Effect of Low Light on Photosynthetic Performance of Tomato Plants—Ailsa Craig and Carotenoid Mutant Tangerine
Abstract
:1. Introduction
2. Results
2.1. Alterations of Pigment Content during Treatment
2.2. Changes of Photosynthetic Performance of Tomato Plants
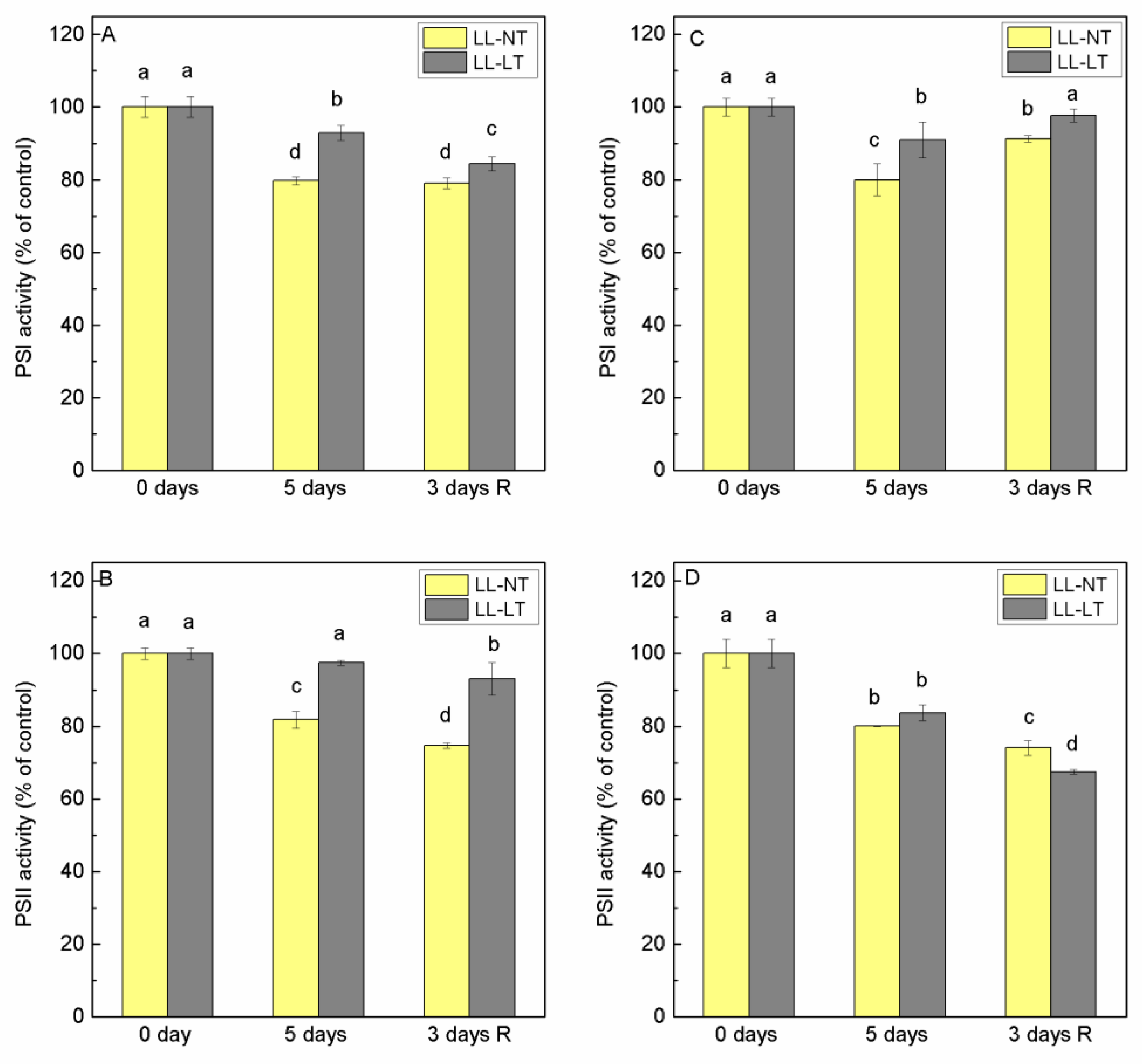
2.3. Photochemical Activity of PSI and PSII of Thylakoid Membranes Isolated from Control and Treated Plants
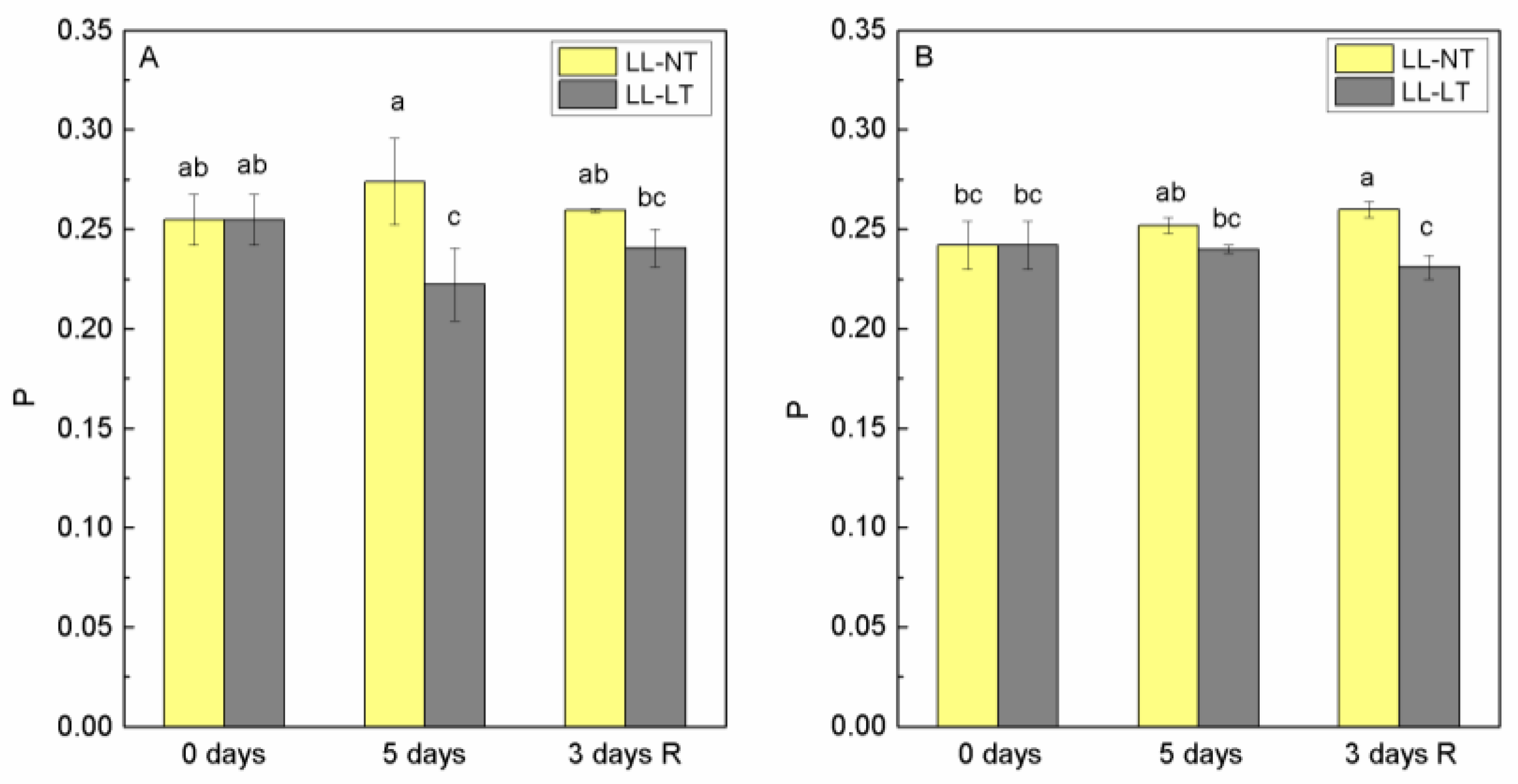
2.4. Effect of Treatment on Parameters of 77K Fluorescence
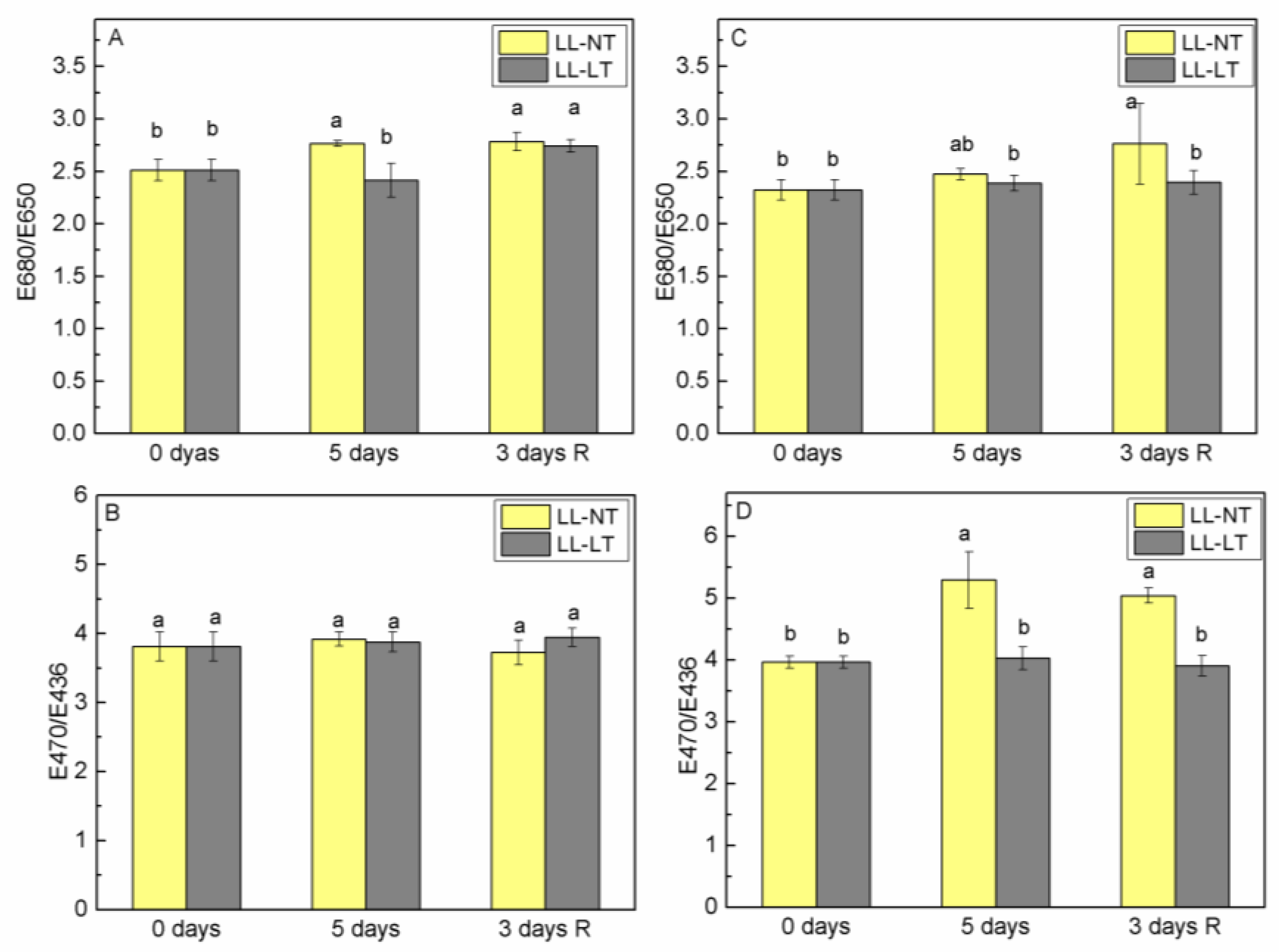
2.5. Lipid Order Alterations during Treatment and after Recovery
2.6. Changes of PSII-LHCII—Associated Polypeptides
3. Discussion
3.1. Pigment Content and Photosynthetic Electron Transport Rate in Leaves of Tomato Plants
3.2. Photochemical Activity of PSI and PSII in Thylakod Membranes of Tomato Plants
3.3. Energy Distribution and Interaction between Both Photosystems, and PSII Antenna Size Altertaions
3.4. Changes of Lipid Order of Thylakoid Membranes from Ailsa Craig and Tangerine
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Determination of Pigment Content
5.3. PAM Chlorophyll a Fluorescence and Determination of Fv/Fm and ETR
5.4. Isolation of Thylakoid Membranes
5.5. Photochemical Activities of PSI and PSII in Isolated Thylakoid Membranes
5.6. Low-Temperature (77 K) Fluorescence Measurements
5.7. Steady-State Fluorescence Polarization Measurements
5.8. SDS–PAGE Electrophoresis and Western Blot
5.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, D.J.; Ort, D.R. Impact of chilling temperatures on photosynthesis in warn-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.M. Chilling Injury in Plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving Plant Growth and Alleviating Photosynthetic Inhibition and Oxidative Stress from Low-Light Stress with Exogenous GR24 in Tomato (Solanum lycopersicum L.) Seedlings. Front. Plant Sci. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Sui, X.-L.; Mao, S.-L.; Wang, L.-H.; Zhang, B.-X.; Zhang, Z.-X. Effect of Low Light on the Characteristics of Photosynthesis and Chlorophyll a Fluorescence during Leaf Development of Sweet Pepper. J. Integr. Agric. 2012, 11, 1633–1643. [Google Scholar] [CrossRef]
- Liu, Q.-H.; Wu, X.; Chen, B.-C.; Ma, J.-Q.; Gao, J. Effects of Low Light on Agronomic and Physiological Characteristics of Rice Including Grain Yield and Quality. Rice Sci. 2014, 21, 243–251. [Google Scholar] [CrossRef]
- Szalai, G.; Majláth, I.; Pál, M.; Gondor, O.K.; Rudnóy, S.; Oláh, C.; Vanková, R.; Kalapos, B.; Janda, T. Janus-Faced Nature of Light in the Cold Acclimation Processes of Maize. Front. Plant Sci. 2018, 9, 850. [Google Scholar] [CrossRef]
- Steinger, T.; Roy, B.A.; Stanton, M.L. Evolution in stressful environments II: Adaptive value and costs of plasticity in response to low light in Sinapis arvensis. J. Evol. Biol. 2003, 16, 313–323. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Responses of Leaf Photosynthesis, Plant Growth and Fruit Production to Periodic Alteration of Plant Density in Winter Produced Single-truss Tomatoes. Hortic. J. 2017, 86, 511–518. [Google Scholar] [CrossRef]
- Höglind, M.; Hanslin, H.M.; Mortensen, L.M. Photosynthesis of Lolium perenne L. at low temperatures under low irradiances. Environ. Exp. Bot. 2011, 70, 297–304. [Google Scholar] [CrossRef]
- Öquist, G. Effects of low temperature on photosynthesis. Plant Cell Environ. 1983, 6, 281–300. [Google Scholar]
- Ruelland, E.; Vaultier, M.-N.; Zachowski, A.; Hurry, V. Cold signalling and cold acclimation in Plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Guzman, I.; Hamby, S.; Romero, J.; Bosland, P.W.; O’connell, M.A. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010, 179, 49–59. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Enfissi, E.M.A.; Nogueira, M.; Bramley, P.M.; Fraser, P.D. The regulation of carotenoid formation in tomato fruit. Plant J. 2017, 89, 774–788. [Google Scholar] [CrossRef]
- Van Der Ploeg, A.; Heuvelink, E. Influence of sub-optimal temperature on tomato growth and yield: A review. J. Hortic. Sci. Biotech. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Barrero-Gil, J.; Huertas, R.; Rambla, J.L.; Granell, A.; Salinas, J. Tomato plants increase their tolerance to low temperature in a chilling acclimation process entailing comprehensive transcriptional and metabolic adjustments. Plant Cell Environ. 2016, 39, 2303–2318. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biochem. 2012, 60, 141–149. [Google Scholar] [CrossRef]
- Murchie, E.H.; Hubbart, S.; Peng, S.; Horton, P. Acclimation of photosynthesis to high irradiance in rice: Gene expression and interactions with leaf development. J. Exp. Bot. 2005, 56, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.-Y.; Anderson, J.M. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. II. Regulation of electron transport capacities, electron carriers, coupling factor (CF1) activity and rates of photosynthesis. Photosynth. Res. 1984, 5, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, W.J.; Yu, H.J. Effects of Exogenous Epibrassinolide on Photosynthetic Characteristics in Tomato (Lycopersicon esculentum Mill) Seedlings under Weak Light Stress. J. Agric. Food Chem. 2010, 58, 3642–3645. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.J.; Ghisla, S.; Hirschberg, J.; Mann, V.; Beyer, P. Plant Carotene Cis-Trans Isomerase CRTISO a new member of the FAD (red)-dependent flavoproteins catalyzing non-redox reactions. J. Biol. Chem. 2011, 286, 8666–8676. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef]
- Gerganova, M.; Stanoeva, D.; Popova, A.V.; Velitchkova, M. Pigment content and oxygen evolution of tomato plants as affected by long term tretamnet at suboptimal temperature. Comptes Rendus L’académie Bulg. Sci. 2016, 69, 1429–1436. [Google Scholar]
- Popova, A.; Velitchkova, M.; Zanev, Y. Effect of Membrane Fluidity on Photosynthetic Oxygen Production Reactions. Z. Naturforsch. 2007, 62, 253–260. [Google Scholar] [CrossRef]
- Tanambell, H.; Bishop, K.S.; Quek, S.Y. Tangerine tomatoes: Origin, biochemistry, potential health benefits and future prospects. Crit. Rev. Food Sci. Nutr. 2021, 61, 2237–2248. [Google Scholar] [CrossRef]
- Nayak, J.J.; Anwar, S.; Krishna, P.; Chen, Z.-H.; Plett, J.M.; Foo, E.; Cazzonelli, C.I. Tangerine tomato roots show increased accumulation of acyclic carotenoids, less abscisic acid, drought sensitivity, and impaired endomycorrhizal colonization. Plant Sci. 2022, 321, 111308. [Google Scholar] [CrossRef]
- Fang, X.; Yang, Z.; Zhua, L. Low temperature and weak light affect greenhouse tomato growth and fruit quality. J. Plant Sci. 2018, 6, 16–24. [Google Scholar]
- Schroner, S.; Krause, G.H. Protective systems against active oxygen species in spinach: Response to cold acclimation in excess light. Planta 1990, 180, 383–389. [Google Scholar] [CrossRef]
- Koç, E.; Ielek, C.; Üstün, A.S. Effect of cold on protein, proline, phenolic compounds and chlorophyll content of two pepper (Capsicum annuum L.) varieties. Gazi Univ. J. Sci. 2010, 23, 1–6. [Google Scholar]
- Tewari, A.K.B.; Tripathy, C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Davaud, A. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of photosystem-II activity: Preferential inactivation of photosystem I. Photosynth. Res. 1994, 40, 75–92. [Google Scholar] [CrossRef]
- Bykowski, M.; Mazur, R.; Wójtowicz, J.; Suski, S.; Garstka, M.; Mostowska, A.; Kowalewska, L. Too rigid to fold: Carotenoid-dependent decrease in thylakoid fluidity hampers the formation of chloroplast grana. Plant Physiol. 2021, 185, 210–227. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Martin, B.; Ort, D.R.; Boyer, J.S. Impairment of photosynthesis by chilling-temperatures in tomato (Lycopersicon esculentum cultivar Rutgers). Plant Physiol. 1981, 68, 329–334. [Google Scholar] [CrossRef]
- Velitchkova, M.; Popova, A.V.; Faik, A.; Gerganova, M.; Ivanov, A.G. Low temperature and high light dependent dynamic photoprotective strategies in Arabidopsis thaliana. Physiol. Plant. 2020, 170, 93–108. [Google Scholar] [CrossRef]
- Faik, A.; Stanoeva, D.; Velitchkova, M. High light enhanced the inhibitory effect of suboptimal temperatures on the oxygen evolving reactions in Arabidopsis thaliana. Comptes Rendus Acad. Bulg. Sci. 2018, 71, 211–219. [Google Scholar]
- Faik, A.; Vasilev, D.; Stanoeva, D.; Velitchkova, M. Suboptimal growth temperature and light intensity effects on photosystem II activity and oxygen evolution of tomato (Solanum lycopersicum). Comptes Rendus Acad. Bulg. Sci. 2017, 70, 1247–1254. [Google Scholar]
- Velitchkova, M.; Popova, A. High light-induced changes of 77 K fluorescence emission of pea thylakoid membranes with altered membrane fluidity. Bioelectrochemistry 2005, 67, 81–90. [Google Scholar] [CrossRef]
- Nogueira, M.; Mora, L.; Enfissi, E.M.; Bramley, P.M.; Fraser, P.D. Subchromoplast Sequestration of Carotenoids Affects Regulatory Mechanisms in Tomato Lines Expressing Different Carotenoid Gene Combinations. Plant Cell 2013, 25, 4560–4579. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aglio, E. Carotenoid composition affects thylakoid morphology and membrane fluidity. Plant Physiol. 2021, 185, 21–22. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Nishida, I.; Murata, N. Chilling sensitivity in plants and cyanobacteria: The Crucial Contribution of Membrane Lipids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 541–568. [Google Scholar] [CrossRef]
- Moon, B.Y.; Higashi, S.T.; Gombos, Z.; Murata, N. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. . Proc. Natl. Acad. Sci. USA 1995, 92, 6219–6223. [Google Scholar] [CrossRef]
- Alfonso, M.; Luján, M.A.; Picorel, R. Role of Lipids and Fatty Acids in the Maintenance of Photosynthesis and the Assembly of Photosynthetic Complexes During Photosystem II Turnover. In Photosynthesis: Molecular Approaches to Solar Energy Conversion. Advances in Photosynthesis and Respiration; Shen, J.R., Satoh, K., Allakhverdiev, S.I., Eds.; Springer: Cham, Switzerland, 2021; Volume 47, pp. 395–426. [Google Scholar]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.-M.; Jansson, S. The Light-Harvesting Chlorophyll a/b Binding Proteins Lhcb1 and Lhcb2 Play Complementary Roles during State Transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Jha, A.; Dasgupta, J. Ultrafast Triplet Generation and its Sensitization Drives Efficient Photoisomerization of Tetra-cis-lycopene to All-trans-lycopene. J. Phys. Chem. B 2015, 119, 8669–8678. [Google Scholar] [CrossRef]
- Park, H.; Kreunen, S.S.; Cuttriss, A.J.; Dean DellaPenna, D.; Pogson, B.J. Identification of the Carotenoid Isomerase Provides Insight into Carotenoid Biosynthesis, Prolamellar Body Formation, and Photomorphogenesis. Plant Cell 2002, 14, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A. Tangerine Dreams: Cloning of Carotenoid Isomerase from Arabidopsis and Tomato. Plant Cell 2002, 14, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Gerganova, M.T.; Faik, A.K.; Velitchkova, M.Y. Acquired tolerance of the photosynthetic apparatus to photoinhibition as a result of growing Solanum lycopersicum at moderately higher temperature and light intensity. Funct. Plant Biol. 2019, 46, 555. [Google Scholar] [CrossRef] [PubMed]
- Velitchkova, M.; Borisova, P.; Vasilev, D.; Popova, A.V. Different impact of high light on the response and recovery of wild type and lut2 mutant of Arabidopsis thaliana at low temperature. Theor. Exp. Plant Physiol. 2021, 33, 95–111. [Google Scholar] [CrossRef]
- Faik, A.; Popova, A.V.; Velitchkova, M. Effects of long-term action of high temperature and high light on the activity and energy interaction of both photosystems in tomato plants. Photosynthetica 2016, 54, 611–619. [Google Scholar] [CrossRef]




| Chl (a+b) | Chl a/b | Car | |
|---|---|---|---|
| Ailsa Craig | |||
| 0 days | 2.24 ± 0.03 a | 3.28 ± 0.10 a | 0.53 ± 0.01 a |
| 5 d NT-LL | 2.06 ± 0.17 a | 3.21 ± 0.11 a | 0.43 ± 0.02 c |
| 5 d LT LL | 1.72 ± 0.09 b | 3.21 ± 0.15 a | 0.41 ± 0.01 c |
| 3 d R NT-LL | 2.12 ± 0.16 a | 3.31 ± 0.13 a | 0.47 ± 0.02 b |
| 3 d R LT-LL | 1.77 ± 0.07 b | 3.29 ± 0.18 a | 0.41 ± 0.01 c |
| Tangerine | |||
| 0 days | 2.11 ± 0.09 a | 3.59 ± 0.03 ac | 0.50 ± 0.02 a |
| 5 d NT-LL | 1.83 ± 0.05 bc | 3.46 ± 0.04 bc | 0.46 ± 0.01 bc |
| 5 d LT LL | 1.69 ± 0.08 c | 3.21 ± 0.08 c | 0.40 ± 0.01 c |
| 3 d R NT-LL | 1.88 ± 0.12 b | 3.52 ± 0.15 a | 0.53 ± 0.03 a |
| 3 d R LT-LL | 1.79 ± 0.06 bc | 3.29 ± 0.14 bc | 0.41 ± 0.01 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velitchkova, M.; Stefanov, M.; Popova, A.V. Effect of Low Light on Photosynthetic Performance of Tomato Plants—Ailsa Craig and Carotenoid Mutant Tangerine. Plants 2023, 12, 3000. https://doi.org/10.3390/plants12163000
Velitchkova M, Stefanov M, Popova AV. Effect of Low Light on Photosynthetic Performance of Tomato Plants—Ailsa Craig and Carotenoid Mutant Tangerine. Plants. 2023; 12(16):3000. https://doi.org/10.3390/plants12163000
Chicago/Turabian StyleVelitchkova, Maya, Martin Stefanov, and Antoaneta V. Popova. 2023. "Effect of Low Light on Photosynthetic Performance of Tomato Plants—Ailsa Craig and Carotenoid Mutant Tangerine" Plants 12, no. 16: 3000. https://doi.org/10.3390/plants12163000
APA StyleVelitchkova, M., Stefanov, M., & Popova, A. V. (2023). Effect of Low Light on Photosynthetic Performance of Tomato Plants—Ailsa Craig and Carotenoid Mutant Tangerine. Plants, 12(16), 3000. https://doi.org/10.3390/plants12163000






