In Vitro Conversion of Coffea spp. Somatic Embryos in SETIS™ Bioreactor System
Abstract
:1. Introduction
2. Results
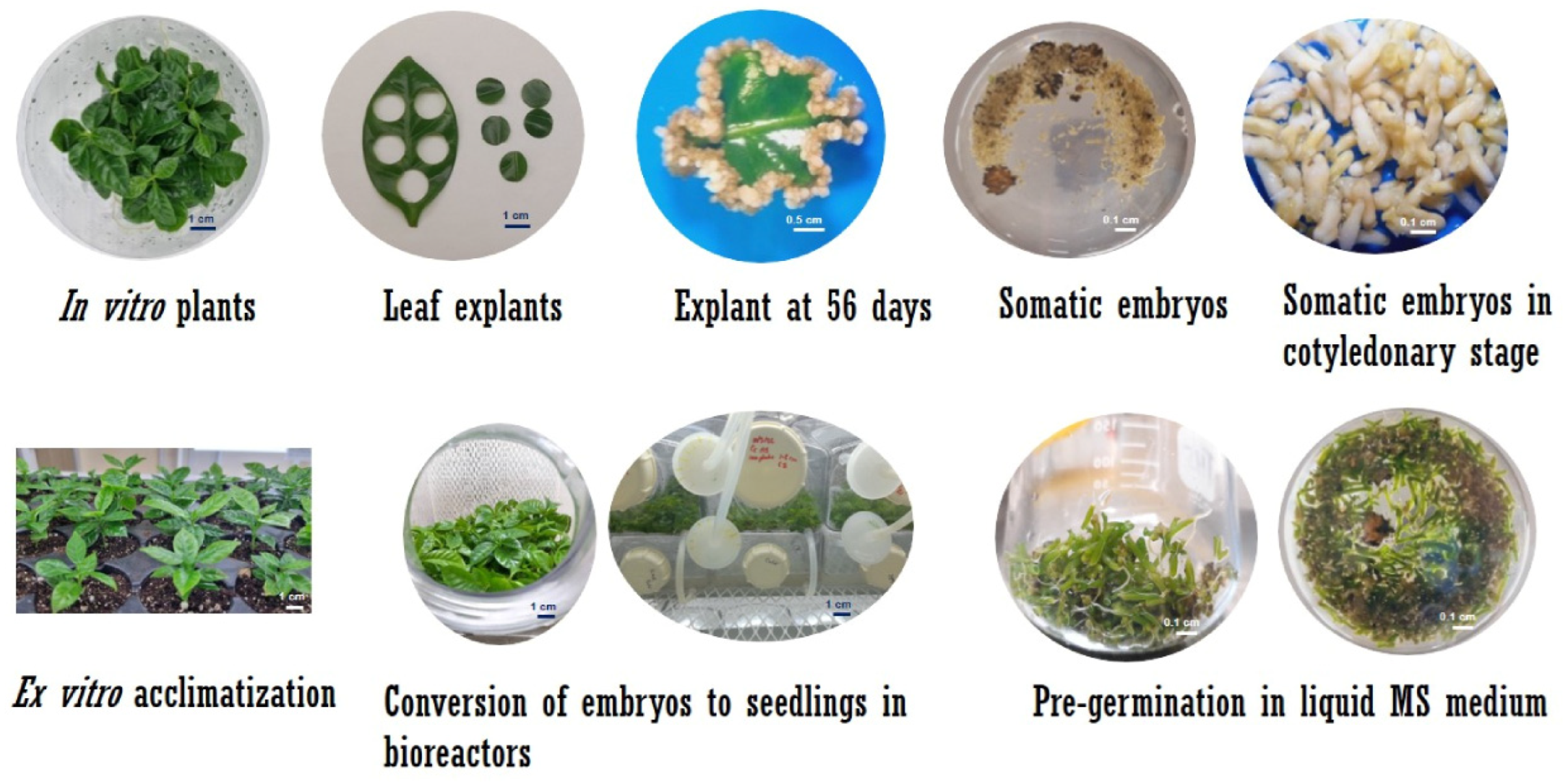
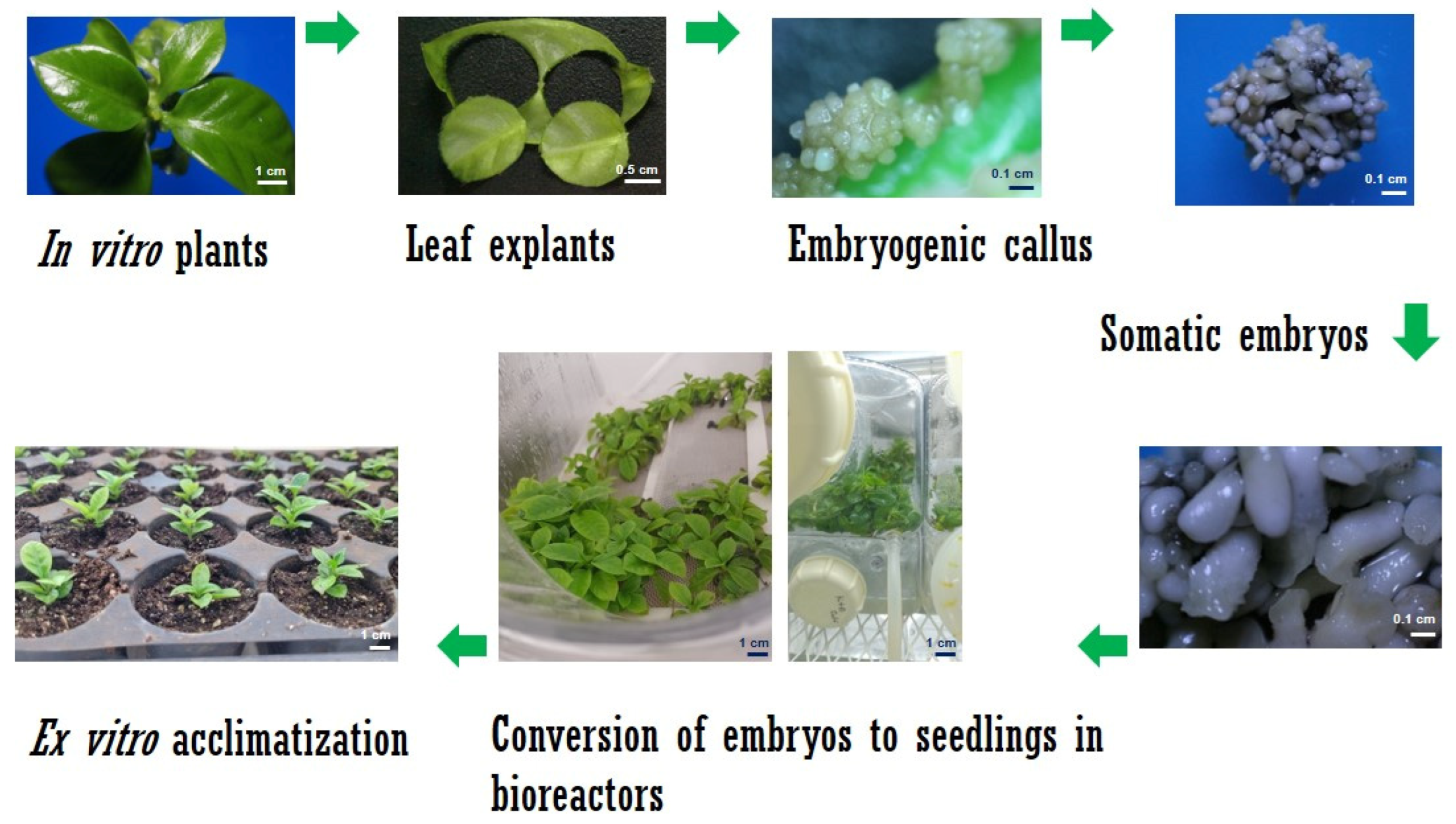
2.1. Somatic Embyrogenesis in C. arabica
2.2. Somatic Embryogenesis in C. canephora
2.3. Establishment of Culture in SETIS™ Bioreactors
2.4. Plant Growth and Development in SETIS™
2.5. Evaluation of the Stomatal Index
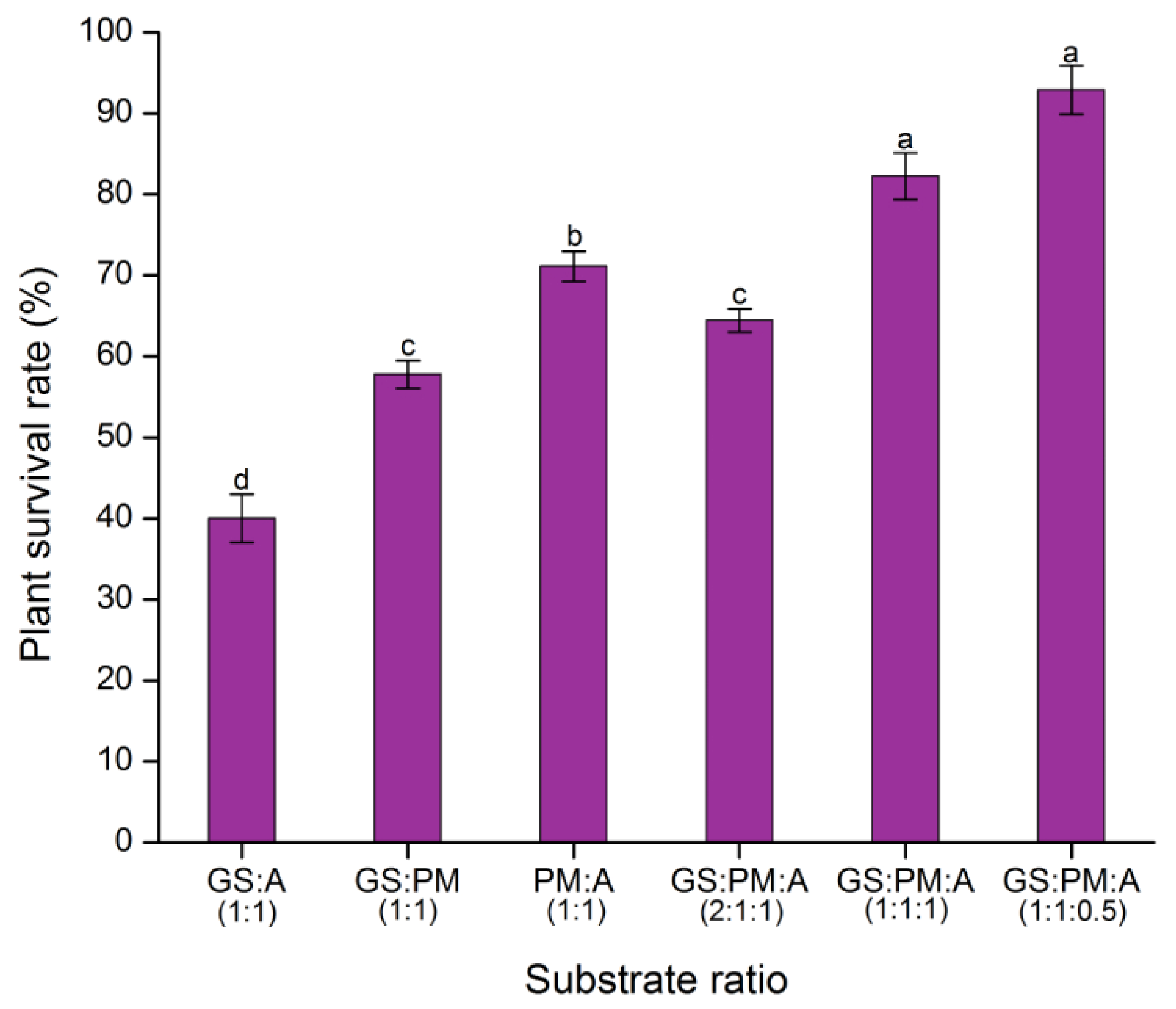
2.6. Substrate Tests
2.7. Ex Vitro Acclimatization of Plantlets
3. Discussion
4. Materials and Methods
4.1. Somatic Embryogenesis Induction in C. arabica
4.2. Somatic Embryogenesis Induction in C. canephora
4.3. Establishment of Culture in SETIS™ Bioreactors
4.4. The Relative Water Content
4.5. Study of the Stomatal Index
4.6. Ex Vitro Acclimatization of Harvested Plants from Bioreactors
4.7. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denoeud, F.; Carretero-Paulet, L.; Dereeper, A.; Droc, G.; Guyot, R.; Pietrella, M.; Zheng, C.; Alberti, A.; Anthony, F.; Aprea, G.; et al. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 2014, 345, 1181–1184. [Google Scholar] [CrossRef]
- Lashermes, P.; Combes, M.C.; Robert, J.; Trouslot, P.; D’Hont, A.; Anthony, F.; Charrier, A. Molecular characterisation and origin of the Coffea arabica L. genome. Mol. Gen. Genet. 1999, 261, 259–266. [Google Scholar] [CrossRef]
- Venial, L.R.; Mendonça, M.A.C.; Amaral-Silva, P.M.; Canal, G.B.; Passos, A.B.R.d.J.; Ferreira, A.; Soares, T.C.B.; Clarindo, W.R. Autotetraploid Coffea canephora and auto-alloctaploid Coffea arabica from in vitro chromosome set doubling: New germplasms for Coffea. Front. Plant Sci. 2020, 11, 154. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Avilez-Montalvo, J.R.; Avilez-Montalvo, R.N.; Márquez-López, R.E.; Galaz-Ávalos, R.M.; Mellado-Mojica, E. Somatic embryogenesis in Coffea spp. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Handel, Switzerland, 2016; pp. 241–266. [Google Scholar]
- Staritsky, G. Embryoid formation in callus tissues of coffee. Acta Bot. Neerl. 1970, 19, 509–514. [Google Scholar] [CrossRef]
- Söndahl, M.R.; Sharp, W.R. High frequency induction of somatic embryos in cultured leaf explants of Coffea arabica L. Z. Pflanzenphysiol. 1977, 81, 395–408. [Google Scholar] [CrossRef]
- Dublin, P. Techniques de reproduction végétative in vitro et amélioration génétique chez les caféiers cultivés. Café Cacao Thé 1984, XXVIII, 231–244. [Google Scholar]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.R.; Monforte-González, M.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Direct somatic embryogenesis in Coffea canephora. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Vázquez-Flota, F.A., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 111–117. [Google Scholar]
- Gatica, A.M.; Arrieta, G.; Espinoza, A.M. Direct somatic embryogenesis in Coffea arabica L. cvs. Caturra and Catuaí: Effect of triacontanol, light condition, and medium cosistency. Agron. Costarric. 2008, 32, 139–147. [Google Scholar]
- Souza Pádua, M.; Vilela Paiva, L.; da Silva, L.C.; Do Livramento, K.G.; Alves, E.; Fonseca Castro, A.H. Morphological characteristics and cell viability of coffee plants calli. Cienc. Rural 2014, 44, 660–665. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Pérez-Hernández, C.A.; Kú-González, Á.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Localization and transport of indole-3-acetic acid during somatic embryogenesis in Coffea canephora. Protoplasma 2018, 255, 695–708. [Google Scholar] [CrossRef]
- Quintana-Escobar, A.O.; Nic-Can, G.I.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M.; Góngora-Castillo, E. Transcriptome analysis of the induction of somatic embryogenesis in Coffea canephora and the participation of arf and AUX/IAA genes. PeerJ 2019, 7, e7752. [Google Scholar] [CrossRef] [PubMed]
- Uc-Chuc, M.Á.; Pérez-Hernández, C.A.; Galaz-Ávalos, R.M.; Brito-Argáez, L.; Aguilar-Hernández, V.; Loyola-Vargas, V.M. YUCCA-mediated biosynthesis of the auxin IAA is required during the somatic embryogenic induction process in Coffea canephora. Int. J. Mol. Sci. 2020, 21, 4751. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hernández, H.A.; Quintana-Escobar, A.O.; Uc-Chuc, M.Á.; Loyola-Vargas, V.M. Genome-wide analysis, modeling, and identification of amino acid binding motifs suggest the involvement of GH3 genes during somatic embryogenesis of Coffea canephora. Plants 2021, 10, 2034. [Google Scholar] [CrossRef] [PubMed]
- Avilez-Montalvo, J.; Quintana-Escobar, A.O.; Méndez-Hernández, H.A.; Uc-Chuc, M.Á.; Brito-Argáez, L.; Aguilar-Hernández, V.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Auxin-cytokinin cross talk in somatic embryogenesis of Coffea canephora. Plants 2022, 11, 2013. [Google Scholar] [CrossRef]
- Zamarripa, C.A.; Ducos, J.P.; Bollon, H.; Dufour, M.; Petiard, V. Production d’embryons somatiques de caféier en milieu liquide: Effects densité d’inoculation et renouvellement du milieu. Café Cacao Thé 1991, XXXV, 233–244. [Google Scholar]
- Etienne-Barry, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Direct sowing of Coffea arabica somatic embryos mass-produced in a bioreactor and regeneration of plants. Plant Cell Rep. 1999, 19, 111–117. [Google Scholar] [CrossRef]
- Bertrand, B.; Montagnon, C.; Georget, F.; Charmetant, P.; Etienne, H. Création et diffusion de variétés de caféiers Arabica: Quelles innovations variétales? Cah. Agric. 2012, 21, 77–88. [Google Scholar]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). Vitr. Cell Dev. Biol. Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Guerra, M.P.; Saare-Surminski, K.; Lieberei, R. A temporary immersion system improves in vitro regeneration of peach palm through secondary somatic embryogenesis. Ann. Bot. 2011, 108, 1463–1475. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Weber, J. Bioreactors for plant cells: Hardware configuration and internal environment optimization as tools for wider commercialization. Biotechnol. Lett. 2014, 36, 1359–1367. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Debnath, S. Bioreactors and molecular analysis in berry crop micropropagation-A review. Can. J. Plant Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mosqueda, M.A.; Bello-Bello, J.J. SETIS™ bioreactor increases in vitro multiplication and shoot length in vanilla (Vanilla planifolia Jacks. Ex Andrews). Acta Physiol. Plant. 2021, 43, 52. [Google Scholar] [CrossRef]
- Ducos, J.P.; Lambot, C.; Pétiard, V. Bioreactors for coffee mass propagation by somatic embryogenesis. Int. J. Pllant Dev. Biol. 2007, 1, 1–12. [Google Scholar]
- Etienne, H.; Solano, W.; Pereira, A.; Bertrand, B.; Berthouly, M. Protocole d’acclimatation de plantules de caféiers produites in vitro. Plant. Rech. Dév. 1997, 4, 304–306. [Google Scholar]
- Robert, M.L.; Herrera-Herrera, J.L.; Herrera-Herrera, G.; Herrera-Alamillo, M.A.; Fuentes-Carrillo, P. A new temporary immersion bioreactor system for micropropagation. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 121–129. [Google Scholar]
- Monja-Mio, K.M.; Herrera-Alamillo, M.Á.; Robert, M.L. Somatic embryogenesis in temporary immersion bioreactors. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 435–454. [Google Scholar]
- Etienne, H.; Bertrand, B.; Georget, F.; Lartaud, M.; Montes, F.; Dechamp, E.; Verdeil, J.L.; Barry-Etienne, D. Development of coffee somatic and zygotic embryos to plants differs in the morphological, histochemical and hydration aspects. Tree Physiol. 2013, 33, 640–653. [Google Scholar] [CrossRef]
- Ducos, J.P.; Labbe, G.; Lambot, C.; Pétiard, V. Pilot scale process for the production of pre-germinated somatic embryos of selected robusta (Coffea canephora) clones. In Vitro Cell. Dev. Biol.-Plant 2007, 43, 652–659. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Takayama, S.; Misawa, M. Mass propagation of Begonia hiemalis plantlet by shake culture. Plant Cell Physiol. 1981, 22, 461–467. [Google Scholar]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Tavakoli Dinanai, E.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Mirzabe, A.H.; Hajiahmad, A.; Fadavi, A.; Rafiee, S. Temporary immersion systems (TISs): A comprehensive review. J. Biotechnol. 2022, 357, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.E.; Wang, X.y.; Escalona, M.; Yan, L.; Huang, L.f. Somatic embryogenesis of Arabica coffee in temporary immersion culture: Advances, limitations, and perspectives for mass propagation of selected genotypes. Front. Plant Sci. 2022, 13, 994578. [Google Scholar] [CrossRef]
- Yasuda, T.; Fujii, Y.; Yamaguchi, T. Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol. 1985, 26, 595–597. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The role of the auxins during somatic embryogenesis. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Handel, Switzerland, 2016; pp. 171–181. [Google Scholar]
- Laux, T.; Würschum, T.; Breuninger, H. Genetic regulation of embryonic pattern formation. Plant Cell 2004, 16, S190–S202. [Google Scholar] [CrossRef]
- Campos, N.A.; Panis, B.; Carpentier, S.C. Somatic embryogenesis in coffee: The evolution of biotechnology and the integration of omics technologies offer great opportunities. Front. Plant Sci. 2017, 8, 1460. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B.; Dechamp, E.; Maurel, P.; Georget, F.; Guyot, R.; Breitler, J.-C. Are genetic and epigenetic instabilities of plant embryogenic cells a fatality? The experience of coffee somatic embryogenesis. Human Genet. Embryol. 2016, 6, 136. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B. Trueness-to-type and agronomic characteristics of Coffea arabica trees micropropagated by the embryogenic cell suspension technique. Tree Physiol. 2001, 21, 1031–1038. [Google Scholar] [CrossRef]
- Aguilar, M.E.; Ortiz, J.L.; Mesén, F.; Jiménez, L.D.; Altmann, F. Cafe arabica Coffea arabica L. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants: Volume II; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 39–62. [Google Scholar]
- Barry-Etienne, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Comparison of somatic embryogenesis-derived coffee (Coffea arabica L.) plantlets regenerated in vitro or ex vitro: Morphological, mineral and water characteristics. Ann. Bot. 2002, 90, 77–85. [Google Scholar] [CrossRef]
- da Silva, J.A.; Solis-Gracia, N.; Jifon, J.; Souza, S.C.; Mandadi, K.K. Use of bioreactors for large-scale multiplication of sugarcane (Saccharum spp.), energy cane (Saccharum spp.), and related species. Vitr. Cell Dev. Biol. Plant 2020, 56, 366–376. [Google Scholar] [CrossRef]
- Alister, B.M.; Finnie, J.; Watt, M.a.; Blakeway, F. Use of the temporary immersion bioreactor system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 425–442. [Google Scholar]
- Arencibia, A.D.; Vergara, C.; Quiroz, K.; Carrasco, B.; García-Gonzales, R. Establishment of photomixotrophic cultures for raspberry micropropagation in Temporary Immersion Bioreactors (TIBs). Sci. Hortic. 2013, 160, 49–53. [Google Scholar] [CrossRef]
- Roels, S.; Noceda, C.; Escalona, M.; Sandoval, J.; Canal, M.J.; Rodriguez, R.; Debergh, P. The effect of headspace renewal in a temporary immersion bioreactor on plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tissue Organ Cult. 2006, 84, 155–163. [Google Scholar] [CrossRef]
- Teisson, C.; Alvard, D. A new concept of plant in vitro cultivation liquid medium: Temporary immersion. In Current Issues in Plant Molecular and Cellular Biology. Current Plant Science and Biotechnology in Agriculture; Terzi, M., Cella, R., Falavigna, A., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 105–110. [Google Scholar]
- Albarrán, J.; Bertrand, B.; Lartaud, M.; Etienne, H. Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell Tissue Organ Cult. 2005, 81, 27–36. [Google Scholar] [CrossRef]
- Mosqueda Frómeta, O.; Escalona Morgado, M.M.; Teixeira da Silva, J.A.; Pina Morgado, D.T.; Daquinta Gradaille, M.A. In vitro propagation of Gerbera jamesonii Bolus ex Hooker f. in a temporary immersion bioreactor. Plant Cell Tissue Organ Cult. 2017, 129, 543–551. [Google Scholar] [CrossRef]
- Söndahl, M.R.; Söndahl, C.N.; Goncalves, W. Field testing of arabica bioreactor-derived plants. In Coffee Biotechnology and Quality; Sera, T., Soccol, C.R., Pandey, A., Roussos, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 143–150. [Google Scholar]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Photoautotrophic culture of Coffea arabusta somatic embryos: Development of a bioreactor for large-scale plantlet conversion from cotyledonary embryos. Ann. Bot. 2002, 90, 21–29. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 2023. [Google Scholar] [CrossRef] [PubMed]
- Barry-Etienne, D.; Bertrand, B.; Schlönvoigt, A.; Etienne, H. The morphological variability within a population of coffee somatic embryos produced in a bioreactor affects the regeneration and the development of plants in the nursery. Plant Cell Tissue Organ Cult. 2002, 68, 153–162. [Google Scholar] [CrossRef]
- Söndahl, M.R. Tissue culture of morphological mutants of coffee. In Plant Tissue Culture 1982; Fujiwara, A., Ed.; The Japanese association for Plant Tissue Culture: Tokyo, Japan, 1982; pp. 417–418. [Google Scholar]
- Söndahl, M.R.; Bragin, A. Somaclonal variation as a breeding tool for coffee improvement. In 14è Colloque Scientifique Internationale sur le Café; Paris, Association Scientifique Internationale du Café: Paris, France, 1991; pp. 701–710. [Google Scholar]
- Sánchez-Teyer, L.F.; Quiroz-Figueroa, F.R.; Loyola-Vargas, V.M.; Infante-Herrera, D. Culture-induced variation in plants of Coffea arabica cv. Caturra Rojo, regenerated by direct and indirect somatic embryogenesis. Mol. Biotechnol. 2003, 23, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla Landey, R.; Cenci, A.; Georget, F.; Bertrand, B.; Camayo, G.; Dechamp, E.; Herrera, J.C.; Santoni, S.; Lashermes, P.; Simpson, J.; et al. High genetic and epigenetic stability in Coffea arabica plants derived from embryogenic suspensions and secondary embryogenesis as revealed by AFLP, MSAP and the phenotypic variation rate. PLoS ONE 2013, 8, e56372. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.; Leonor Osório, M.; Manuela Chaves, M.; Amancio, S. Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and chestnut plantlets under ex vitro acclimatization. Plant Cell Tissue Organ Cult. 2001, 67, 271–280. [Google Scholar] [CrossRef]
- Revathi, J.; Manokari, M.; Shekhawat, M.S. Optimization of factors affecting in vitro regeneration, flowering, ex vitro rooting and foliar micromorphological studies of Oldenlandia corymbosa L.: A multipotent herb. Plant Cell Tissue Organ Cult. 2018, 134, 1–13. [Google Scholar] [CrossRef]
- Shekhawat, M.; Manokari, M.; Kannan, N. Micromorphological response towards altered environmental conditions in subsequent stages of in vitro propagation of Morinda coreia. Environ. Exp. Biol. 2017, 15, 37. [Google Scholar]
- Ziv, M. In vitro acclimatization. In Automation and Environmental Control in Plant Tissue Culture; Aitken-Christie, J., Kozai, T., Smith, M.A.L., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 493–516. [Google Scholar]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation. Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 71–93. [Google Scholar]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D. Challenges for the large-scale propagation of forest trees by somatic embryogenesis-a review. In Proceedings of the 3rd International Conference of the IUFRO Unit on “Woody Plant Production Integrating Genetic and Vegetative Propagation Yechnologies”, Vitoria-Gasteiz, Spain, 8–12 September 2014; Park, Y.S., Bonga, J.M., Eds.; IUFRO: Vitoria-Gasteiz, Spain, 2014; pp. 81–91. [Google Scholar]
- Paek, K.Y.; Hahn, E.J.; Son, S.H. Application of bioreactors for large-scale micropropagation systems of plants. In Vitro Cell. Dev. Biol. Plant 2001, 37, 149–157. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zygielbaum, A.I.; Gitelson, A.A.; Arkebauer, T.J.; Rundquist, D.C. Non-destructive detection of water stress and estimation of relative water content in maize. Geophys. Res. Lett. 2009, 36, L12403. [Google Scholar] [CrossRef]
- Paul, V.; Sharma, L.; Pandey, R.; Meena, R.C. Measurements of stomatal density and stomatal index on leaf/plant surfaces. In Physiological Techniques to Analyze the Impact on Crop Plants; Paul, V., Pandey, R., Pal, M., Eds.; Division of Plant Physiology, ICAR-Indian Agricultural Research Institute (IARI): New Delhi, India, 2017; pp. 27–30. [Google Scholar]





| Condition | Species | Stomata | Epidermal Cells | Stomatal Index | Stomatal Density |
|---|---|---|---|---|---|
| In vitro | C. arabica | 7 | 30 | 18.3 | 45.3 |
| C. canephora | 5 | 24 | 16.2 | 42.3 | |
| Ex vitro | C. arabica | 2 | 10 | 18.8 | 11.7 |
| C. canephora | 2 | 7 | 20.6 | 14.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Hernández, H.A.; Galaz-Ávalos, R.M.; Quintana-Escobar, A.O.; Pech-Hoil, R.; Collí-Rodríguez, A.M.; Salas-Peraza, I.Q.; Loyola-Vargas, V.M. In Vitro Conversion of Coffea spp. Somatic Embryos in SETIS™ Bioreactor System. Plants 2023, 12, 3055. https://doi.org/10.3390/plants12173055
Méndez-Hernández HA, Galaz-Ávalos RM, Quintana-Escobar AO, Pech-Hoil R, Collí-Rodríguez AM, Salas-Peraza IQ, Loyola-Vargas VM. In Vitro Conversion of Coffea spp. Somatic Embryos in SETIS™ Bioreactor System. Plants. 2023; 12(17):3055. https://doi.org/10.3390/plants12173055
Chicago/Turabian StyleMéndez-Hernández, Hugo A., Rosa M. Galaz-Ávalos, Ana O. Quintana-Escobar, Rodolfo Pech-Hoil, Ana M. Collí-Rodríguez, Itzamná Q. Salas-Peraza, and Víctor M. Loyola-Vargas. 2023. "In Vitro Conversion of Coffea spp. Somatic Embryos in SETIS™ Bioreactor System" Plants 12, no. 17: 3055. https://doi.org/10.3390/plants12173055
APA StyleMéndez-Hernández, H. A., Galaz-Ávalos, R. M., Quintana-Escobar, A. O., Pech-Hoil, R., Collí-Rodríguez, A. M., Salas-Peraza, I. Q., & Loyola-Vargas, V. M. (2023). In Vitro Conversion of Coffea spp. Somatic Embryos in SETIS™ Bioreactor System. Plants, 12(17), 3055. https://doi.org/10.3390/plants12173055








