Effects of Reduced Phosphate Fertilizer and Increased Trichoderma Application on the Growth, Yield, and Quality of Pepper
Abstract
:1. Introduction
2. Results
2.1. Effects of Phosphorus Fertilizer and Trichoderma Applications on Plant Growth
2.2. Effects of Phosphorus Fertilizer and Trichoderma Applications on Plant Nutrition
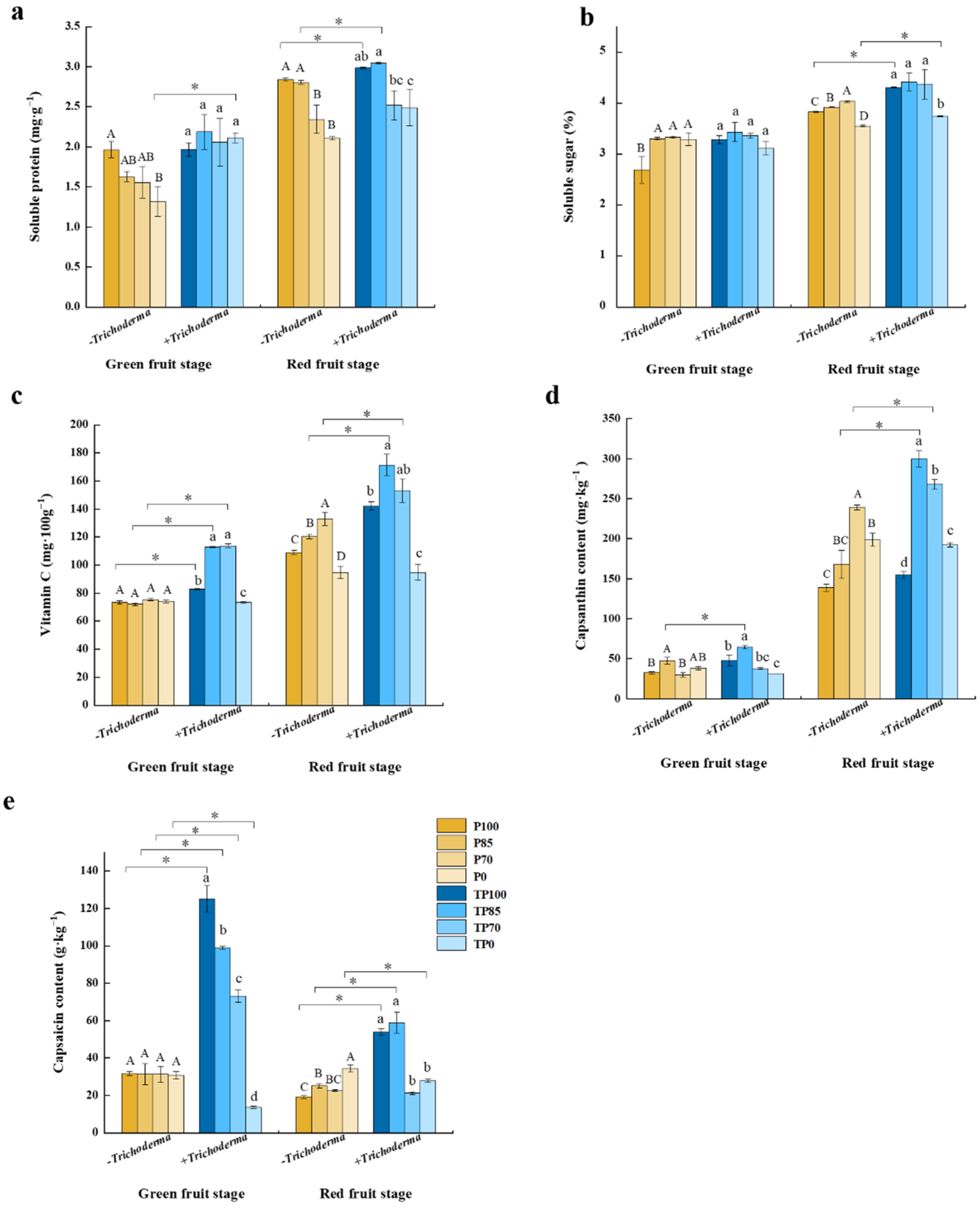
2.3. Effects of Phosphorus Fertilizer and Trichoderma Applications on Pepper Fruit Quality
2.4. Effects of Phosphorus Fertilizer and Trichoderma Applications on Yield per Plant
2.5. Effects of Phosphorus Fertilizer and Trichoderma Applications on Soil Fertility
3. Discussion
4. Material and Methods
4.1. Plant Material and Experimental Conditions
4.2. Experimental Design and Treatments
4.3. Measurement of Plant Growth Indices
4.4. Determination of the N, P, and K Content of Pepper Plants
4.5. Determination of Fruit Yield and Quality Indexes
4.6. Determination of the N, P, and K Content of Soil
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thampi, P.S.S. A Glimpse of the World Trade in Capsicum. In Capsicum; CRC Press: Boca Raton, FL, USA, 2003; pp. 36–44. [Google Scholar]
- Buckenhüskes, H.J. Current Requirements on Paprika Powder for Food Industry. In Capsicum; CRC Press: Boca Raton, FL, USA, 2003; pp. 243–250. [Google Scholar]
- Baenas, N.; Belović, M.; Ilic, N.; Morenoc, D.A.; García-Viguerac, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [PubMed]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar]
- Du, Y.; Jin, T.; Zhao, H.; Han, C.; Chen, Q.; Yue, F.; Luo, Z.; Fu, M. Synergistic inhibitory effect of 1-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) treatment on chlorophyll degradation of green pepper fruit during storage. Postharvest Biol. Technol. 2021, 171, 111363. [Google Scholar]
- Buczkowska, H.; Michalojc, Z.; Nurzynska-Wierdak, R. Yield and fruit quality of sweet pepper depending on foliar application of calcium. Turk. J. Agric. For. 2016, 40, 222–228. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Aroiee, H.; Azizi, M.; Nemati, H.; Jaafar, H.Z.E. Effect of humic acid on antioxidant activities and fruit quality of hot pepper (Capsicum annuum L.). J. Herbs Spices Med. Plants 2012, 18, 360–369. [Google Scholar]
- Fiasconaro, M.L.; Lovato, M.E.; Antolín, M.C.; Clementi, L.A.; Torres, N.; Gervasio, S.; Martín, C.A. Role of proline accumulation on fruit quality of pepper (Capsicum annuum L.) grown with a K-rich compost under drought conditions. Sci. Hortic. 2019, 249, 280–288. [Google Scholar]
- Zhang, J.; Lv, J.; Dawuda, M.M.; Xie, J.; Yu, J.; Li, J.; Zhang, X.; Tang, C.; Wang, C.; Gan, Y. Appropriate ammonium-nitrate ratio improves nutrient accumulation and fruit quality in pepper (Capsicum annuum L.). Agronomy 2019, 9, 683. [Google Scholar] [CrossRef]
- Mardanluo, S.; Souri, M.K.; Ahmadi, M. Plant growth and fruit quality of two pepper cultivars under different potassium levels of nutrient solutions. J. Plant Nutr. 2018, 41, 1604–1614. [Google Scholar]
- Buddhi, C.W.; Min-Ho, Y. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: A review. Afr. J. Microbiol. Res. 2012, 6, 6600–6605. [Google Scholar]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera Abel. Forests 2019, 10, 348. [Google Scholar]
- Janati, W.; Benmrid, B.; Elhaissoufi, W.; Zeroual, Y.; Nasielski, J.; Bargaz, A. Will Phosphate Bio-Solubilization Stimulate Biological Nitrogen Fixation in Grain Legumes? Front. Agron. 2021, 3, 637196. [Google Scholar] [CrossRef]
- Gurdeep, K.; Reddy, M.S. Effects of phosphate-solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 2015, 25, 428–437. [Google Scholar]
- Toro, M. Phosphate Solubilizing Microorganisms in the Rhizosphere of Native Plants from Tropical Savannas: An Adaptive Strategy to Acid Soils. In First International Meeting on Microbial Phosphate Solubilization; Springer: Dordrecht, The Netherlands, 2007; pp. 249–252. [Google Scholar]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- El Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 2015, 31, 50. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, S.; Ray, S.; Raghuwansh, R.; Singh, H.B. Differential reprogramming of defense network in Capsicum annum L. plants against colletotrichum truncatum infection by phyllospheric and rhizospheric trichoderma strains. J. Plant Growth Regul. 2020, 39, 751–763. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; Rosa, A.D.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop. Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Arasu, V.S.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.S.; Zou, Z.R.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, J.; Hu, X.; Wu, J. Effect of aluminum toxicity and phosphorus deficiency on the growth and photosynthesis of oil tea (Camellia oleifera Abel.) seedlings in acidic red soils. Acta Physiol. Plant 2011, 33, 1285–1292. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Prasuhn, V.; Stamm, C.; Schulin, R. Phosphorus losses in runoff from manured grassland of different soil P status at two rainfall intensities. Agr. Ecosyst. Environ. 2012, 153, 65–74. [Google Scholar] [CrossRef]
- Sharma, B.L.; Singh, S.P.; Sharma, M.L. Bio-degradation of crop residues by Trichoderma species vis-a vis nutrient quality of the prepared compost. Sugar Technol. 2012, 14, 174–180. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Gurunathan, S.; Rangappa, S.; Huntrike, S.S.; Srivastava, R.K. Elicitation of resistance and associated defense responses in Trichoderma hamatum induced protection against pearl millet downy mildew pathogen. Sci. Rep. 2017, 7, 43991. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using non-destructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant. Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Bedine, M.A.B.; Iacomi, B.; Tchameni, S.N.; Sameza, M.L.; Fekam, F.B. Harnessing the phosphate-solubilizing ability of Trichoderma strains to improve plant growth, phosphorus uptake and photosynthetic pigment contents in common bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2022, 45, 102510. [Google Scholar] [CrossRef]
- Nzanza, B.; Marais, D.; Soundy, P. Yield and nutrient content of tomato (Solanum lycopersicum L.) as influenced by Trichoderma harzianum and Glomus mosseae inoculation. Sci. Hortic. 2012, 144, 55–59. [Google Scholar] [CrossRef]
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017, 29, 67–75. [Google Scholar] [CrossRef]
- Anam, G.B.; Reddy, M.S.; Ahn, Y.H. Characterization of Trichoderma asperellum RM-28 for its sodic/saline-alkali tolerance and plant growth promoting activities to alleviate toxicity of red mud. Sci. Total. Environ. 2019, 662, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Perazzolli, M.; Palmieri, M.C.; Matafora, V.; Achi, A.; Pertot, I. Phosphoproteomic analysis of induced resistance reveals activation of signal transduction processes by beneficial and pathogenic interaction in grapevine. J. Plant Physiol. 2016, 195, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma applications on strawberry plants modulate the physiological processes positively affecting fruit production and quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Javed, H.U.; Zahid, M.S.; Wu, Z.; Ma, C.; Jiu, S.; Xu, W.; Zhang, C.; Wang, S. Nutrient solution with high nitrogen content, a suitable facilitator of growth and berry quality in hydroponic ‘Shine Muscat’grapevine (Vitis vinifera × V. labrusca). Sci. Hortic. 2023, 310, 111749. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun. Soil. Sci. Plan. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Kitson, R.E.; Mellon, M.G. Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind. Eng. Chem. Anal. Ed. 1944, 16, 379–383. [Google Scholar] [CrossRef]
- Johnston, B.R.; Duncan, C.W.; Lawton, K.; Benne, E.J. Determination of potassium in plant materials with a flame photometer. J. Ass. Offic. Agr. Chem. 1952, 35, 813. [Google Scholar] [CrossRef]
- Sun, M.J.; Chao, Y.; He, W.; Kang, X.; Yang, Q.; Wang, H.; Pan, H.; Lou, Y.; Zhuge, Y. Changes in Foxtail Millet (Setaria italica L.) Yield, Quality, and Soil Microbiome after Replacing Chemical Nitrogen Fertilizers with Organic Fertilizers. Sustainability 2022, 14, 16412. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Cruz, J.; Carrillo-Rodríguez, J.C. Phytochemical evaluation of wild and cultivated pepper (Capsicum annuum L. and C. pubescens Ruiz & Pav.) from Oaxaca, Mexico. Chil. J. Agr. Res. 2011, 71, 578. [Google Scholar]
- Wolf, A.M.; Baker, D.E. Colorimetric method for phosphorus measurement in ammonium oxalate soil extracts. Commun. Soil. Sci. Plan. 1990, 21, 2257–2263. [Google Scholar] [CrossRef]
- Koralage, I.S.A.; Silva, N.R.N.; De Silva, C.S. The determination of available phosphorus in soil: A quick and simple method. OUSL J. 2015, 8, 1–17. [Google Scholar]
- Turner, B.L.; Newman, S.; Reddy, K.R. Overestimation of organic phosphorus in wetland soils by alkaline extraction and molybdate colorimetry. Environ. Sci. Technol. 2006, 40, 3349–3354. [Google Scholar] [CrossRef]
- Tang, X.; Xia, M.; Guan, F.; Fan, S. Spatial distribution of soil nitrogen, phosphorus and potassium stocks in Moso bamboo forests in subtropical China. Forests 2016, 7, 267. [Google Scholar] [CrossRef]






| Red Fruit Stage | Different Levels of Phosphate | Trichoderma | Different Levels of Phosphate * Trichoderma | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Yield per plant | 492.556 | *** | 432.774 | *** | 27.692 | *** |
| pH | EC (μm·g−1) | Total N Content (g·kg−1) | Total P Content (g·kg−1) | Total K Content (g·kg−1) | Alkali Hydrolyzed N Content (mg·kg−1) | Available P Content (mg·kg−1) | Available K Content (mg·kg−1) | Organic Matter (%) |
|---|---|---|---|---|---|---|---|---|
| 6.64 | 217.82 | 1.81 | 1.18 | 10.54 | 102.28 | 103.97 | 81.6 | 4.45 |
| Base Fertilizer (g) | Top Dressing (g) | |||||
|---|---|---|---|---|---|---|
| Treatment | Calcium Superphosphate | Urea | Potassium Sulfate | Calcium Superphosphate | Urea | Potassium Sulfate |
| P100 | 562.50 | 146.74 | 135.00 | 168.84 | 178.92 | 40.50 |
| P85 | 478.08 | 146.74 | 135.00 | 143.51 | 178.92 | 40.50 |
| P70 | 393.84 | 146.74 | 135.00 | 118.19 | 178.92 | 40.50 |
| P0 | 0.00 | 146.74 | 135.00 | 0.00 | 178.92 | 40.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Zou, C.; Jiang, Y.; Yu, X.; Ye, X. Effects of Reduced Phosphate Fertilizer and Increased Trichoderma Application on the Growth, Yield, and Quality of Pepper. Plants 2023, 12, 2998. https://doi.org/10.3390/plants12162998
Duan X, Zou C, Jiang Y, Yu X, Ye X. Effects of Reduced Phosphate Fertilizer and Increased Trichoderma Application on the Growth, Yield, and Quality of Pepper. Plants. 2023; 12(16):2998. https://doi.org/10.3390/plants12162998
Chicago/Turabian StyleDuan, Xiaoyu, Chunlei Zou, Yifan Jiang, Xuejing Yu, and Xueling Ye. 2023. "Effects of Reduced Phosphate Fertilizer and Increased Trichoderma Application on the Growth, Yield, and Quality of Pepper" Plants 12, no. 16: 2998. https://doi.org/10.3390/plants12162998






