Soil Moisture Content Dominates the Photosynthesis of C3 and C4 Plants in a Desert Steppe after Long-Term Warming and Increasing Precipitation
Abstract
1. Introduction
2. Results
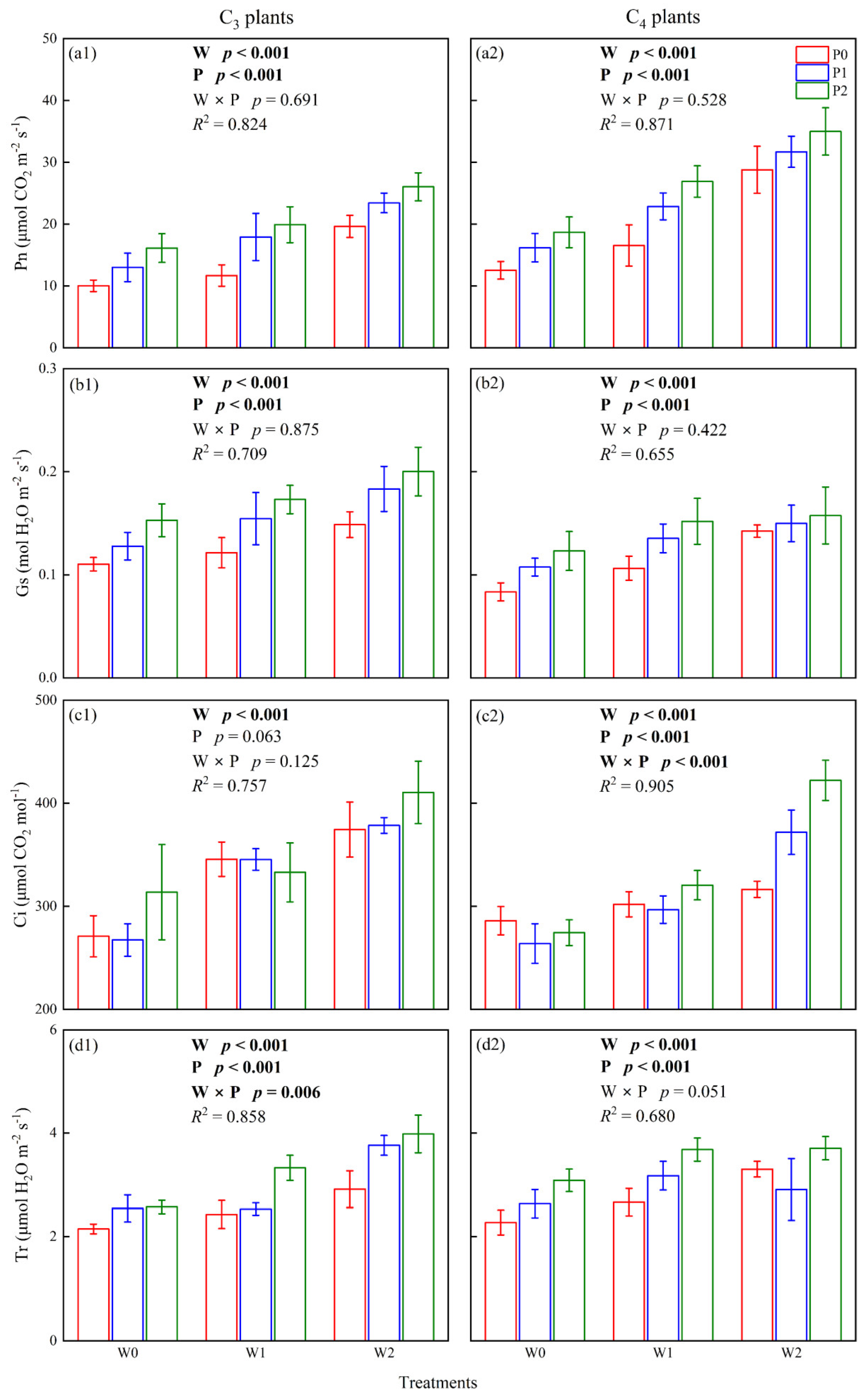
2.1. Photosynthesis of C3 and C4 Plants
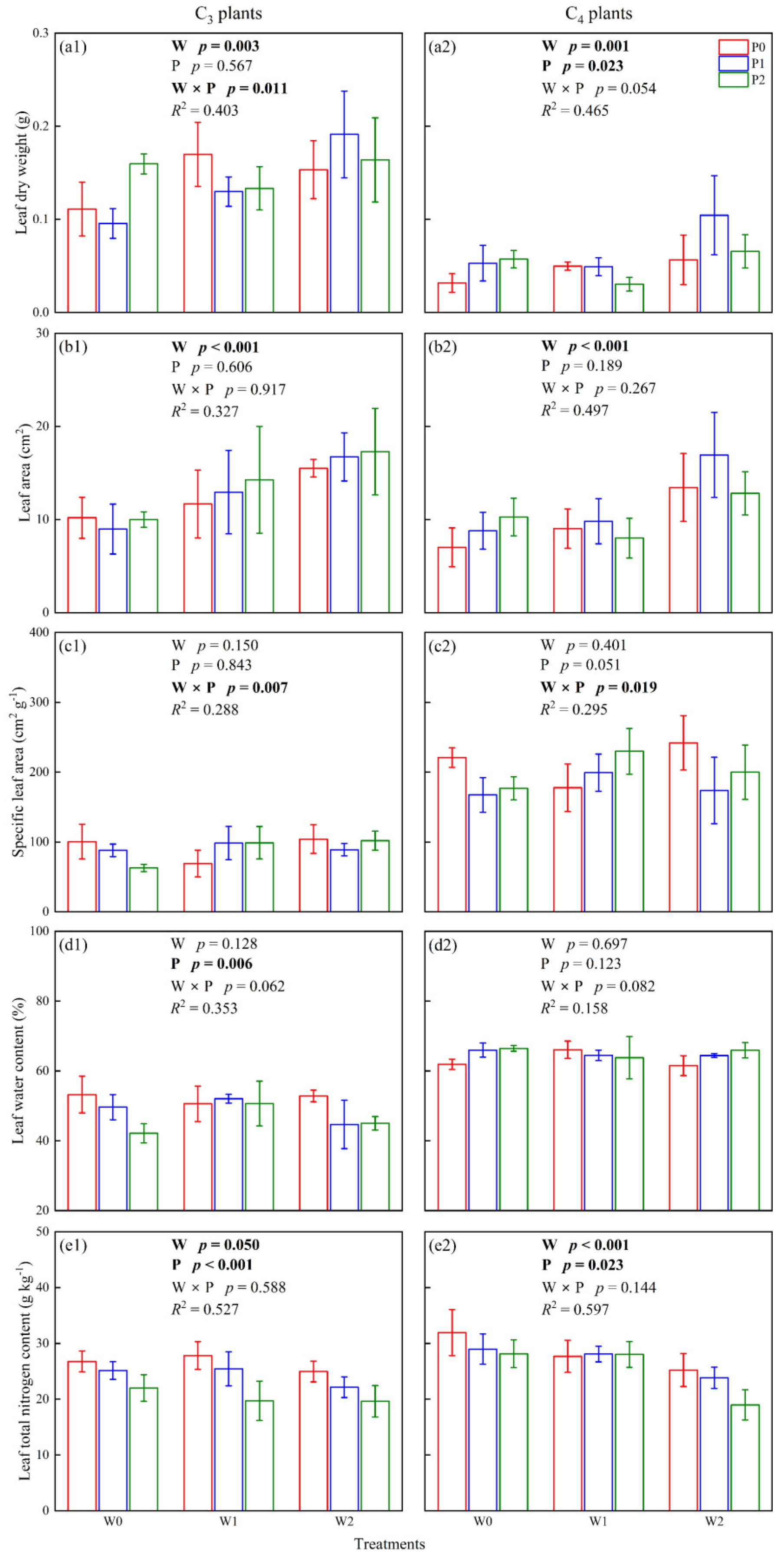
2.2. Functional Traits of C3 and C4 Leaves
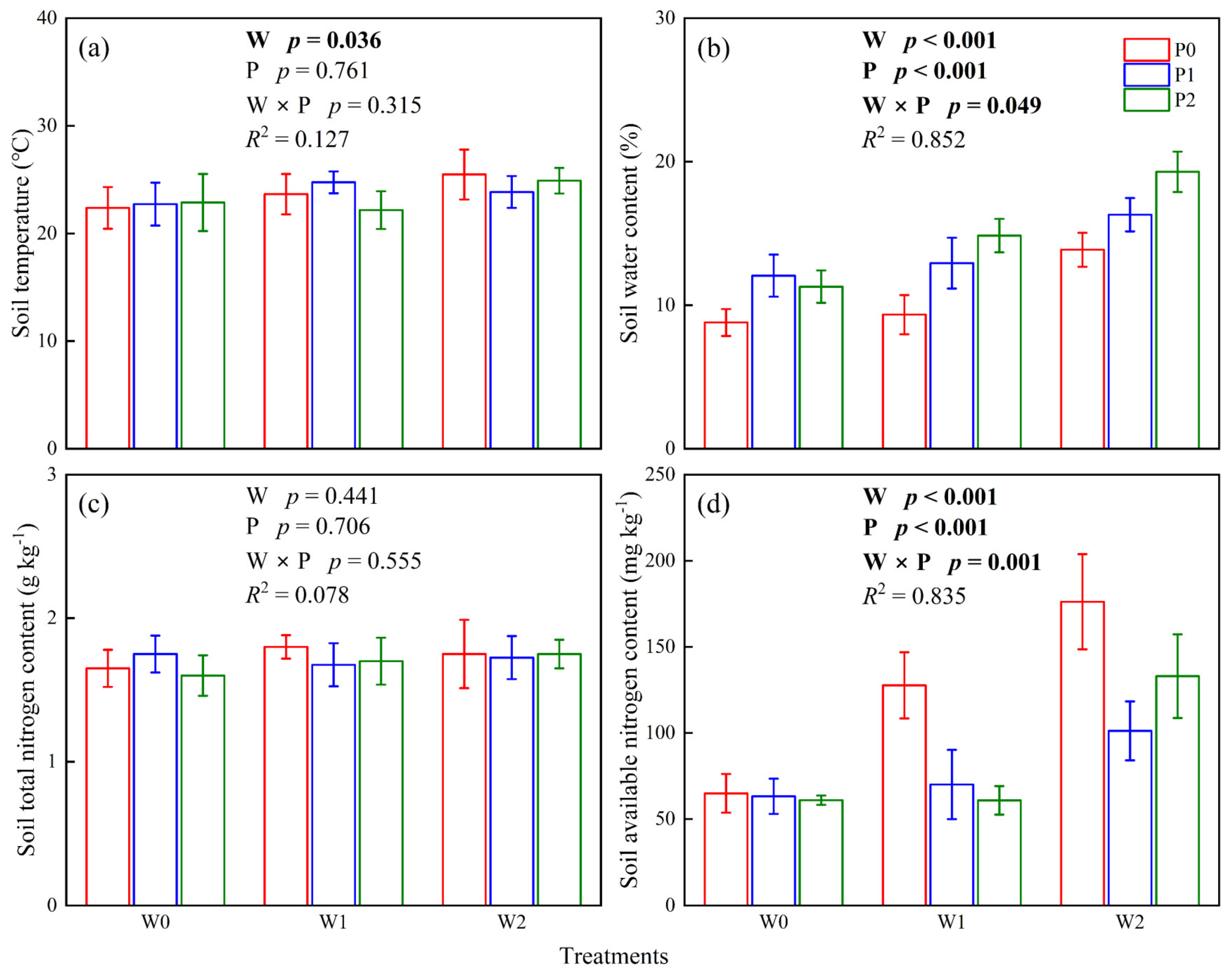
2.3. Soil Physicochemical Parameters
2.4. Redundancy Analysis
3. Discussion
4. Materials and Methods
4.1. Study Site
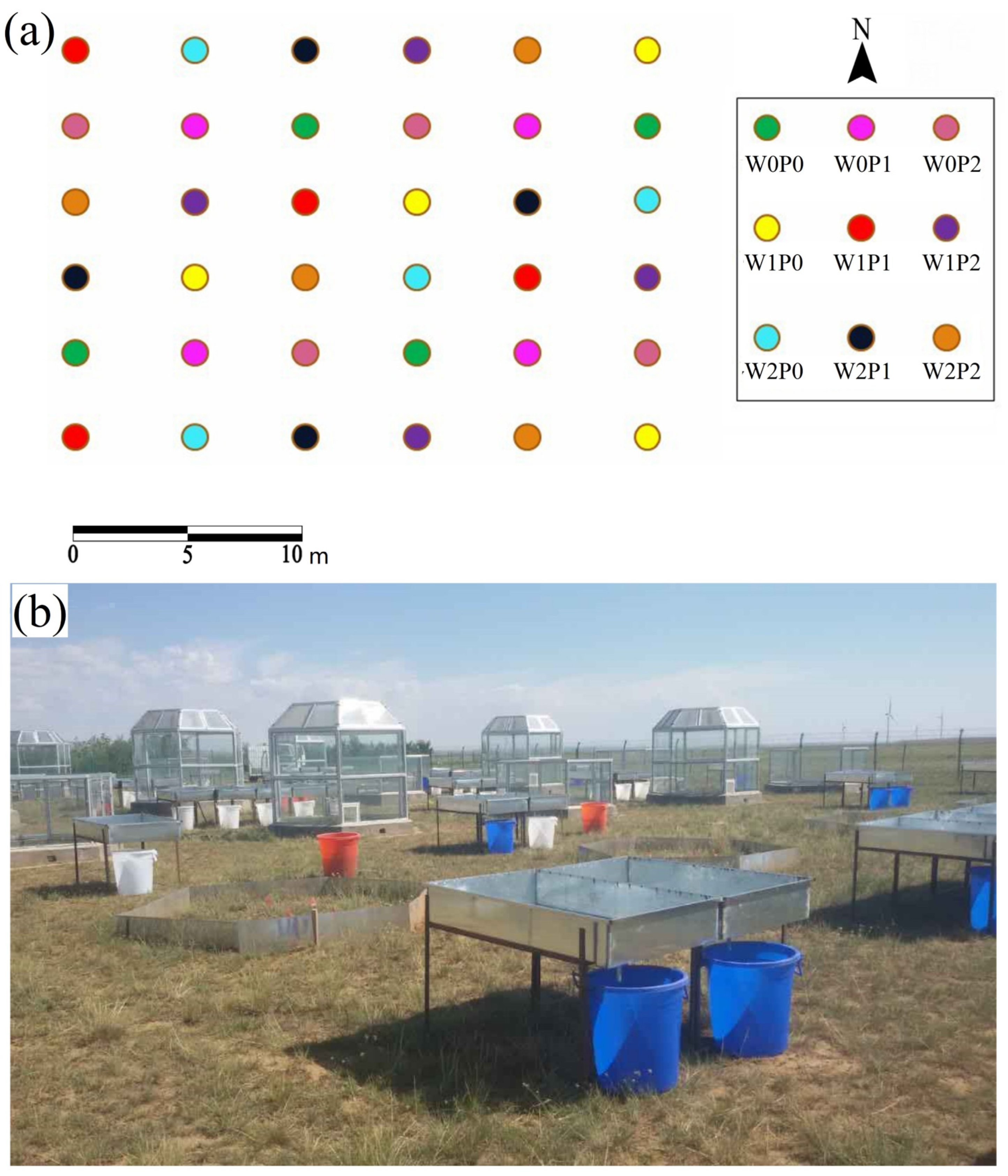
4.2. Experimental Design
4.3. Plant and Soil Sampling
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilhelm, C.; Selmar, D. Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef]
- Simkin, A.J. Genetic engineering for global food security: Photosynthesis and biofortification. Plants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ren, H.Y.; Bisseling, T.; Chang, S.X.; Wang, Z.; Li, Y.H.; Pan, Z.L.; Liu, Y.H.; Cahill, J.F.; Cheng, X.; et al. Long-term N addition, not warming, increases net ecosystem CO2 exchange in a desert steppe in northern China. Environ. Sci. Technol. 2021, 55, 7256–7265. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.; Kelly, S.; von Caemmerer, S. Photosynthesis and food security: The evolving story of C4 rice. Photosynth. Res. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.C. Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol. 2001, 127, 1439–1448. [Google Scholar] [CrossRef]
- Wittmer, M.H.; Auerswald, K.A.R.L.; Bai, Y.; Schaeufele, R.; Schnyder, H.A.N.S. Changes in the abundance of C3/C4 species of Inner Mongolia grassland: Evidence from isotopic composition of soil and vegetation. Glob. Chang. Biol. 2010, 16, 605–616. [Google Scholar] [CrossRef]
- Pau, S.; Edwards, E.J.; Still, C.J. Improving our understanding of environmental controls on the distribution of C3 and C4 grasses. Glob. Chang. Biol. 2013, 19, 184–196. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Ehleringer, J.R. Implications of Quantum Yield Differences on the Distributions of C3 and C4 Grasses. Oecologia 1978, 31, 255–267. [Google Scholar] [CrossRef]
- Brown, R.H. A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci. 1978, 18, 93–98. [Google Scholar] [CrossRef]
- Winslow, J.C.; Hunt, E.R., Jr.; Piper, S.C. The influence of seasonal water availability on global C3 versus C4 grassland biomass and its implications for climate change research. Ecol. Model. 2003, 163, 153–173. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E. C3 and C4 photosynthesis. Encycl. Glob. Environ. Change 2002, 2, 186–190. [Google Scholar]
- Hatch, M.D. C4 photosynthesis: A unique elend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta-Rev. Bioenerg. 1987, 895, 81–106. [Google Scholar] [CrossRef]
- Sage, R.F.; Pearcy, R.W. The nitrogen use efficiency of C3 and C4 plants: II. Leaf nitrogen effects on the gas exchange characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987, 84, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Street-Perrott, F.A.; Metcalfe, S.E.; Brenner, M.; Moreland, M.; Freeman, K.H. Climate change as the dominant control on glacial-interglacial variations in C3 and C4 plant abundance. Science 2001, 293, 1647–1651. [Google Scholar] [CrossRef]
- Taylor, S.H.; Hulme, S.P.; Rees, M.; Ripley, B.S.; Ian Woodward, F.; Osborne, C.P. Ecophysiological traits in C3 and C4 grasses: A phylogenetically controlled screening experiment. New Phytol. 2010, 185, 780–791. [Google Scholar] [CrossRef]
- Niu, S.; Yuan, Z.; Zhang, Y.; Liu, W.; Zhang, L.; Huang, J.; Wan, S. Photosynthetic responses of C3 and C4 species to seasonal water variability and competition. J. Exp. Bot. 2005, 56, 2867–2876. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Habermann, E.; Dias de Oliveira, E.A.; Contin, D.R.; Delvecchio, G.; Viciedo, D.O.; de Moraes, M.A.; Martinez, C.A. Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiol. Plant. 2019, 165, 383–402. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018, 562, 263–267. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Jacob, D.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Zougmoré, R.B. Impacts of 1.5 °C global warming on natural and human systems. In Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Habermann, E.; Contin, D.R.; Afonso, L.F.; Barosela, J.R.; de Pinho Costa, K.A.; Viciedo, D.O.; Martinez, C.A. Future warming will change the chemical composition and leaf blade structure of tropical C3 and C4 forage species depending on soil moisture levels. Sci. Total Environ. 2022, 821, 153342. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.Y.; Wang, Z.Y.; Guo, N.; Xu, X.B.; Liu, P.P.; Wang, C.J. Status of Stipa breviflora as the constructive species will be lost under climate change in the temperate desert steppe in the future. Ecol. Indic. 2021, 126, 107715. [Google Scholar] [CrossRef]
- Volder, A.; Tjoelker, M.G.; Briske, D.D. Contrasting physiological responsiveness of establishing trees and a C4 grass to rainfall events, intensified summer drought, and warming in oak savanna. Glob. Chang. Biol. 2010, 16, 3349–3362. [Google Scholar] [CrossRef]
- Nippert, J.B.; Fay, P.A.; Carlisle, J.D.; Knapp, A.K.; Smith, M.D. Ecophysiological responses of two dominant grasses to altered temperature and precipitation regimes. Acta Oecologica 2009, 35, 400–408. [Google Scholar] [CrossRef]
- Xu, Z.; Shimizu, H.; Ito, S.; Yagasaki, Y.; Zou, C.; Zhou, G.; Zheng, Y. Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 2014, 239, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R.; Cerling, T.E.; Helliker, B.R. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 1997, 112, 285–299. [Google Scholar] [CrossRef]
- Song, B.; Niu, S.; Wan, S. Precipitation regulates plant gas exchange and its long-term response to climate change in a temperate grassland. J. Plant Ecol. 2016, 9, 531–541. [Google Scholar] [CrossRef]
- Wertin, T.M.; Reed, S.C.; Belnap, J. C3 and C4 plant responses to increased temperatures and altered monsoonal precipitation in a cool desert on the Colorado Plateau, USA. Oecologia 2015, 177, 997–1013. [Google Scholar] [CrossRef]
- Smith, N.G.; McNellis, R.; Dukes, J.S. No acclimation: Instantaneous responses to temperature maintain homeostatic photosynthetic rates under experimental warming across a precipitation gradient in Ulmus americana. AoB Plants 2020, 12, plaa027. [Google Scholar] [CrossRef]
- Angerer, J.; Han, G.D.; Fujisaki, I.; Havstad, K.M. Climate change and ecosystems of Asia with Emphasis on Inner Mongolia and Mongolia. Rangelands 2008, 30, 46–51. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, H.Y.; Wang, Z.W.; Li, Z.G.; Liu, Y.H.; Wang, Z.; Liu, Y.H.; Zhang, R.Y.; Zhao, M.L.; Chang, S.X.; et al. Additive negative effects of decadal warming and nitrogen addition on grassland community stability. J. Ecol. 2020, 108, 1442–1452. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, Y.; Yuan, Z.; Liu, W.; Huang, J.; Wan, S. Effects of interspecific competition and nitrogen seasonality on the photosynthetic characteristics of C3 and C4 grasses. Environ. Exp. Bot. 2006, 57, 270–277. [Google Scholar] [CrossRef]
- Niu, S.; Li, Z.; Xia, J.; Han, Y.; Wu, M.; Wan, S. Climatic warming changes plant photosynthesis and its temperature dependence in a temperate steppe of northern China. Environ. Exp. Bot. 2008, 63, 91–101. [Google Scholar] [CrossRef]
- Areejit, S.; Martin, O.C. Shining fresh light on the evolution of photosynthesis. eLife 2013, 2, 01403. [Google Scholar]
- Hadi, A.; Naz, N.; Rehman, F.U.; Kalsoom, M.; Tahir, R.; Adnan, M.; Mehta, J. Impact of climate change drivers on C4 plants: A review. Curr. Res. Agric. Farming 2020, 1, 13–18. [Google Scholar] [CrossRef]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef]
- Collatz, G.J.; Berry, J.A.; Clark, J.S. Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: Present, past, and future. Oecologia 1998, 114, 441–454. [Google Scholar] [CrossRef]
- Togawa-Urakoshi, Y.; Ueno, O. Photosynthetic nitrogen-and water-use efficiencies in C3 and C4 subtype grasses grown under two nitrogen supply levels. Plant Prod. Sci. 2022, 25, 183–194. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, W.; Niu, S.; Wan, S. Plant nitrogen dynamics and nitrogen-use strategies under altered nitrogen seasonality and competition. Ann. Bot. 2007, 100, 821–830. [Google Scholar] [CrossRef]
- Yang, H.J.; Wu, M.Y.; Liu, W.X.; Zhang, Z.; Zhang, N.L.; Wan, S.Q. Community structure and composition in response to climate change in a temperate steppe. Glob. Chang. Biol. 2011, 17, 452–465. [Google Scholar] [CrossRef]
- Lattanzi, F.A. C3/C4 grasslands and climate change. Grassl. Sci. Eur. 2010, 15, 3–13. [Google Scholar]
- Ghannoum, O.; Conroy, J.P.; Driscoll, S.P.; Paul, M.J.; Foyer, C.H.; Lawlor, D.W. Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytol. 2003, 159, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; He, M.; Wang, C.; Wang, Z. The stability of perennial grasses mediates the negative impacts of long-term warming and increasing precipitation on community stability in a desert steppe. Front. Plant Sci. 2023, 14, 1235510. [Google Scholar] [CrossRef]
- Lv, G.; He, M.; Li, Q.; Wang, Z.; Wang, C. Experimental climate change in desert steppe reveals the importance of C4 plants for ecosystem carbon exchange. Ecol. Indic. 2023, 154, 110705. [Google Scholar] [CrossRef]
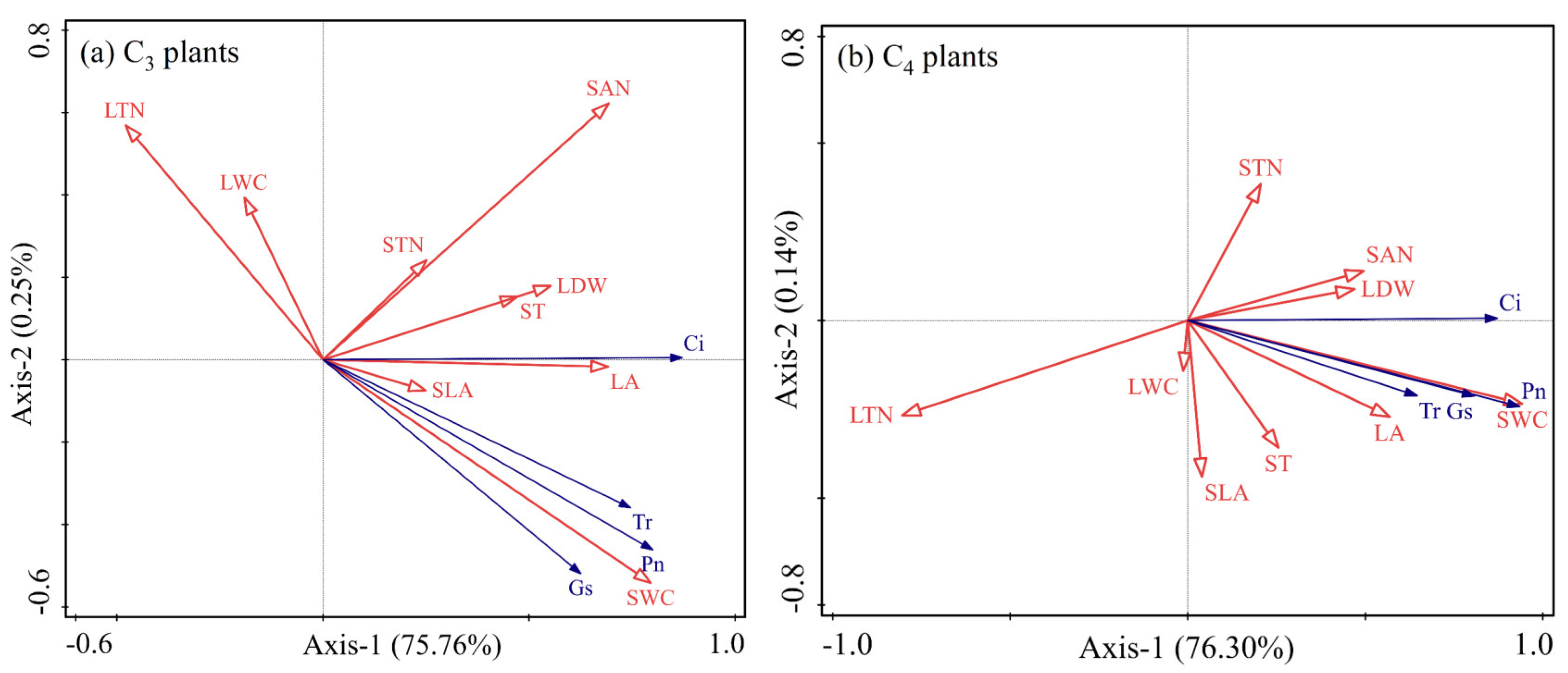
| Photosynthesis in C3 Plants | Photosynthesis in C4 Plants | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | Explains % | F | p | Factors | Explains % | F | p | |
| SWC | 48.0 | 31.4 | 0.002 ** | SWC | 67.7 | 71.2 | 0.001 ** | |
| SAN | 19.6 | 20.0 | 0.002 ** | SAN | 5.3 | 6.5 | 0.024 * | |
| LTN | 3.6 | 4.1 | 0.050 * | LTN | 1.4 | 1.8 | 0.220 | |
| LDW | 1.6 | 1.8 | 0.184 | STN | 0.5 | 0.6 | 0.466 | |
| ST | 2.5 | 3.0 | 0.094 | LWC | 0.4 | 0.5 | 0.528 | |
| SLA | 0.3 | 0.3 | 0.572 | LDW | 0.3 | 0.4 | 0.520 | |
| STN | 0.2 | 0.3 | 0.636 | ST | 0.4 | 0.4 | 0.528 | |
| LA | 0.1 | 0.1 | 0.696 | LA | 0.4 | 0.4 | 0.550 | |
| LWC | <0.1 | <0.1 | 0.756 | SLA | <0.1 | <0.1 | 0.786 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Jin, J.; He, M.; Wang, C. Soil Moisture Content Dominates the Photosynthesis of C3 and C4 Plants in a Desert Steppe after Long-Term Warming and Increasing Precipitation. Plants 2023, 12, 2903. https://doi.org/10.3390/plants12162903
Lv G, Jin J, He M, Wang C. Soil Moisture Content Dominates the Photosynthesis of C3 and C4 Plants in a Desert Steppe after Long-Term Warming and Increasing Precipitation. Plants. 2023; 12(16):2903. https://doi.org/10.3390/plants12162903
Chicago/Turabian StyleLv, Guangyi, Jing Jin, Mengting He, and Chengjie Wang. 2023. "Soil Moisture Content Dominates the Photosynthesis of C3 and C4 Plants in a Desert Steppe after Long-Term Warming and Increasing Precipitation" Plants 12, no. 16: 2903. https://doi.org/10.3390/plants12162903
APA StyleLv, G., Jin, J., He, M., & Wang, C. (2023). Soil Moisture Content Dominates the Photosynthesis of C3 and C4 Plants in a Desert Steppe after Long-Term Warming and Increasing Precipitation. Plants, 12(16), 2903. https://doi.org/10.3390/plants12162903






