Saline–Alkaline Stress Resistance of Cabernet Sauvignon Grapes Grafted on Different Rootstocks and Rootstock Combinations
Abstract
1. Introduction
2. Results
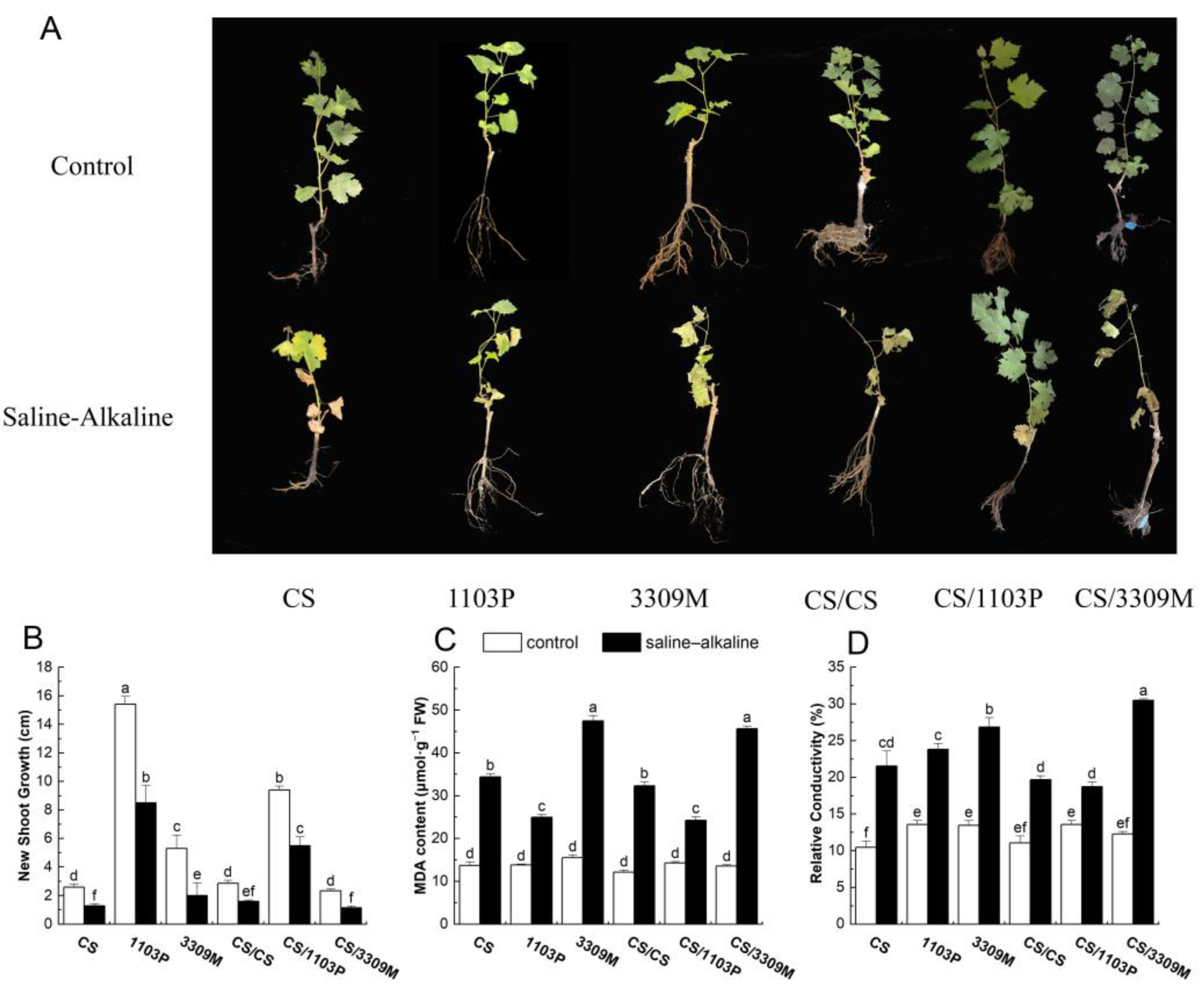
2.1. Effect of Saline–Alkaline Stress on New Shoot Growth and the Membrane System of Different Rootstocks and Rootstock Combinations
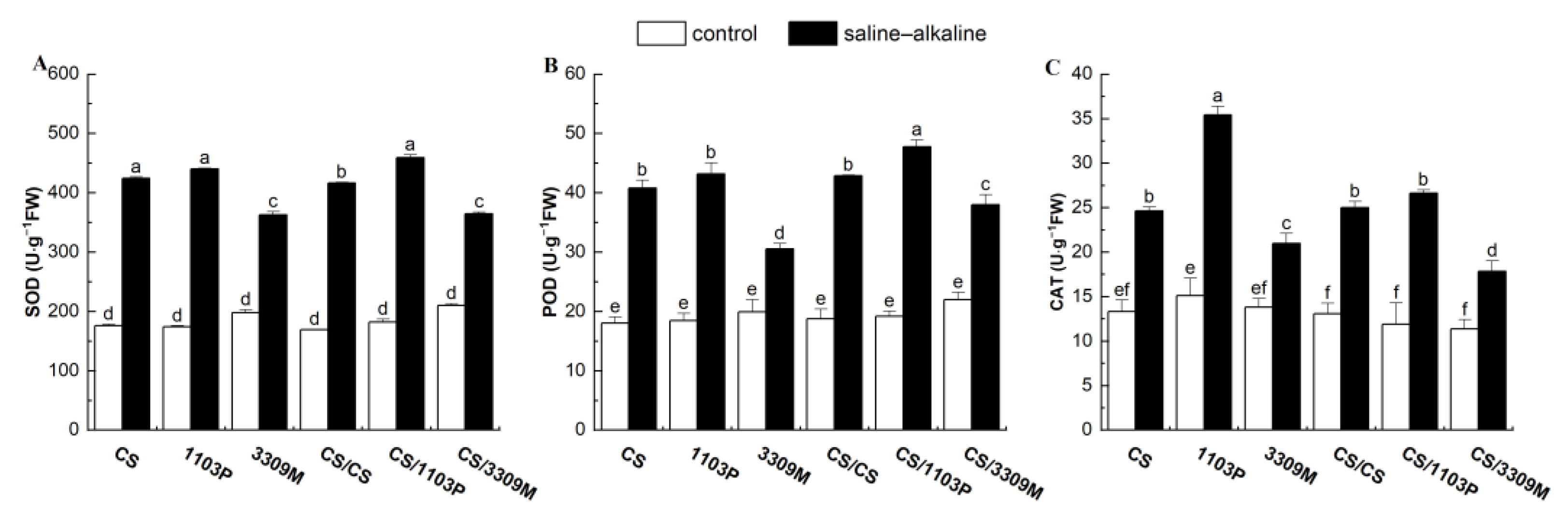
2.2. Effect of Saline–Alkaline Stress on the Antioxidant Enzyme Activity of Different Rootstocks and Rootstock Combinations
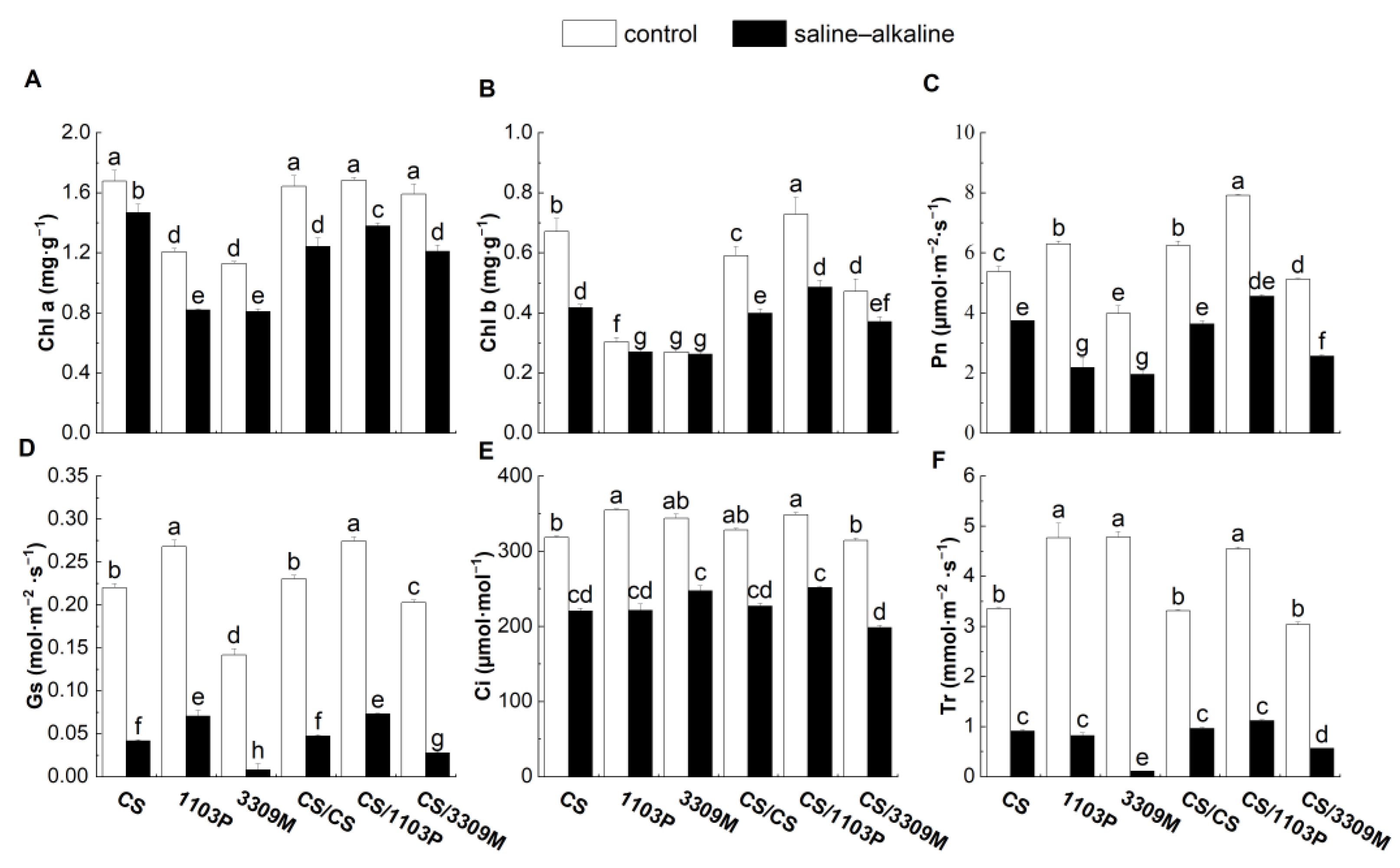
2.3. Effect of Saline–Alkaline Stress on the Chlorophyll Content and Photosynthetic Properties of Different Rootstocks and Rootstock Combinations
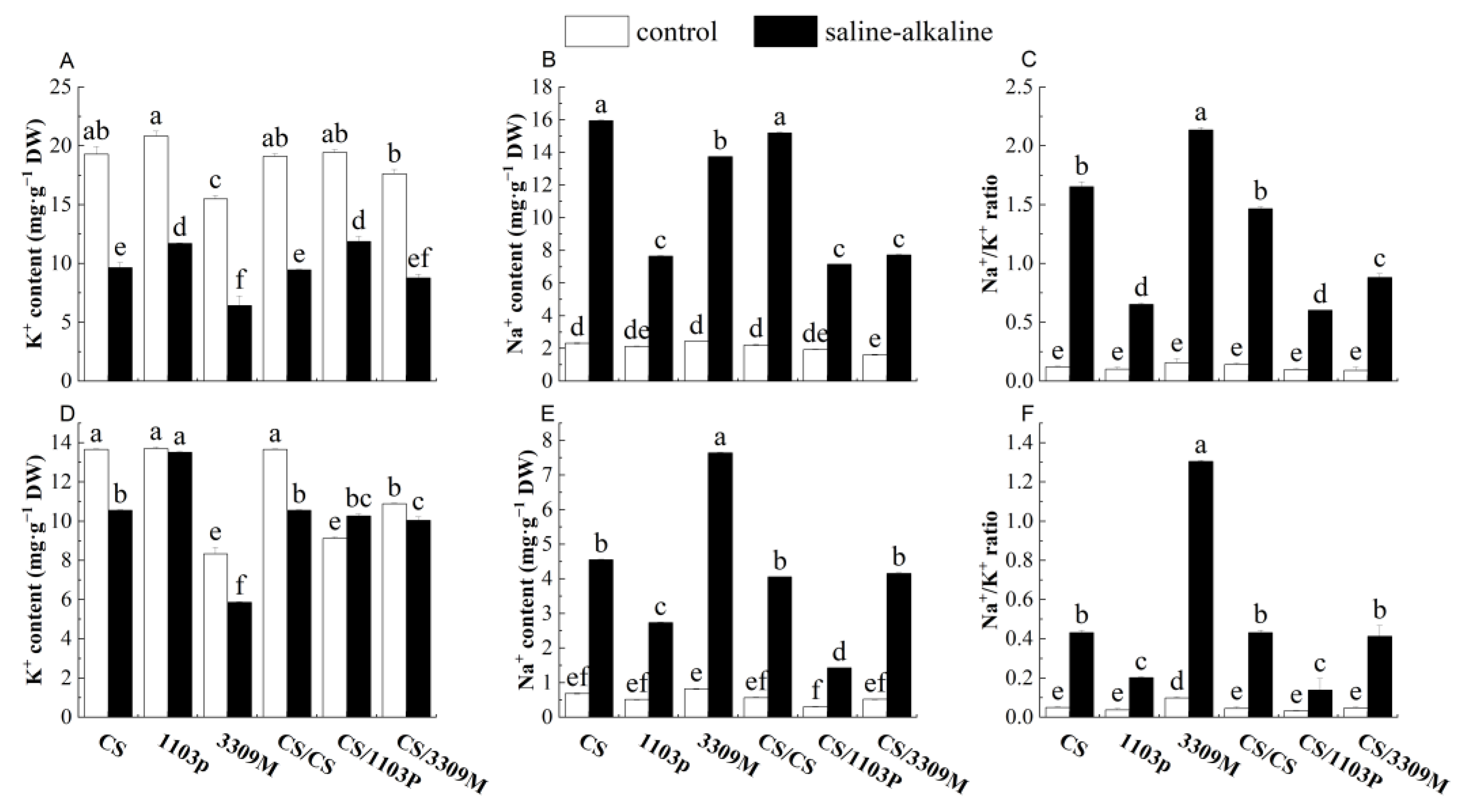
2.4. Effect of Saline–Alkaline Stress on the Ion Content in the Roots and Leaves of Different Rootstocks and Rootstock Combinations
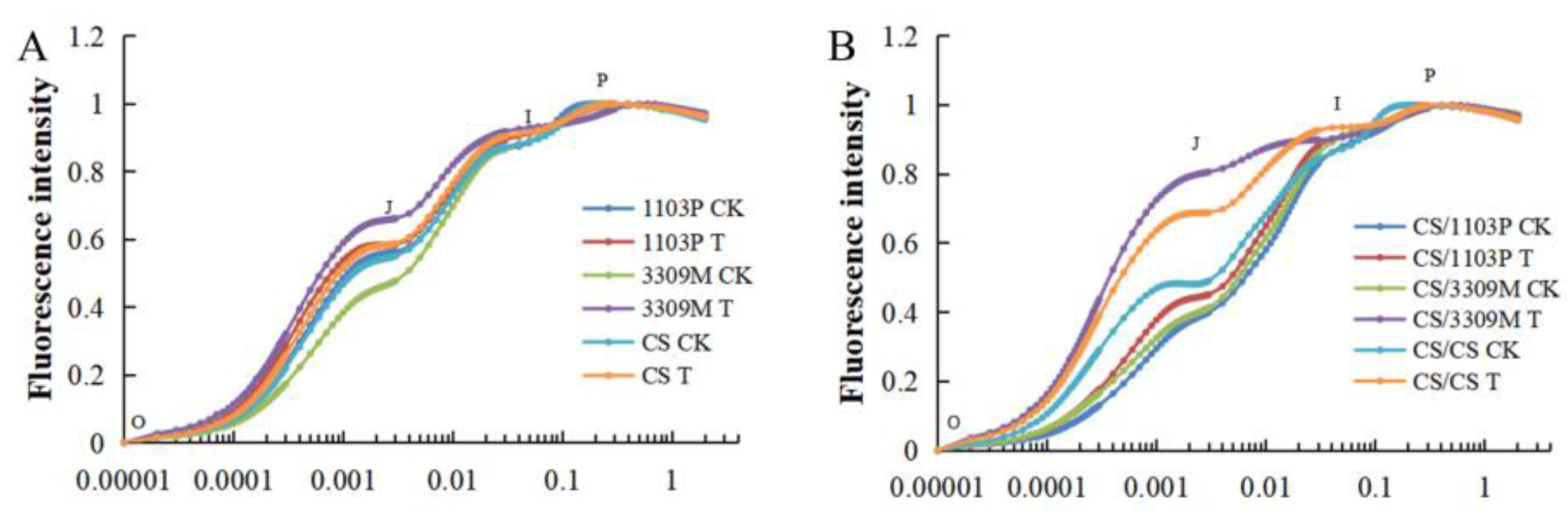
2.5. Effect of Saline–Alkaline Stress on the Rapid Chlorophyll Fluorescence Induction Curve of Different Rootstocks and Rootstock Combinations
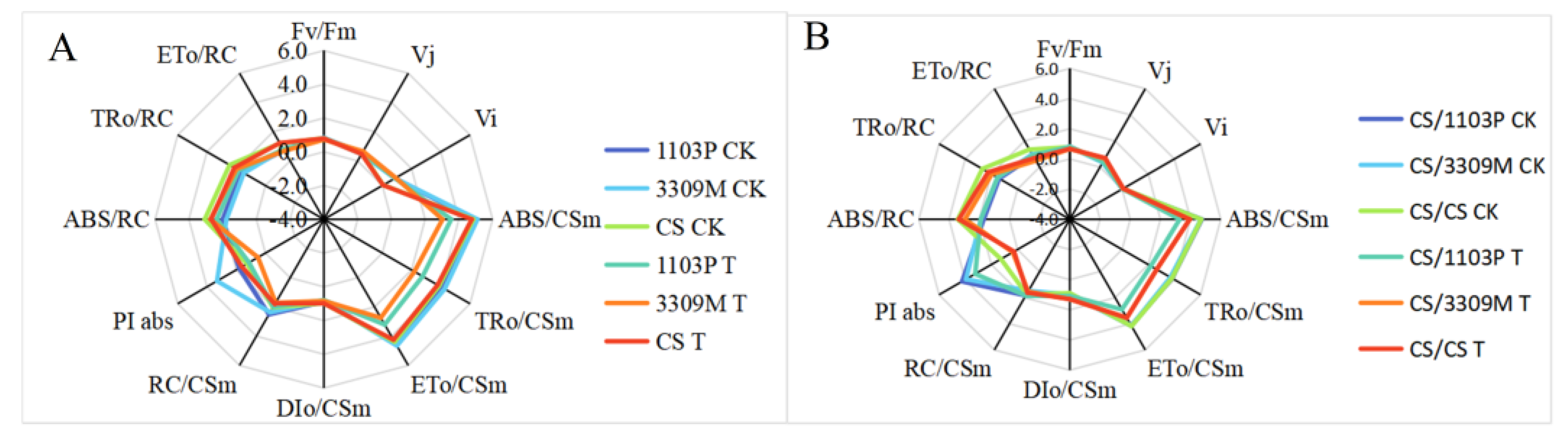
2.6. Effect of Saline–Alkaline Stress on the Fluorescence Parameters of Different Rootstocks and Rootstock Combinations
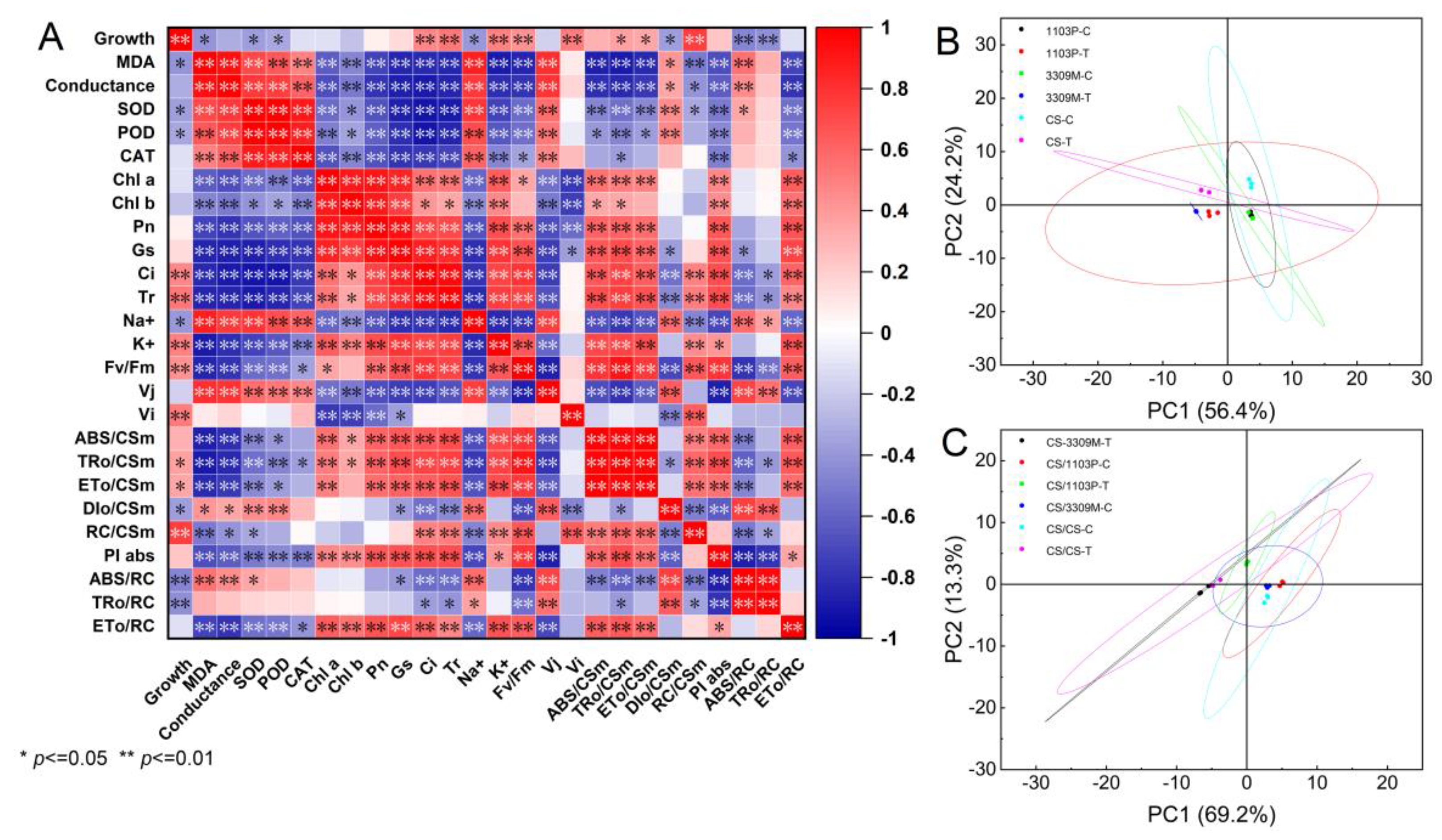
2.7. Correlation and Principal Component Analysis of Several Indicators of Different Rootstocks and Rootstock Combinations under Saline–Alkaline Stress
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Huang, Z.; Li, M.; Hou, Z. Growth, ionic homeostasis, and physiological responses of cotton under different salt and alkali stresses. Sci. Rep. 2020, 10, 21844. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B. Protection of Halophytes and Their Uses for Cultivation of Saline-Alkali Soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Dong, Q.; Zheng, W.; Duan, D.; Huang, D.; Wang, Q.; Liu, C.; Li, C.; Gong, X.; Li, C.; Mao, K.; et al. MdWRKY30, a group IIa WRKY gene from apple, confers tolerance to salinity and osmotic stresses in transgenic apple callus and Arabidopsis seedlings. Plant Sci. 2020, 299, 110611. [Google Scholar] [CrossRef]
- Yin, R.; Bai, T.; Ma, F.; Wang, X.; Li, Y.; Yue, Z. Physiological responses and relative tolerance by Chinese apple rootstocks to NaCl stress. Sci. Hortic. 2010, 126, 247–252. [Google Scholar] [CrossRef]
- Ma, C.; Bian, C.; Liu, W.; Sun, Z.; Xi, X.; Guo, D.; Liu, X.; Tian, Y.; Wang, C.; Zheng, X. Strigolactone alleviates the salinity-alkalinity stress of Malus hupehensis seedlings. Front. Plant Sci. 2022, 13, 901782. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2020, 15, e0244365. [Google Scholar] [CrossRef]
- Xie, R.; He, W.; Chai, J.; Luo, L.; Wang, Y.; Chen, Q.; Tang, H.; Wang, X. A Study of Scion Phenotypes in Pummelo Grafted onto a New Citrus Rootstock Citrus junos ‘Pujiang Xiangcheng’. Horticulturae 2022, 8, 1039. [Google Scholar] [CrossRef]
- dos Santos, I.C.; de Almeida, A.-A.F.; Pirovani, C.P.; Costa, M.G.C.; da Conceição, A.S.; Filho, W.D.S.S.; Filho, M.A.C.; Gesteira, A.D.S. Physiological, biochemical and molecular responses to drought conditions in field-grown grafted and ungrafted citrus plants. Environ. Exp. Bot. 2019, 162, 406–420. [Google Scholar] [CrossRef]
- Ranjbar, A.; Imani, A.; Piri, S.; Abdoosi, V. Grafting commercial cultivars of almonds on accurate rootstocks mitigates adverse effects of drought stress. Sci. Hortic. 2021, 293, 110725. [Google Scholar] [CrossRef]
- Bayoumi, Y.; Abd-Alkarim, E.; El-Ramady, H.; El-Aidy, F.; Hamed, E.-S.; Taha, N.; Prohens, J.; Rakha, M. Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses. Horticulturae 2021, 7, 61. [Google Scholar] [CrossRef]
- Fu, S.; Chen, J.; Wu, X.; Gao, H.; Lü, G. Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato. Horticulturae 2022, 8, 595. [Google Scholar] [CrossRef]
- Gaion, L.A.; Monteiro, C.C.; Cruz, F.J.R.; Rossatto, D.R.; López-Díaz, I.; Carrera, E.; Lima, J.E.; Peres, L.E.P.; Carvalho, R.F. Constitutive gibberellin response in grafted tomato modulates root-to-shoot signaling under drought stress. J. Plant Physiol. 2018, 221, 11–21. [Google Scholar] [CrossRef] [PubMed]
- López-Serrano, L.; Canet-Sanchis, G.; Selak, G.V.; Penella, C.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Pepper Rootstock and Scion Physiological Responses Under Drought Stress. Front. Plant Sci. 2019, 10, 38. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, F.; Huang, Y.; Bie, Z. Grafting Watermelon onto Pumpkin Increases Chilling Tolerance by up Regulating Arginine Decarboxylase to Increase Putrescine Biosynthesis. Front. Plant Sci. 2022, 12, 812396. [Google Scholar] [CrossRef]
- Feng, X.H.; Guo, K.; Yang, C.; Li, J.S.; Chen, H.Y.; Liu, X.J. Growth and fruit production of tomato grafted onto wolfberry (Lycium chinense) rootstock in saline soil. Sci. Hortic. 2019, 255, 298–305. [Google Scholar] [CrossRef]
- Lo’at, A.A.; Ghazi, D.A.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Hassan, S.; Abdein, M.A. Growth, Yield, and Bunch Quality of “Superior Seedless” Vines Grown on Different Rootstocks Change in Response to Salt Stress. Plants 2021, 10, 2215. [Google Scholar] [CrossRef]
- Walker, R.; Blackmore, D.; Clingeleffer, P.; Holt, H.; Pearson, W.; Francis, I. Effect of rootstock on yield, grape composition and wine sensory attributes of Shiraz grown in a moderately saline environment. Aust. J. Grape Wine Res. 2019, 25, 414–429. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, K.; Yang, X.; Li, J.; Li, X. Comparative study of the key aromatic compounds of Cabernet Sauvignon wine from the Xinjiang region of China. J. Food Sci. Technol. 2021, 58, 2109–2120. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhen, W.; Sun, T.; Hu, X. Abscisic acid alleviates harmful effect of saline–alkaline stress on tomato seedlings. Plant Physiol. Biochem. 2022, 175, 58–67. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Y.; Xiang, G.; Yao, Y. Effects of SA15 and SA17 rootstocks on growth and berry quality of ‘Merlot’ grape under salt and alkali treatment. J. Nanjing Agric. Univ. 2019, 42, 1022–1029. (In Chinese) [Google Scholar]
- Migicovsky, Z.; Cousins, P.; Jordan, L.M.; Myles, S.; Striegler, R.K.; Verdegaal, P.; Chitwood, D.H. Grapevine rootstocks affect growth-related scion phenotypes. Plant Direct 2021, 5, e00324. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, L.; Ma, Y.; Liu, Y.; Zhao, Y.; Zhao, B.; Sun, J. Effects of saline-alkali stress on photosynthetic and chlorophyll fluores-cence characteristics of different grape rootstocks. J. Fruit Sci. 2022, 39, 773–783. (In Chinese) [Google Scholar] [CrossRef]
- An, Y.; Gao, Y.; Tong, S.; Liu, B. Morphological and Physiological Traits Related to the Response and Adaption of Bolboschoenus planiculmis Seedlings Grown under Salt-Alkaline Stress Conditions. Front. Plant Sci. 2021, 12, 567782. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Wu, Y.; Guo, A.; Hu, Y.; Zhu, Y.; Jia, X. Photosynthetic and physiological response to biochemical improvement of ‘Red Globe’ grape under saline-alkali stress. Agric. Res. Arid. Areas 2018, 36, 214–222. (In Chinese) [Google Scholar]
- Yu, X.; Yue, Q.Y.; Yu, M.; Du, Y.P.; Yao, Y.X. Physiological responses of grape rootstocks SA15, SA17 and 1103P to salt-alkali stress. Plant Physiol. J. 2020, 56, 57–65. (In Chinese) [Google Scholar] [CrossRef]
- Martin, L.; Vila, H.; Bottini, R.; Berli, F. Rootstocks increase grapevine tolerance to NaCl through ion compartmentalization and exclusion. Acta Physiol. Plant. 2020, 42, 145. [Google Scholar] [CrossRef]
- Luo, X.; Dai, Y.; Zheng, C.; Yang, Y.; Chen, W.; Wang, Q.; Chandrasekaran, U.; Du, J.; Liu, W.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2020, 229, 950–962. [Google Scholar] [CrossRef]
- Lu, Q.; Feng, L.; Wang, S.; Gu, L.; Chu, R.; Zhou, L. Effects of Compound Saline-alkali Stress on Physiological and Biochemical Indexes of Table Grapes. Chin. Agric. Sci. Bull. 2023, 39, 62–70. (In Chinese) [Google Scholar]
- Sui, C.C. The Relieving Effect of Exogenous Spermidine on Saline-alkali Stress in Grape Seedlings; Shandong Agriculture University: Taian, China, 2021. [Google Scholar]
- Yang, Y.; Wang, L.; Tian, J.; Li, J.; Sun, J.; He, L.; Guo, S.; Tezuka, T. Proteomic study participating the enhancement of growth and salt tolerance of bottle gourd rootstock-grafted watermelon seedlings. Plant Physiol. Biochem. 2012, 58, 54–65. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zheng, W.; Tian, Y.; Wu, Y.; Zhou, D.W. Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 2011, 49, 275–284. [Google Scholar] [CrossRef]
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.-W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qin, H.; Zhao, Y.; Liu, Y.; Ai, J.; Cao, J.; Yang, Y.; Shen, Y. Photosynthetic characteristics and growth development of amur grape (Vitis amurensis rupr.) under saline-alkali stress. Northwest J. Bot. 2017, 37, 339–345. (In Chinese) [Google Scholar]
- Soylemezoglu, G.; Demir, K.; Inal, A.; Gunes, A. Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci. Hortic. 2009, 123, 240–246. [Google Scholar] [CrossRef]
- Sun, J.; Li, S.; Guo, H.; Hou, Z. Ion homeostasis and Na+ transport-related gene expression in two cotton (Gossypium hirsutum L.) varieties under saline, alkaline and saline-alkaline stresses. PLoS ONE 2021, 16, e0256000. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shaw, B.P. Biochemical and molecular characterisations of salt tolerance components in rice varieties tolerant and sensitive to NaCl: The relevance of Na+ exclusion in salt tolerance in the species. Funct. Plant Biol. 2020, 48, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- López-Aguilar, R.; Orduño-Cruz, A.; Lucero-Arce, A.; Murillo-Amador, B.; Troyo-Diéguez, E. Response to salinity of three grain legumes for potential cultivation in arid areas. Soil Sci. Plant Nutr. 2003, 49, 329–336. [Google Scholar] [CrossRef]
- Sun, J.; Cao, H.; Cheng, J.; He, X.; Sohail, H.; Niu, M.; Huang, Y.; Bie, Z. Pumpkin CmHKT1;1 Controls Shoot Na+ Accumulation via Limiting Na+ Transport from Rootstock to Scion in Grafted Cucumber. Int. J. Mol. Sci. 2018, 19, 2648. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root respiratory burst oxidase homologue-dependent H2O2 production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure. J. Exp. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef] [PubMed]
- Asins, M.J.; Raga, V.; Roca, D.; Belver, A.; Carbonell, E.A. Genetic dissection of tomato rootstock effects on scion traits under moderate salinity. Theor. Appl. Genet. 2015, 128, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2011, 352, 353–362. [Google Scholar] [CrossRef]
- Alfocea, F.P.; Balibrea, M.E.; Alarcón, J.J.; Bolarín, M.C. Composition of Xylem and Phloem Exudates in Relation to the Salt-tolerance of Domestic and Wild Tomato Species. J. Plant Physiol. 2000, 156, 367–374. [Google Scholar] [CrossRef]
- Wen, X.; Qiu, N.; Lu, Q.; Lu, C. Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta 2004, 220, 486–497. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, L.; Yang, Z.; Mubarak, H.; Tang, C. Chlorophyll Fluorescence in Leaves of Ficus tikoua under Arsenic Stress. Bull. Environ. Contam. Toxicol. 2016, 97, 576–581. [Google Scholar] [CrossRef]
- Han, J.; Gu, L.; Wen, J.; Sun, Y. Inference of photosynthetic capacity parameters from chlorophyll a fluorescence is affected by redox state of PSII reaction centers. Plant Cell Environ. 2022, 45, 1298–1314. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Detection of Chilling Injury in Pickling Cucumbers Using Dual-Band Chlorophyll Fluorescence Imaging. Foods 2021, 10, 1094. [Google Scholar] [CrossRef]
- Amorim, T.L.; Santos, H.R.B.; Neto, J.B.; Hermínio, P.J.; Silva, J.R.I.; Silva, M.M.A.; Simões, A.N.; Souza, E.; Ferreira-Silva, S.L. Resistant rootstocks mitigate ionic toxicity with beneficial effects for growth and photosynthesis in grapevine grafted plants under salinity. Sci. Hortic. 2023, 317, 112053. [Google Scholar] [CrossRef]
- Rao, L.; Li, S.; Cui, X. Leaf morphology and chlorophyll fluorescence characteristics of mulberry seedlings under waterlogging stress. Sci. Rep. 2021, 11, 13379. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Romero, J.; Lagorio, M. Effects of sub-optimal illumination in plants. Comprehensive chlorophyll fluorescence analysis. J. Photochem. Photobiol. B Biol. 2021, 218, 112182. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef]
- Luo, J.; Liu, H.; Liu, X.; Li, X.; Liao, H.; Fu, X. Long distance signal transduction in response to environmental changes in plants. Yi Chuan/Hered. 2022, 44, 556–566. [Google Scholar] [CrossRef]
- Lo’ay, A.; Doaa, M. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Hu, W.H.; Yan, X.H.; Li, X.H.; Cao, D.G. Effects of 24-Epibrassinolide on the Chlorophyll Fluorescence Transient in Leaves of Pepper under Drought Stress. Bull. Bot. Res. 2021, 41, 53–59. (In Chinese) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Liu, Z.; Zhu, C.; Zhang, Z.; Shi, W.; Lu, Q.; Sun, J. Saline–Alkaline Stress Resistance of Cabernet Sauvignon Grapes Grafted on Different Rootstocks and Rootstock Combinations. Plants 2023, 12, 2881. https://doi.org/10.3390/plants12152881
Zhao B, Liu Z, Zhu C, Zhang Z, Shi W, Lu Q, Sun J. Saline–Alkaline Stress Resistance of Cabernet Sauvignon Grapes Grafted on Different Rootstocks and Rootstock Combinations. Plants. 2023; 12(15):2881. https://doi.org/10.3390/plants12152881
Chicago/Turabian StyleZhao, Baolong, Zhiyu Liu, Chunmei Zhu, Zhijun Zhang, Wenchao Shi, Qianjun Lu, and Junli Sun. 2023. "Saline–Alkaline Stress Resistance of Cabernet Sauvignon Grapes Grafted on Different Rootstocks and Rootstock Combinations" Plants 12, no. 15: 2881. https://doi.org/10.3390/plants12152881
APA StyleZhao, B., Liu, Z., Zhu, C., Zhang, Z., Shi, W., Lu, Q., & Sun, J. (2023). Saline–Alkaline Stress Resistance of Cabernet Sauvignon Grapes Grafted on Different Rootstocks and Rootstock Combinations. Plants, 12(15), 2881. https://doi.org/10.3390/plants12152881




