Valorization of Olive Mill Byproducts: Recovery of Biophenol Compounds and Application in Animal Feed
Abstract
:1. Introduction
2. Results
2.1. Ingredients and Chemical Composition of the Diets and OMW
2.2. Phenolic Profiles of Spray-Dried Olive Oil Mill Wastewater
2.3. Nutritional Composition of Milk
2.4. Milk Fatty Acid Composition
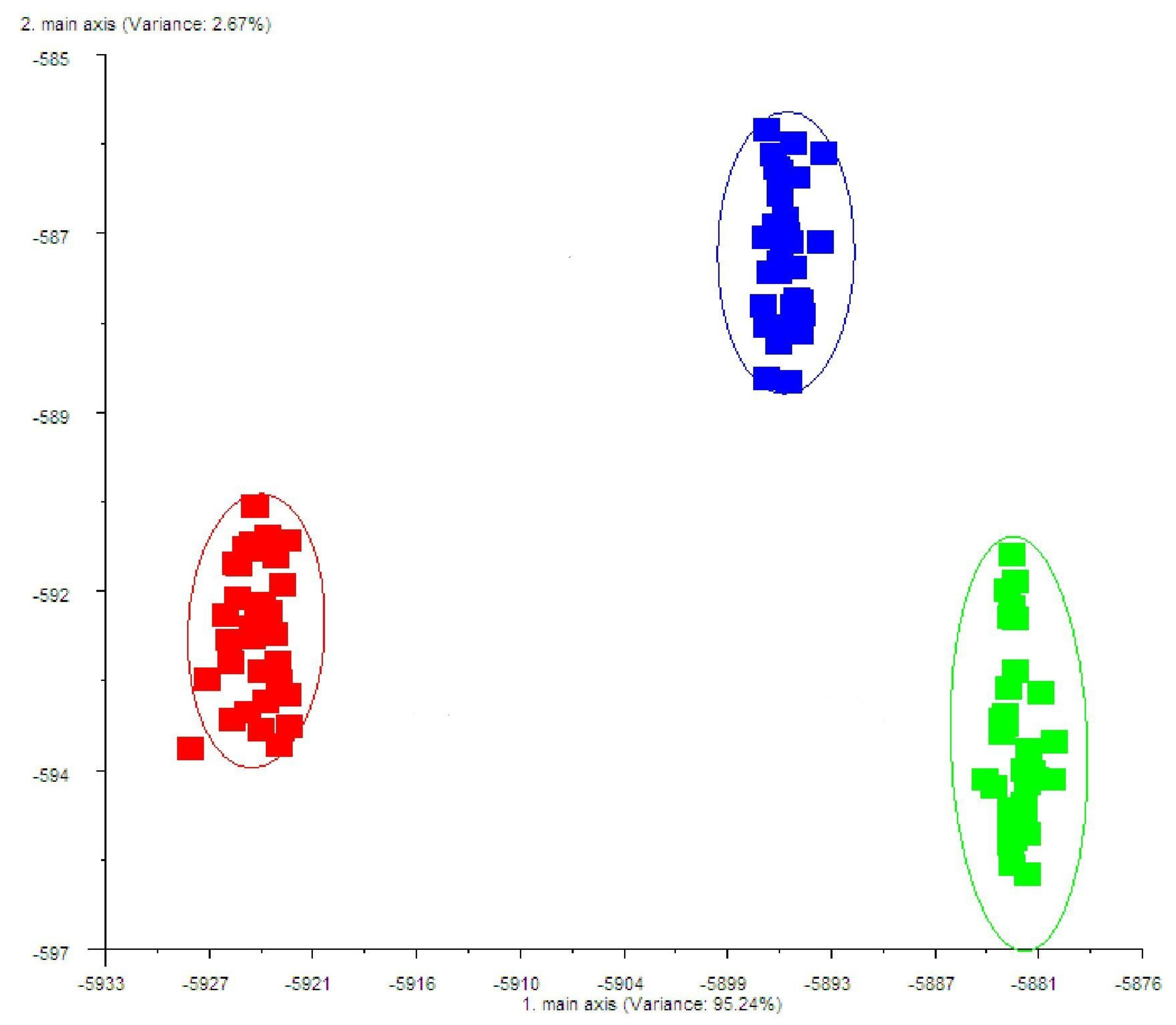
2.5. E-Nose Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design, Treatment, and Sample Collection
4.2. Sampling Olive Oil Mill Wastewater
4.3. Spray-Dried Olive Mill Wastewater Phenols Analysis
4.4. Chemical Analysis of Milk and Feed
4.5. Milk Fatty Acid Composition
4.6. E-Nose Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FaoStat. Food and Agriculture Organization of the United Nations. Crops 2024. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 13 January 2023).
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Ghabbour, N.; Rharrabti, Y. Physicochemical and microbiological characterization of olive mill wastewater (OMW) from different regions of northern Morocco. Environ. Technol. 2020, 41, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.; Anastasiou, C.C.; Oflaherty, V.; Mitchell, R. Bioremediation of olive mill wastewater. Int. Biodeter. Biodegr. 2008, 61, 127–134. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill waste waters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Benali, T.; Rharrabti, Y. Antibacterial and antioxidant potentials of phenolic extracts from olive mill wastewater and their use to enhance the stability of olive oil. Riv. Ital. Delle Sostanze Grasse 2020, 97, 31–42. [Google Scholar]
- Benincasa, C.; Pellegrino, M.; Romano, E.; Claps, S.; Fallara, C.; Perri, E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front. Nutr. 2021, 8, 782693. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Zhao, J.; Kagami, M.; Yano, K.; Kawasaki, K. Evaluation of the Effect of Incorporating Olive Mill Wastewater on Nutrients, Quality, and Bacterial Flora in Fermented Total Mixed Ration. Fermentation 2023, 9, 665. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Monahan, F.J.; Hervás, G.; Frutos, P.; Moloney, A.P. Review: Modulating ruminal lipid metabolism to improve the fatty acid composition of meat and milk. Challenges and opportunities. Animal 2018, 12, s272–s281. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vitro and in vivo studies. Vet. Parasitol. 2001, 99, 205–219. [Google Scholar] [CrossRef]
- Pathak, A.K. Potential of using condensed tannins to control gastrointestinal nematodes and improve small ruminant performance. Int. J. Mol. Vet. Res. 2013, 3, 36–50. [Google Scholar]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; Messa, C.; Perri, E.; Notarnicola, M. Antiproliferative, antioxidant and anti-inflammatory effects of hydroxytyrosol on human hepatoma HepG2 and Hep3B cell lines. Anticancer Res. 2012, 32, 5371–5377. [Google Scholar]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F. Antioxidant and other biological activities of olive mill wastewater. J Agric Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Stoner, G.D.; Mukhtar, H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. 1995, 59, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol exerts anti-inflammatory and anti-oxidant activities in a mouse model of systemic inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic Flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Nudda, A.; Buffa, G.; Atzori, A.S.; Cappai, M.G.; Caboni, P.; Fais, G.; Pulina, G. Small amounts of agro-industrial byproducts in dairy ewes diets affects milk production traits and haematological parameters. Anim. Feed Sci. Technol. 2019, 251, 76–85. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Bichi, E.; Belenguer, Á.; Frutos, P. Tannins as feed additives to modulate ruminal biohydrogenation: Effects on animal performance, milk fatty acid composition and ruminal fermentation in dairy ewes fed a diet containing sunflower oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Buccioni, A.; Serra, A.; Minieri, S.; Mannelli, F.; Cappucci, A.; Benvenuti, D.; Rapaccini, S.; Conte, G.; Mele, M. Milk production, composition, and milk fatty acid profile from grazing sheep fed diets supplemented with chestnut tannin extract and extruded linseed. Small Rumin. Res. 2015, 130, 200–207. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Atzori, A.S.; Marzano, A.; Battacone, G.; Nicolussi, P.; Bonelli, P.; Pulina, G. Whole exhausted berries of Myrtus communis L. supplied to dairy ewes: Effects on milk production traits and blood metabolites. Small Rumin. Res. 2017, 155, 33–38. [Google Scholar] [CrossRef]
- Pulina, G.; Battacone, G.; Mazzette, A.; Acciaro, M.; Decandia, M.; Sitzia, M.; Nudda, A. The effects of hydrolyzable tannins on rumen fluid traits and production performances in dairy sheep fed on pasture. In Proceedings of the 3rd EAAP International Symposium on Energy and Protein Metabolism and Nutrition, Parma, Italy, 6–10 September 2010. [Google Scholar]
- Castañares, N.; Mazzette, A.; Lovicu, M.; Mazza, A.; Nudda, A. Milk production of Sarda ewes fed chestnut and quebracho tannins. Ital. J. Anim. Sci. 2011, 10, 96. [Google Scholar]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Valenti, B.; Luciano, G.; Morbidini, L.; Rossetti, U.; Codini, M.; Avondo, M.; Priolo, A.; Bella, M.; Natalello, A.; Pauselli, M. Dietary pomegranate pulp: Effect on ewe milk quality during late lactation. Animals 2019, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Cappucci, A.; Alves, S.P.; Bessa, R.J.B.; Buccioni, A.; Mannelli, F.; Mariano Pauselli, M.; Viti, C.; Pastorelli, R.; Roscini, V.; Serra, A.; et al. Effect of increasing amounts of olive crude phenolic concentrate in the diet of dairy ewes on rumen liquor and milk fatty acid composition. J. Dairy Sci. 2018, 101, 4992–5005. [Google Scholar] [CrossRef] [PubMed]
- Khiaosa-Ard, R.; Bryner, S.F.; Scheeder, M.R.L.; Wettstein, H.-R.; Leiber, F.; Kreuzer, M.; Soliva, C.R. Evidence for the inhibition of the terminal step of ruminal α-linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 2009, 92, 177–188. [Google Scholar] [CrossRef]
- Corl, B.A.; Baumgard, L.H.; Dwyer, D.A.; Griinari, J.M.; Phillips, B.S.; Bauman, D.E. The role of Delta(9)-desaturase in the production of cis-9, trans-11 CLA. J. Nutr. Biochem. 2001, 12, 622–630. [Google Scholar] [CrossRef]
- Lock, A.L.; Bauman, D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 2004, 12, 1197–1206. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Dufour, C.; van Vuuren, A.M.; Cabrita, A.R.J.; Dewhurst, R.J.; Demeyer, D.; Fievez, V. Use of odd and branched-chain fatty acids in rumen contents and milk as a potential microbial marker. J Dairy Sci. 2005, 88, 1031–1042. [Google Scholar] [CrossRef]
- Or-Rashid, M.M.; Odongo, N.E.; McBride, B.W. Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid, vaccenic acid, and odd-chain and branched-chain fatty acids. J. Anim. Sci. 2007, 85, 1228–1234. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J. Nutritional indices for assessing fatty acids: A mini review. Int. J. Mol. Sci. 2020, 21, 56–95. [Google Scholar]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; 149p, Available online: https://apps.who.int/iris/handle/10665/42665 (accessed on 15 April 2023).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. Off. J. Eur. Union 2010, 276, 33–79. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=IT (accessed on 25 February 2023).
- IDF 148–2:2006; Milk—Enumeration of Somatic Cells—Part 2: Guidance on the Operation of Fluoro-Opto-Electronic Counters. International Dairy Federation: Brussels, Belgium, 2006.
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Gray, I.K.; Rumsby, M.G.; Hawke, J.C. The variations in linolenic acid and galactolipid levels in Graminaceae species with age of tissue and light environment. Phytochemistry 1967, 6, 107–113. [Google Scholar] [CrossRef]
- Cifuni, G.F.; Claps, S.; Signorelli, F.; Di Francia, A.; Di Napoli, M.A. Fatty acid and terpenoid profile: A signature of mountain milk. Int. Dairy J. 2022, 127, 105301. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry. Commission on Oils, Fats and Derivatives. In Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed.; Paquot, C., Hautfenne, A., Eds.; Blackwell Scientific: Oxford, UK, 1987. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
| Diets | ||||
|---|---|---|---|---|
| C | T0.1 | T0.2 | OMW | |
| Maize grain | 27.5 | 27.5 | 27.5 | |
| Wheat bran | 20.2 | 20.1 | 20 | |
| Maize flour | 8 | 8 | 8 | |
| Maize dried distillers’ grain | 8 | 8 | 8 | |
| Hulled sunflower seed flour | 8 | 8 | 8 | |
| Barley grain | 8 | 8 | 8 | |
| Soybean meal | 7 | 7 | 7 | |
| Molasses | 5.8 | 5.8 | 5.8 | |
| Dried beet pulp | 3.9 | 3.9 | 3.9 | |
| Spray-dried olive mill wastewater phenolics | - | 0.1 | 0.2 | |
| Calcium carbonate | 1.47 | 1.47 | 1.47 | |
| Magnesium oxide | 0.43 | 0.43 | 0.43 | |
| Sodium bicarbonate | 0.94 | 0.94 | 0.94 | |
| Sodium chloride | 0.47 | 0.47 | 0.47 | |
| Vitamin-mineral supplement | 0.29 | 0.29 | 0.29 | |
| Chemical composition | ||||
| Dry matter | 85.11 | 85.84 | 85.84 | 93 |
| Crude protein | 16.52 | 16.51 | 16.50 | 3.1 |
| Ether extract | 3.50 | 3.50 | 3.50 | 2.4 |
| Ash | 6.50 | 6.52 | 6.54 | 8.5 |
| NDF | 25.36 | 25.31 | 25.26 | 9.50 |
| ADF | 10.58 | 10.57 | 10.55 | 4.25 |
| ADL | 2.35 | 2.35 | 2.34 | - |
| Fatty acids (g/100 g of fatty acids) | ||||
| C16:0 | 9.45 | 9.45 | 9.44 | 16.5 |
| C18:0 | 3.36 | 3.37 | 3.37 | 2.5 |
| C18:1 n-9 | 18.13 | 18.17 | 18.21 | 62 |
| C18:2 n-6 | 43.85 | 43.81 | 43.78 | 16 |
| C18:3 n-3 | 2.10 | 2.10 | 2.10 | 0.45 |
| Others | 23.11 | 23.11 | 23.11 | 2.55 |
| Diets 1 | ||
|---|---|---|
| Phenolic Compound | T0.1 | T0.2 |
| Catecol | 0.245 | 0.490 |
| Tyrosol | 81.715 | 163.429 |
| Vanillin | 1.108 | 2.216 |
| Hydroxytyrosol | 59.236 | 118.472 |
| Hydroxytyryloleate | 22.559 | 45.117 |
| p-Coumaric acid | 0.200 | 0.401 |
| Caffeic acid | 0.116 | 0.231 |
| Apigenin | 0.382 | 0.764 |
| Luteolin | 2.495 | 4.990 |
| Diosmetin | 0.143 | 0.286 |
| Luteolin-7-O-glucoside | 3.542 | 7.084 |
| Luteolin-4-O-glucoside | 0.459 | 0.918 |
| Oleuropein | 4.120 | 8.239 |
| Oleuropein derivatives | 262.223 | 524.446 |
| Ligstroside derivatives | 11.159 | 22.319 |
| Rutin | 1.941 | 3.881 |
| Verbascoside | 27.998 | 55.996 |
| Sum of phenols | 479.640 | 959.280 |
| Diets 1 | |||||
|---|---|---|---|---|---|
| C | T0.1 | T0.2 | SEM 2 | p-Value | |
| Milk yield, g/d | 445 | 455 | 448 | 0.030 | 0.965 |
| Fat, g/100 g | 5.82 | 5.61 | 5.54 | 0.154 | 0.729 |
| Protein, g/100 g | 4.51 | 4.44 | 4.33 | 0.251 | 0.164 |
| Lactose, g/100 g | 4.34 | 4.42 | 4.40 | 0.041 | 0.378 |
| Casein, g/100 g | 3.411 | 3.357 | 3.282 | 0.131 | 0.7874 |
| Urea, mg/100 mL | 51.239 A | 52.617 A | 43.177 B | 1.534 | <0.0001 |
| SCC 3, 103 cells/mL | 2163 A | 1012 AB | 879 B | 313 | 0.00082 |
| Diets 1 | |||||
|---|---|---|---|---|---|
| C | T0.1 | T0.2 | SEM 2 | p-Value | |
| C4 | 3.059 | 2.748 | 2.574 | 0.174 | 0.159 |
| C6 | 2.898 | 2.747 | 2.669 | 0.161 | 0.599 |
| C8 | 2.971 ab | 2.543 b | 3.146 a | 0.162 | 0.044 |
| C10 | 8.682 | 9.073 | 9.512 | 0.428 | 0.400 |
| C11 | 0.288 | 0.253 | 0.254 | 0.005 | 0.742 |
| C12:0 | 4.085 | 4.235 | 4.561 | 0.174 | 0.168 |
| C12:1 | 0.128 | 0.127 | 0.138 | 0.008 | 0.609 |
| C13:0 | 0.100 | 0.0092 | 0.090 | 0.005 | 0.354 |
| C14:0 iso | 0.178 | 0.165 | 0.167 | 0.011 | 0.708 |
| C14:0 | 10.479 | 10.908 | 10.721 | 0.184 | 0.279 |
| C14:1 trans | 0.155 B | 0.200 A | 0.211 A | 0.009 | 0.0008 |
| C14:1 cis | 0.414 | 0.419 | 0.430 | 0.025 | 0.877 |
| C15:0 iso | 0.587 a | 0.506 b | 0.511ab | 0.027 | 0.008 |
| C15:0 | 1.176 | 1.052 | 1.061 | 0.047 | 0.149 |
| C15:1 | 0.352 | 0.303 | 0.317 | 0.019 | 0.215 |
| C16:0 iso | 0.089 | 0.100 | 0.088 | 0.006 | 0.381 |
| C16:0 | 26.819 a | 25.141 b | 25.044 b | 0.513 | 0.039 |
| C16:1 trans | 0.305 | 0.324 | 0.319 | 0.013 | 0.593 |
| C16:1 cis | 0.623 | 0.574 | 0.690 | 0.112 | 0.950 |
| C17:0 ante iso | 0.552 | 0.502 | 0.491 | 0.044 | 0.588 |
| C17:0 | 0.491 | 0.418 | 0.427 | 0.025 | 0.100 |
| C17:1 | 0.667 | 0.609 | 0.602 | 0.04 | 0.413 |
| C18:0 iso | 0.237 | 0.231 | 0.230 | 0.02 | 0.943 |
| C18:0 | 9.233 | 9.546 | 9.570 | 0.029 | 0.677 |
| C18:1 t9 | 0.075 B | 0.100 A | 0.096 A | 0.003 | 0.0002 |
| C18:1 t-11 | 0.526 B | 0.833 A | 0.848 A | 0.007 | 0.0001 |
| C18:1 n-9 | 18.538 | 19.635 | 19.926 | 0.505 | 0.317 |
| C18:1 n-7 | 0.508 | 0.428 | 0.431 | 0.025 | 0.059 |
| C18:2 t-9t-12 | 0.284 | 0.286 | 0.318 | 0.019 | 0.424 |
| C18:2 t-9c-12 | 0.042 | 0.079 | 0.052 | 0.026 | 0.582 |
| C18:2 c-9t-12 | 0.437 | 0.525 | 0.516 | 0.038 | 0.232 |
| C18:2 n-6 | 2.438 | 2.600 | 2.491 | 0.083 | 0.394 |
| C18:3 n-6 | 0.106 | 0.107 | 0.103 | 0.010 | 0.956 |
| C18:3 n-3 | 0.495 | 0.421 | 0.413 | 0.60 | 0.069 |
| C18:2 c-9t-11 | 0.690 b | 0.809 a | 0.750 a | 0.022 | 0.040 |
| C20:0 | 0.297 | 0.336 | 0.317 | 0.020 | 0.434 |
| C20:1 | 0.052 | 0.061 | 0.058 | 0.005 | 0.549 |
| C21:0 | 0.081 A | 0.036 B | 0.050 B | 0.005 | 0.0001 |
| C20:2 n-6 | 0.061 | 0.059 | 0.058 | 0.005 | 0.906 |
| C20:3 n-6 | 0.069 | 0.073 | 0.071 | 0.006 | 0.872 |
| C20:4 n-6 | 0.048 B | 0.051 A | 0.052 A | 0.007 | 0.0022 |
| C20:3 n-3 | 0.159 | 0.188 | 0.178 | 0.016 | 0.446 |
| C20:5 n-3 | 0.053 | 0.053 | 0.052 | 0.019 | 0.503 |
| C22:0 | 0.158 | 0.175 | 0.161 | 0.011 | 0.536 |
| C22:2 n-6 | 0.053 | 0.057 | 0.057 | 0.005 | 0.878 |
| C22:4 n-6 | 0.051 | 0.056 | 0.052 | 0.004 | 0.767 |
| C22:5 n-3 | 0.108 | 0.106 | 0.096 | 0.008 | 0.528 |
| C22:6 n-3 | 0.083 | 0.089 | 0.082 | 0.006 | 0.760 |
| SFA 3 | 72.470 | 70.815 | 71.666 | 0.586 | 0.161 |
| MUFA 4 | 22.347 | 23.620 | 22.990 | 0.525 | 0.252 |
| PUFA 5 | 5.182 | 5.564 | 5.343 | 0.144 | 0.197 |
| n-3 6 | 0.900 | 0.857 | 0.822 | 0.031 | 0.232 |
| n-6 7 | 2.828 | 3.004 | 2.884 | 0.083 | 0.328 |
| P/S 8 | 0.071 | 0.079 | 0.075 | 0.002 | 0.138 |
| Total trans | 1.345 B | 1.746 A | 1.793 A | 0.022 | 0.001 |
| Branched-chain | 1.645 | 1.505 | 1.496 | 0.09 | 0.463 |
| Odd-chain | 2.138 A | 1.853 B | 1.894 B | 0.074 | 0.027 |
| n-6/n-3 | 3.148 | 3.519 | 3.535 | 0.150 | 0.069 |
| AI 9 | 2.803 | 2.667 | 2.733 | 0.095 | 0.611 |
| TI 10 | 2.987 | 2.827 | 2.900 | 0.071 | 0.307 |
| Sensor Number | Sensor Name 1 | Sensor Sensitives | Detection Limits |
|---|---|---|---|
| 1 | W1C | Aromatic organic compounds | Toluene, 10 mg kg−1 |
| 2 | W5S | Very sensitive, broad range sensitivity, reacts to nitrogen oxides, very sensitive, with negative signal | NO2, 1 mg kg−1 |
| 3 | W3C | Ammonia, also used as sensor for aromatic compounds | Benzene, 10 mg kg−1 |
| 4 | W6S | Detects mainly hydrogen gas | H2, 0.1 mg kg−1 |
| 5 | W5C | Alkanes, aromatic compounds, and nonpolar organic compounds | Propane, 1 mg kg−1 |
| 6 | W1S | Sensitive to methane. Broad range of organic compounds detected | CH3, 100 mg kg−1 |
| 7 | W1W | Detects inorganic sulphur compounds, e.g., H2S. Also sensitive to many terpenes and sulphur-containing organic compounds | H2S, 1 mg kg−1 |
| 8 | W2S | Detects alcohol, partially sensitive to aromatic compounds, broad range | CO, 100 mg kg−1 |
| 9 | W2W | Aromatic compounds, inorganic sulphur and organic compounds | H2S, 1 mg kg−1 |
| 10 | W3S | Reacts to high concentrations (>100 mg kg−1) of methane and aliphatic organic compounds | Not determined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuni, G.F.; Claps, S.; Morone, G.; Sepe, L.; Caparra, P.; Benincasa, C.; Pellegrino, M.; Perri, E. Valorization of Olive Mill Byproducts: Recovery of Biophenol Compounds and Application in Animal Feed. Plants 2023, 12, 3062. https://doi.org/10.3390/plants12173062
Cifuni GF, Claps S, Morone G, Sepe L, Caparra P, Benincasa C, Pellegrino M, Perri E. Valorization of Olive Mill Byproducts: Recovery of Biophenol Compounds and Application in Animal Feed. Plants. 2023; 12(17):3062. https://doi.org/10.3390/plants12173062
Chicago/Turabian StyleCifuni, Giulia Francesca, Salvatore Claps, Giuseppe Morone, Lucia Sepe, Pasquale Caparra, Cinzia Benincasa, Massimiliano Pellegrino, and Enzo Perri. 2023. "Valorization of Olive Mill Byproducts: Recovery of Biophenol Compounds and Application in Animal Feed" Plants 12, no. 17: 3062. https://doi.org/10.3390/plants12173062







