Google Scholar] [CrossRef]
- Fokar, M.; Nguyen, H.T.; Blum, A. Heat tolerance in spring wheat. I. Estimating cellular thermotolerance and its heritability. Euphytica 1998, 104, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jing, Q.; Jiang, D.; Cao, W. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Huerta-Espino, J.; Kehel, Z.; Autrique, E. Characterization of heat- and drought-stress tolerance in high-yielding spring wheat. Crop Sci. 2015, 55, 709. [Google Scholar] [CrossRef]
- Telfer, P.; Edwards, J.; Bennett, D.; Ganesalingam, D.; Able, J.; Kuchel, H. A field and controlled environment evaluation of wheat (Triticum aestivum) adaptation to heat stress. Field Crops Res. 2018, 229, 55–65. [Google Scholar] [CrossRef]
- Jenner, C.F. Starch synthesis in the kernel of wheat under high temperature conditions. Aust. J. Plant Physiol. 1994, 21, 791–806. [Google Scholar] [CrossRef]
- Dias, A.S.; Bagulho, A.S.; Lidon, F.C. Ultrastructure and biochemical traits of bread and durum wheat grains under heat stress. Braz. J. Plant Physiol. 2008, 20, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Keeling, P.L.; Bacon, P.J.; Holt, D.C. Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 1993, 191, 342–348. [Google Scholar] [CrossRef]
- Shah, N.H.; Paulsen, G.M. Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 2003, 257, 219–226. [Google Scholar] [CrossRef]
- Cossani, C.M.; Reynolds, M.P. Physiological traits for improving heat tolerance in wheat. Plant Physiol. 2012, 160, 1710–1718. [Google Scholar] [CrossRef] [Green Version]
- Elbasyoni, I.S. Performance and stability of commercial wheat cultivars under terminal heat stress. Agronomy 2018, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.C.; Tiwary, A.K.; Ortiz-Ferrara, G. Reduction in kernel weight as a potential indirect selection criterion for wheat grain yield under terminal heat stress. Plant Breed. 2008, 127, 241–248. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Mondal, S.; Gerard, G.S.; Hernández-Espinosa, N.; Singh, R.P.; Crossa, J.; Guzmán, C. Identification of CIMMYT spring bread wheat germplasm maintaining superior grain yield and quality under heat-stress. J. Cereal Sci. 2020, 93, 102981. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Comm. 2015, 6, 5989. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.S.P. Heat stress effects on source–sink relationships and metabolome dynamics in wheat. J. Exp. Bot. 2020, 71, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Prabhakaran, V.T.; Mehra, R.B. Genotype-by-environment interaction incrop improvement. In Plant Breeding-Mendelian to Molecular Approaches; Jain, H.K., Kharkwal, M.C., Eds.; Narosa Publishing House: New Delhi, India, 2004; pp. 535–572. [Google Scholar]
- Hyles, J.; Bloomfield, M.T.; Hunt, J.R.; Trethowan, R.M.; Trevaskis, B. Phenology and related traits for wheat adaptation. Heredity 2020, 125, 417–430. [Google Scholar] [CrossRef]
- Phogat, B.S.; Kumar, S.; Kumari, J.; Kumar, N.; Pandey, A.C.; Singh, T.P.; Kumar, S.; Tyagi, R.K.; Jacob, S.R.; Singh, A.K.; et al. Characterization of wheat germplasm conserved in the Indian National Genebank and establishment of a composite core collection. Crop Sci. 2021, 61, 604–620. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Guarino, L.; Cruz, M.; Rojas, E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genet. Resour. Newslett. 2001, 127, 15–19. [Google Scholar]
- Pask, A.J.D.; Pietragalla, J.; Mullan, D.M.; Reynolds, M.P. Physiological Breeding II: A Field Guide to Wheat Phenotyping; D.F. CIMMYT: Texococo, Mexico, 2012. [Google Scholar]
- SAS Institute. Statistical Analysis System for Windows, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013.
- IBM SPSS. IBM SPSS (Statistical Package for the Social Sciences) Statistics Software for Windows, Version 20.0; IBM Corp.: Armonk, NY, USA, 2011. Available online: https://hadoop.apache.org (accessed on 15 January 2021).
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agr. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Rutkoski, J.E.; Velu, G.; Singh, P.K.; Crespo-Herrera, L.A.; Guzman, C.; Bhavani, S.; Lan, C.; He, X.; Singh, R.P. Harnessing diversity in wheat to enhance grain yield, climate resilience, disease and insect pest resistance and nutrition through conventional and modern breeding approaches. Front. Plant Sci. 2016, 7, 991. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.K.; Basu, S.; Kumar, S.; Kumar, G.; Prakash, V.; Kumar, S.; Mishra, J.S.; Bhatt, B.P.; Malviya, N.; Singh, G.P.; et al. Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in eastern Indo-Gangetic Plains. Field Crops Res. 2017, 206, 106–114. [Google Scholar] [CrossRef]
- Ullah, S.; Bramley, H.; Mahmood, T.; Trethowan, R. A strategy of ideotype development for heat-tolerant wheat. J. Agro. Crop Sci. 2020, 206, 229–241. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, D.S.S.; Singh, G.P.; Singh, A.M. Relationship between canopy temperature depression, membrane stability, relative water content and grain yield in bread wheat (Triticum aestivum) under heat-stress environments. Indian J. Agric. Sci. 2011, 81, 197–202. [Google Scholar]
- Pinto, R.S.; Molero, G.; Reynolds, M.P. Identification of heat tolerant wheat lines showing genetic variation in leaf respiration and other physiological traits. Euphytica 2017, 213, 76. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Lepekhov, S.B. Canopy temperature depression for drought- and heat stress tolerance in wheat breeding. Vavilov J. Genet. Breed. 2022, 26, 196–201. [Google Scholar] [CrossRef]
- Tomás, D.; Coelho, L.P.; Rodrigues, J.C.; Viegas, W.; Silva, M. Assessment of four Portuguese wheat landrace diversity to cope with global warming. Front. Plant Sci. 2020, 11, 594977. [Google Scholar] [CrossRef]
- Agarwal, V.P.; Gupta, N.K.; Gupta, S.; Singh, G. Screening of wheat germplasm for terminal heat tolerance under hyper-arid conditions. Cereal Res. Commun. 2021, 49, 375–383. [Google Scholar] [CrossRef]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Mohammed, S.; Huggins, T.D.; Beecher, F.; Chick, C.; Sengodon, P.; Mondal, S.; Paudel, A.; Ibrahim, A.M.; Tilley, M.; Hays, D.B. The role of leaf epicuticular wax in the adaptation of wheat (Triticum aestivum L.) to high temperatures and moisture deficit conditions. Crop Sci. 2018, 58, 679–689. [Google Scholar] [CrossRef]
- Soni, A.; Munjal, R. Characterisation and evaluation of wheat genetic resources for heat stress tolerance using stay-green traits. Crop Pasture Sci. 2023. [Google Scholar] [CrossRef]
- Mondal, S.; Dutta, S.; Crespo-Herrera, L.; Huerta-Espino, J.; Braun, H.J.; Singh, R.P. Fifty years of semi-dwarf spring wheat breeding at CIMMYT: Grain yield progress in optimum, drought and heat stress environments. Field Crops Res. 2020, 250, 107757. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alotaibi, M.; Refay, Y.; Ghazy, A.; Zakri, A.; Al-Doss, A. Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS ONE 2020, 15, e0236351. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Pierre, C.S.; Saad, A.S.I.; Vargas, M.; Condon, A.G. Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Sci. 2007, 47, S172–S189. [Google Scholar] [CrossRef]
- Fu, J.; Bowden, R.L.; Jagadish, S.V.K.; Prasad, P.V.V. Genetic variation for terminal heat stress tolerance in winter wheat. Front. Plant Sci. 2023, 14, 1132108. [Google Scholar] [CrossRef]
- Ayeneh, A.; van Ginkel, M.; Reynolds, M.P.; Ammar, K. Comparison of leaf, spike, peduncle and canopy temperature depression in wheat under heat stress. Field Crops Res. 2002, 79, 173–184. [Google Scholar] [CrossRef]
- Bahar, B.; Yildirim, M.; Yucel, C. Heat and drought resistance criteria in spring bread wheat (Triticum aestivum L.): Morpho- physiological parameters for heat tolerance. Sci. Res. Essays. 2011, 6, 2212–2220. [Google Scholar] [CrossRef] [Green Version]
- Lordkaew, S.; Yimyam, N.; Wongtamee, A.; Jamjod, S.; Rerkasem, B. Evaluating a heat-tolerant wheat germplasm in a heat stress environment. Plant Genet. Resour. 2019, 17, 339–345. [Google Scholar] [CrossRef]
- Chaubey, R.K.; Bhutia, D.D.; Navathe, S.; Mishra, V.K.; Singh, A.K.; Chand, R. Interrelationships among different grain characteristics of wheat grown under optimum and late sowing date conditions in the Eastern Indo-Gangetic plains of India. Cereal Res. Commun. 2021, 49, 449–455. [Google Scholar] [CrossRef]
- Chiotelli, E.; Le Meste, M. Effect of small and large wheat starch granules on thermomechanical behavior of starch. Cereal Chem. 2002, 79, 286–293. [Google Scholar] [CrossRef]
- Uthayakumaran, S.; Wrigley, C. Wheat: Grain-quality Characteristics and Management of Quality Requirements. In Cereal Grains-Assessing and Managing Quality; Batey, C.W.I., Miskelly, D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 91–134. [Google Scholar] [CrossRef]
- Liu, P.; Guo, W.; Jiang, Z.; Pu, H.; Feng, C.; Zhu, X.; Peng, Y.; Kuang, A.; Little, C.R. Effects of high temperature after anthesis on starch granules in grains of wheat (Triticum aestivum L.). J. Agric. Sci. 2011, 149, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; Hanumantharao, B.; Nair, R.M.; Prasad, P.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, H.J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. In Climate Change and Crop Production; Reynolds, M.P., Ed.; CABI: Wallingford, UK, 2010; pp. 115–138. [Google Scholar]
- Powell, N.; Ji, X.; Ravash, R.; Edlinton, J.; Dolferus, R. Yield stability for cereals in a changing climate. Funct. Plant Biol. 2012, 39, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mehta, G.; Kumar, S.; Ramdas, S.; Tiwari, R.; Singh, G.P.; Sharma, P. AMMI and GGE biplot analysis of yield under terminal heat tolerance in wheat. Mol. Biol. Rep. 2023, 50, 3459–3467. [Google Scholar] [CrossRef] [PubMed]





| S. No. | Traits Studied | Code | How Was the Trait Measured? |
|---|---|---|---|
| 1. | Chlorophyll Content | CC | Estimated on flag leaves of five random main tillers in each accession with hand-held Chlorophyll Content Meter (Model-CCM-200 plus, Opti-Sciences, Hudson, NH, USA). |
| 2. | Canopy Temperature Depression (°C) | CTD | Measured on warm, sunny and clear day using portable Infrared Thermometer (Fisher Scientific, Loughborough, Leicestershire, UK). |
| 3. | Normalized Difference Vegetation Index | NDVI | NDVI was recorded using hand-held crop sensor (Green Seeker®, Trimble, Westminster, CO, USA). It ranged from 0 to 1; 0 refers to no green area and 1 to maximum greenness. |
| 4. | Membrane Stability Index (%) | MSI | MSI was estimated with small leaf discs of uniform size cut from 0.1 g leaf samples of each wheat accession and calculated using the following formula: MSI = [1 − (C1/C2)] × 100, where C1 and C2 represent readings of EC (Electrical Conductivity) recorded using digital conductivity meter at 45 °C and 100 °C, respectively. |
| 5. | Days to 50% Anthesis | DA | Recorded as the period between the date of sowing and the dateat which 50% of spikes start to extrude their anthers. |
| 6. | Grain Filling Period (days) | GFP | GFP calculated as the difference between days to 50% anthesis and days to physiological maturity. |
| 7. | Plant Waxiness (0–10 scale) | PW | PW was measured with visual observations of whole plot during mid of GFP and scored using scale from 0 (0%) to 10 (100%) in an increment of 10%. |
| 8. | Leaf Rolling (0–10 scale) | LR | LR was measured at mid of GFP with visual observation of whole plot and scored as proportion of the leaves showing rolling effect using a scale from 0 (0%) to 10 (100%) in an increment of 10%. |
| 9. | Plant Height (cm) | PH | PH was measured from base of the plant to top of the spike excluding awns of the main tiller at maturity. |
| 10. | Peduncle Length (cm) | PL | Measured from uppermost node to the spike collar of the main tiller at maturity in three plants per accession. |
| 11. | Flag Leaf Area (cm2) | FLA | Derived from five randomly chosen plants’ flag leaves using the equation Leaf area = Length × Breadth × 0.75. |
| 12. | Spike Length (cm) | SL | Measured from the spike collar to tip of the spike excluding awns of the main tiller in three plants per accession. |
| 13. | Number of Spikelets per Spike | NSS | Spikelets per spike were counted on the main tiller spike of three plants per accession. |
| 14. | Grain Length (mm) | GL | Measured on five grains per accession with Digimatic Caliper (Model-CD-6″ASX, Mitutoyo Corporation, Kawasaki, Kanagawa, Japan). |
| 15. | Grain Width (mm) | GW | Grain width was measured from five grains randomly selected per accession with Digimatic Caliper. |
| 16. | 1000-Grain Weight (g) | TGW | TGW was recorded from 1000 grains randomly selected from plot yield and weighted using sensitive electronic balance (d = 0.1 mg, Sartorius, model CPA64, Göttingen, Lower Saxony, Germany). |
| 17. | Harvest Index (%) | HI | HI was calculated using the following formula: HI = (Grain yield per plant/Biological yield per plant) ×100. |
| 18. | Grain Yield (g/m2) | GY | Plot yield of each accession harvested, threshed manually, and weight of grains recorded with electronic balance. GY is expressed as yield per unit area. |
| Trait | Environment | Range | Mean ± S.E. | SD | CV (%) | PCV (%) | GCV (%) | H2 (%) | GA (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | |||||||||
| CCI | NS | 17.4 | 38.5 | 26.9 ± 0.43 | 4.21 | 15.69 | 18.1 | 15.0 | 68.3 | 25.5 |
| HS | 13.5 | 37.2 | 22.7 ± 0.49 | 4.10 | 18.10 | 18.6 | 16.2 | 75.9 | 29.0 | |
| CTD (°C) | NS | 2.6 | 9.7 | 6.2 ± 0.18 | 1.80 | 29.19 | 23.3 | 10.5 | 20.3 | 9.7 |
| HS | 4.4 | 12.0 | 6.9 ± 0.15 | 1.47 | 21.24 | 26.1 | 11.0 | 17.8 | 9.6 | |
| NDVI (0–1) | NS | 0.54 | 0.72 | 0.62 ± 0.01 | 0.04 | 6.03 | 7.5 | 5.1 | 42.9 | 6.6 |
| HS | 0.32 | 0.64 | 0.48 ± 0.01 | 0.06 | 12.61 | 10.9 | 8.5 | 63.8 | 14.3 | |
| MSI (%) | NS | 50.0 | 77.5 | 66.6 ± 0.70 | 6.89 | 10.35 | 10.5 | 6.0 | 33.0 | 7.2 |
| HS | 43.7 | 72.6 | 59.4 ± 0.68 | 6.70 | 11.29 | 8.5 | 2.5 | 8.7 | 1.5 | |
| PW (0–10) | NS | 1.0 | 10.0 | 6.2 ± 0.20 | 1.94 | 31.35 | 23.8 | 19.6 | 67.6 | 33.2 |
| HS | 2.0 | 10.0 | 6.9 ± 0.17 | 1.68 | 24.47 | 21.4 | 17.2 | 64.6 | 28.5 | |
| LR (0–10) | NS | 2.5 | 9.5 | 5.8 ± 0.13 | 1.28 | 22.04 | 20.8 | 17.5 | 71.4 | 30.5 |
| HS | 4.0 | 10.0 | 6.5 ± 0.13 | 1.26 | 19.29 | 19.1 | 15.0 | 61.4 | 24.1 | |
| Days to 50% anthesis | NS | 83.4 | 119.0 | 91.9 ± 0.48 | 4.72 | 5.13 | 6.1 | 5.6 | 84.9 | 10.6 |
| HS | 66.6 | 94.0 | 74.6 ± 0.40 | 3.88 | 5.20 | 6.8 | 6.3 | 85.4 | 11.9 | |
| GFP (days) | NS | 24.0 | 39.5 | 34.2 ± 0.25 | 2.45 | 7.16 | 19.9 | 10.7 | 29.0 | 11.9 |
| HS | 23.5 | 32.0 | 28.1 ± 0.20 | 1.94 | 6.91 | 8.7 | 5.7 | 43.0 | 7.7 | |
| Plant height (cm) | NS | 84.9 | 150.9 | 106.6 ± 1.31 | 12.83 | 12.03 | 5.9 | 2.5 | 17.8 | 2.2 |
| HS | 73.2 | 127.0 | 96.9 ± 1.25 | 12.23 | 12.63 | 7.1 | 4.4 | 39.1 | 5.7 | |
| Peduncle length (cm) | NS | 29.4 | 60.1 | 39.2 ± 0.61 | 6.00 | 15.33 | 14.7 | 12.1 | 67.7 | 20.6 |
| HS | 27.0 | 53.8 | 35.1 ± 0.54 | 5.27 | 15.04 | 14.5 | 12.9 | 79.4 | 23.7 | |
| Flag leaf area (cm2) | NS | 21.9 | 77.0 | 37.4 ± 0.88 | 8.61 | 23.01 | 20.4 | 16.1 | 62.4 | 26.2 |
| HS | 14.2 | 51.7 | 23.8 ± 0.61 | 5.95 | 24.96 | 17.8 | 14.5 | 66.3 | 24.3 | |
| Spike length (cm) | NS | 8.7 | 15.6 | 11.7 ± 0.12 | 1.18 | 10.14 | 6.8 | 3.0 | 19.4 | 2.7 |
| HS | 8.1 | 13.5 | 10.6 ± 0.11 | 1.10 | 10.38 | 4.8 | 1.3 | 7.1 | 0.7 | |
| Spikelets per spike | NS | 16.0 | 24.0 | 20.2 ± 0.15 | 1.46 | 7.23 | 5.9 | 2.1 | 12.7 | 1.6 |
| HS | 15.5 | 22.7 | 18.7 ± 0.15 | 1.49 | 7.98 | 5.9 | 2.4 | 16.3 | 2.0 | |
| Grain length (mm) | NS | 5.97 | 8.76 | 6.90 ± 0.04 | 0.39 | 5.59 | 5.6 | 4.9 | 77.9 | 9.0 |
| HS | 5.85 | 8.62 | 6.68 ± 0.04 | 0.38 | 5.63 | 6.1 | 5.8 | 89.3 | 11.3 | |
| Grain width (mm) | NS | 2.84 | 4.02 | 3.49 ± 0.02 | 0.19 | 5.31 | 3.5 | 2.0 | 33.0 | 2.4 |
| HS | 2.64 | 3.68 | 3.27 ± 0.02 | 0.18 | 5.52 | 3.3 | 1.4 | 17.9 | 1.2 | |
| 1000-grain weight (g) | NS | 30.0 | 52.2 | 41.5 ± 0.53 | 5.19 | 12.51 | 6.0 | 3.0 | 24.1 | 3.0 |
| HS | 24.9 | 46.6 | 35.5 ± 0.48 | 4.73 | 13.34 | 8.0 | 3.3 | 16.7 | 2.8 | |
| Harvest index (%) | NS | 21.6 | 50.6 | 39.7 ± 0.49 | 4.84 | 12.19 | 9.4 | 1.7 | 13.3 | 0.6 |
| HS | 23.4 | 48.0 | 34.4 ± 0.45 | 4.38 | 12.76 | 9.3 | 3.2 | 11.9 | 2.3 | |
| Grain yield (g/m2) | NS | 300.0 | 802.5 | 562.2 ± 8.77 | 86.00 | 15.30 | 11.3 | 7.3 | 42.1 | 9.8 |
| HS | 176.7 | 598.1 | 423.6 ± 7.34 | 71.95 | 16.97 | 10.5 | 5.8 | 30.2 | 6.6 | |
| Adaptation | Wheat Accession | TGW | Grain Yield | TGW | Grain Yield | HSI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µ | βi | S2Di | µ | βi | S2Di | NS | HS | NS | HS | |||
| Unfavorable (heat-stressed) environment | IC543425 | 37.4 | 1.32 | 0.09 | 487.2 | −0.56 | 319.85 | 39.7 | 31.9 | 508.0 | 498.0 | 0.08 |
| IC128454 | 33.6 | 1.05 | 1.91 | 543.6 | −0.22 | 376.52 | 34.9 | 29.1 | 546.0 | 538.1 | 0.05 | |
| IC265318 | 39.1 | 0.71 | 11.30 | 500.7 | −0.15 | 363.40 | 38.5 | 36.4 | 508.0 | 504.1 | 0.03 | |
| IC252348 | 46.0 | 1.38 | 16.61 | 480.1 | −0.15 | 529.35 | 46.9 | 41.9 | 488.0 | 476.7 | 0.09 | |
| IC566223 | 36.3 | 0.76 | 23.13 | 589.5 | 0.04 | 119.09 | 40.4 | 34.7 | 604.3 | 598.1 | 0.04 | |
| IC335792 | 33.6 | 1.02 | 7.37 | 559.7 | 0.20 | 446.45 | 34.0 | 29.9 | 593.4 | 539.4 | 0.37 | |
| IC290191 | 37.3 | 1.12 | 1.93 | 500.5 | 0.26 | 9.76 | 39.7 | 34.5 | 522.2 | 482.7 | 0.31 | |
| IC401976 | 48.0 | 0.73 | 4.67 | 493.3 | 0.28 | 21.89 | 49.7 | 45.2 | 510.5 | 478.1 | 0.26 | |
| IC446713 | 44.7 | 0.62 | 1.02 | 522.7 | 0.32 | 11.41 | 46.6 | 41.7 | 545.2 | 505.4 | 0.30 | |
| EC576707 | 33.7 | 0.15 | 2.20 | 552.1 | 0.36 | 37.58 | 33.8 | 32.4 | 579.2 | 534.7 | 0.37 | |
| IC075240 | 42.9 | 0.88 | 6.98 | 474.5 | 0.33 | 63.50 | 44.7 | 39.9 | 501.9 | 460.1 | 0.34 | |
| IC416019 | 43.6 | 1.71 | 1.29 | 510.0 | 0.41 | 55.05 | 49.1 | 36.8 | 542.5 | 490.1 | 0.40 | |
| EC574731 | 42.0 | 0.99 | 0.22 | 520.7 | 0.43 | 62.51 | 45.2 | 37.6 | 549.2 | 500.7 | 0.36 | |
| IC535176 | 46.7 | 0.19 | 0.94 | 556.0 | 0.47 | 61.91 | 47.1 | 45.0 | 593.9 | 532.1 | 0.43 | |
| IC539531 | 46.1 | 0.75 | 2.11 | 478.3 | 0.50 | 49.48 | 48.5 | 42.6 | 517.2 | 452.1 | 0.52 | |
| IC529207 | 37.7 | 1.06 | 26.18 | 563.5 | 0.55 | 5.10 | 39.3 | 35.7 | 604.9 | 527.4 | 0.52 | |
| Both environments | IC416018 | 43.0 | 0.89 | 13.29 | 553.8 | 0.78 | 36.99 | 47.1 | 37.6 | 605.9 | 508.1 | 0.66 |
| IC443661 | 40.8 | −0.06 | 1.18 | 463.9 | 0.85 | 24.38 | 41.1 | 39.2 | 523.2 | 413.4 | 0.86 | |
| EC534487 | 43.2 | 1.24 | 2.62 | 551.6 | 0.92 | 80.85 | 47.7 | 37.6 | 609.9 | 496.1 | 0.76 | |
| IC539221 | 49.8 | 0.86 | 3.33 | 529.0 | 0.94 | 12.34 | 51.8 | 46.6 | 593.2 | 468.7 | 0.86 | |
| IC393878 | 45.3 | 1.21 | 3.85 | 572.2 | 0.96 | 109.62 | 49.3 | 43.9 | 633.7 | 516.7 | 0.75 | |
| Favorable (non-stressed) environment | IC573461 | 41.8 | 2.86 | 46.15 | 558.5 | 1.39 | 34.86 | 52.2 | 31.1 | 654.9 | 462.7 | 1.20 |
| IC535717 | 39.4 | 0.69 | 17.82 | 553.6 | 1.45 | 104.97 | 43.4 | 38.0 | 649.0 | 464.1 | 1.17 | |
| IC144911 | 33.3 | 0.81 | 0.05 | 537.0 | 1.56 | 79.27 | 35.0 | 31.1 | 648.9 | 425.4 | 1.41 | |
| EC576175 | 40.0 | 1.32 | 3.41 | 557.9 | 1.62 | 176.60 | 44.4 | 38.1 | 661.7 | 456.7 | 1.27 | |
| EC277134 | 40.4 | 1.32 | 71.39 | 570.8 | 1.69 | 154.00 | 48.0 | 35.3 | 680.4 | 464.7 | 1.30 | |
| IC252619 | 35.0 | 1.39 | 1.74 | 545.9 | 1.70 | 112.16 | 40.2 | 32.2 | 659.5 | 441.7 | 1.35 | |
| IC529242 | 34.3 | 1.56 | 2.87 | 482.2 | 1.82 | 169.29 | 40.5 | 30.7 | 600.9 | 369.1 | 1.58 | |
| IC443694 | 33.6 | 0.89 | 5.34 | 505.4 | 1.93 | 240.81 | 37.7 | 31.9 | 630.9 | 387.7 | 1.58 | |
| IC553599 | 39.6 | 0.66 | 2.56 | 550.5 | 1.98 | 57.60 | 42.4 | 39.3 | 685.7 | 424.7 | 1.56 | |
| IC524299 | 42.5 | 1.56 | 117.81 | 565.8 | 2.02 | 114.97 | 50.5 | 34.1 | 709.5 | 422.7 | 1.65 | |
| IC252431 | 34.1 | 0.76 | 1.61 | 607.9 | 2.05 | 269.05 | 37.7 | 33.0 | 739.5 | 478.4 | 1.44 | |
| EC190899 | 35.8 | 1.81 | 3.98 | 588.3 | 2.18 | 155.19 | 41.1 | 33.1 | 732.4 | 448.7 | 1.58 | |
| EC576585 | 45.4 | 0.75 | 7.26 | 492.9 | 2.19 | 178.97 | 48.5 | 44.7 | 637.5 | 353.7 | 1.82 | |
| CUO/79/Pru 11A | 45.1 | 1.10 | 75.79 | 580.5 | 2.38 | 102.43 | 52.1 | 40.7 | 741.0 | 423.4 | 1.75 | |
| IC277741 | 37.5 | 0.68 | 42.16 | 597.6 | 2.74 | 76.59 | 38.0 | 35.8 | 802.5 | 416.1 | 1.97 | |
| Nationalchecks | RAJ3765 | 40.8 | 0.80 | 4.03 | 514.2 | 0.61 | 35.67 | 43.0 | 38.7 | 552.5 | 472.0 | 0.60 |
| HD2932 | 39.2 | 1.02 | 7.19 | 538.1 | 1.14 | 49.90 | 41.7 | 36.6 | 614.7 | 458.8 | 1.04 | |
| WR544 | 41.1 | 0.99 | 3.10 | 562.8 | 1.31 | 25.24 | 44.6 | 37.7 | 655.1 | 472.1 | 1.14 | |
| HD2967 | 40.5 | 1.25 | 3.38 | 592.7 | 1.03 | 46.12 | 44.9 | 36.2 | 665.2 | 518.7 | 0.90 | |
| Population mean | 38.6 | - | - | 489.4 | - | - | 41.5 | 41.5 | 562.2 | 423.6 | 1.00 | |
| LSD (5%) | - | - | - | - | - | - | 2.1 | 2.6 | 49.0 | 21.1 | - | |
| Sl. No. | Traits for Mapping Population | Parents with Desirable Traits for Heat-Stress Tolerance | |
|---|---|---|---|
| (a). | Bi-Parent Population | Parent (Higher Value) | Parent (Lower Value) |
| 1. | Plant waxiness | IC529207, IC528965 | IC252431, IC252444 |
| 2. | Leaf rolling | IC416019, IC416055 | IC553599, IC252816 |
| 3. | Earliness | IC296383 | IC542509 |
| 4. | Grain filling period | IC252725 | EC577013 |
| 5. | Grain width | IC401976 | IC112258 |
| 6. | 1000-grain weight | IC539221 | IC542544 |
| 7. | Harvest index | IC443653 | IC542509 |
| 8. | Grain yield | IC566223 | EC577013 |
| (b). | MAGIC Population | Parents with Desirable Traits | |
| 1. | 4-parent MAGIC | IC566223, IC529207, IC416019, IC296383 | |
| 2. | 8-parent MAGIC | IC128454, IC519900, IC528965, IC416055, | |
| IC539221, IC401976, IC535176, IC566223 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patidar, A.; Yadav, M.C.; Kumari, J.; Tiwari, S.; Chawla, G.; Paul, V. Identification of Climate-Smart Bread Wheat Germplasm Lines with Enhanced Adaptation to Global Warming. Plants 2023, 12, 2851. https://doi.org/10.3390/plants12152851
Patidar A, Yadav MC, Kumari J, Tiwari S, Chawla G, Paul V. Identification of Climate-Smart Bread Wheat Germplasm Lines with Enhanced Adaptation to Global Warming. Plants. 2023; 12(15):2851. https://doi.org/10.3390/plants12152851
Chicago/Turabian StylePatidar, Anil, Mahesh C. Yadav, Jyoti Kumari, Shailesh Tiwari, Gautam Chawla, and Vijay Paul. 2023. "Identification of Climate-Smart Bread Wheat Germplasm Lines with Enhanced Adaptation to Global Warming" Plants 12, no. 15: 2851. https://doi.org/10.3390/plants12152851






