Covered Rutile-TiO2 Nanoparticles Enhance Tomato Yield and Growth by Modulating Gas Exchange and Nutrient Status
Abstract
:1. Introduction
2. Results
2.1. Characterization of nTiO2
2.2. Effects of nTiO2 on Fruit Yield and Quality
2.3. Effects of nTiO2 on Growth and Biomass
2.4. Effect of NPs-TiO2 on Gas Exchange Parameters
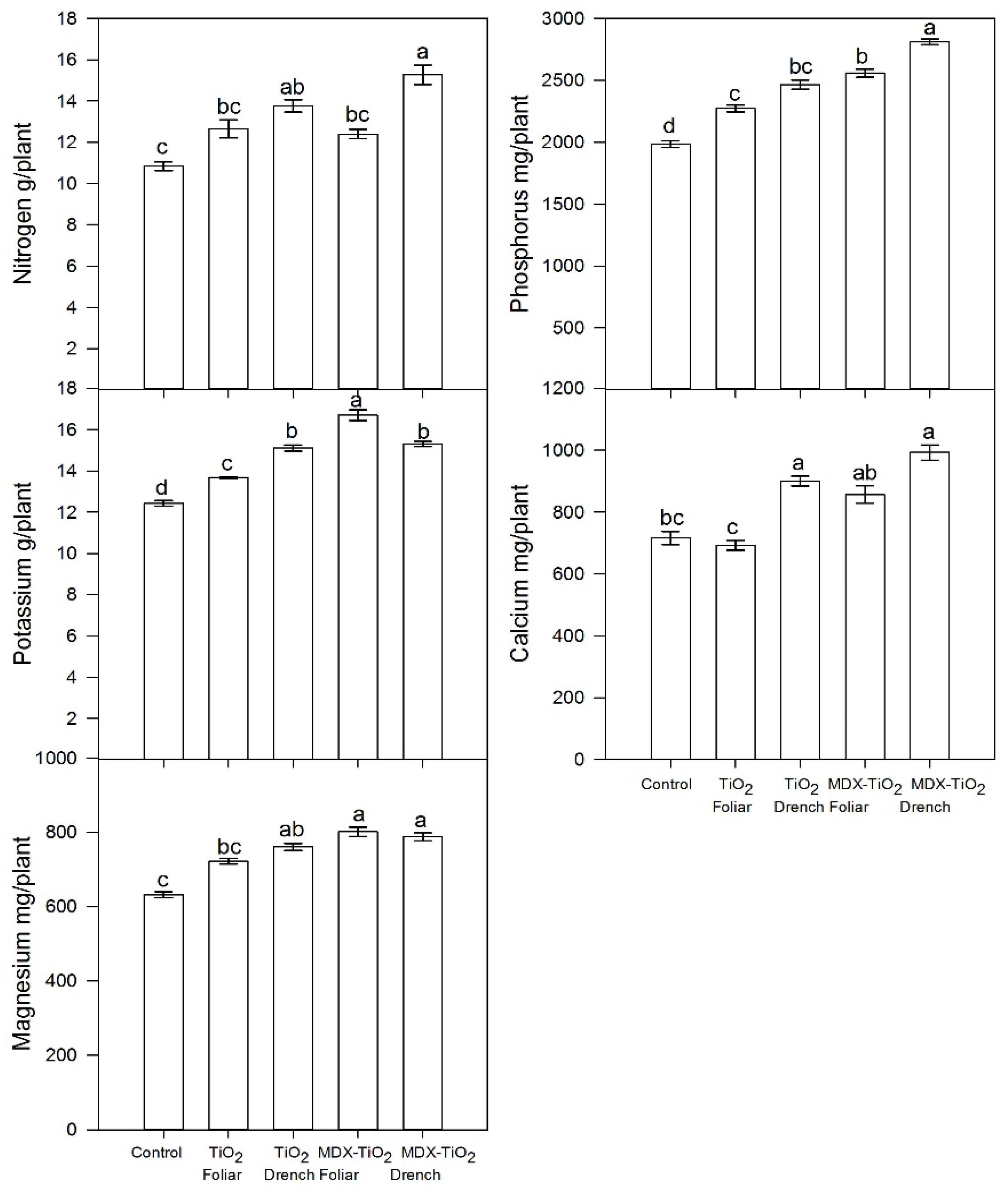
2.5. Effect of nTiO2 on Nutrient Status of Petiole Sap
2.6. Effect of nTiO2 on Macronutrient Status in Fruits
2.7. Effect of nTiO2 on Micronutrient and Titanium Status in Fruits
3. Discussion
3.1. Effect of nTiO2 on Fruit Production and Quality
3.2. Effect of nTiO2 on Plant Growth
3.3. Effect of nTiO2 on SPAD and Gas Exchange
3.4. Effect of nTiO2 on Macronutrient Status
3.5. Effect of nTiO2 on Micronutrient Status
3.6. Effect of nTiO2 on Fruit Titanium
4. Materials and Methods
4.1. Surface Covering of nTiO2
4.2. Characterization of nTiO2
4.3. Study Site and Growing Conditions
4.4. Plant Material and Experimental Conduction
4.5. Foliar and Drench Applications of nTiO2
4.6. Fruit Yield and Quality
4.7. Plant Growth, Dry Weight and SPAD
4.8. Gas Exchange Parameters
4.9. Fruit Nutrient Status
4.10. Petiole Sap Nutrient Status
4.11. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 20 July 2023).
- Chen, H.; Yada, R. Nanotechnologies in agriculture: New tools for sustainable development. Trends. Food. Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6, 1243. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef]
- Chhipa, H.; Joshi, P. Nanofertilisers, nanopesticides and nanosensors in agriculture. In Nanoscience in Food and Agriculture 1; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 247–282. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Nanofertilisers, nanopesticides, nanosensors of pest and nanotoxicity in agriculture. Sustain. Agric. Rev. 2016, 19, 307–330. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total. Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Assessing plant uptake and transport mechanisms of engineered nanomaterials from soil. MRS Bull. 2017, 42, 379–384. [Google Scholar] [CrossRef]
- Lyu, Y.; Pu, K. Recent advances of activatable molecular probes based on semiconducting polymer nanoparticles in sensing and imaging. Adv. Sci. 2017, 4, 1600481. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hanif, H.U.; Muhammad, A.; Ali, M.A.; Niaz, A.; Qazi, I.A. Phyto-availability of phosphorus to Lactuca sativa in response to soil applied TiO2 nanoparticles. Pak. J. Agric. Sci. 2015, 52, 177–182. [Google Scholar]
- Mattiello, A.; Marchiol, L. Application of nanotechnology in agriculture: Assessment of TiO2 nanoparticle effects on barley. In Application of Titanium Dioxide; Janus, M., Ed.; In Tech: London, UK, 2017; pp. 23–39. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace. Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef]
- Ahmad, B.; Shabbir, A.; Jaleel, H.; Khan, M.M.A.; Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant. Biol. 2018, 13, 6–15. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Reyes-Díaz, M.; Nunes-Nesi, A.; Recio, G.; Carmona, E.; Corgne, A.; Rangel, Z.; Inostroza-Blancheteau, C. Titanium dioxide nanoparticles provoke transient increase in photosynthetic performance and differential response in antioxidant system in Raphanus sativus L. Sci. Hortic. 2020, 269, 109418. [Google Scholar] [CrossRef]
- Morteza, E.; Moaveni, P.; Farahani, H.A.; Kiyani, M. Study of photosynthetic pigments changes of maize (Zea mays L.) under nano TiO2 spraying at various growth stages. SpringerPlus 2013, 2, 247. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. PProg. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- An, C.; Jiang, W.; Wang, J.; Wang, S.; Ma, Z.; Li, Y. Synthesis of three-dimensional AgI@ TiO2 nanoparticles with improved photocatalytic performance. Dalton Trans. 2013, 42, 8796–8801. [Google Scholar] [CrossRef] [PubMed]
- Jemaa, I.B.; Chaabouni, F.; Ranguis, A. Cr doping effect on the structural, optoelectrical and photocatalytic properties of RF sputtered TiO2 thin films from a powder target. J. Alloys Compd. 2020, 825, 153988. [Google Scholar] [CrossRef]
- Kim, K.M.; Ko, J.M.; Park, N.G.; Ryu, K.S.; Chang, S.H. Characterization of poly (vinylidenefluoride-co-hexafluoropropylene)-based polymer electrolyte filled with rutile TiO2 nanoparticles. Solid. State. Ion. 2003, 161, 121–131. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A. Characterization of TiO2 and TiO2–MnO oxides prepared by sol–gel method. Solid State Ion. 2001, 138, 227–232. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics. J. Agric. Food. Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef]
- Irshad, M.A.; ur Rehman, M.Z.; Anwar-ul-Haq, M.; Rizwan, M.; Nawaz, R.; Shakoor, M.B.; Wijaya, L.; Alyemeni, L.M.; Ahmad, P.; Ali, S. Effect of green and chemically synthesized titanium dioxide nanoparticles on cadmium accumulation in wheat grains and potential dietary health risk: A field investigation. J. Hazard. Mater. 2021, 415, 125585. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.S. Effect of titanium nanoparticles (TiO2) on growth, yield and chemical constituents of coriander plants. Arab. J. Nucl. Sci. Appl. 2015, 48, 187–194. [Google Scholar]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Grajkowski, J.; Ochmian, I. Influence of three biostymulants on yielding and fruit quality of three primocane raspberry cultivars. Acta Sci. Pol. Hortorum Cultus 2007, 6, 29–36. [Google Scholar]
- Kleiber, T.; Markiewicz, B. Application of “Tytanit” in greenhouse tomato growing. Acta Sci. Pol. Hortorum Cultus 2013, 12, 117–126. [Google Scholar]
- Dağhan, H. Effects of TiO2 nanoparticles on maize (Zea mays L.) growth, chlorophyll content and nutrient uptake. Appl. Ecol. Environ. Res. 2018, 16, 6873–6883. [Google Scholar] [CrossRef]
- Rafique, R.; Arshad, M.; Khokhar, M.F.; Qazi, I.A.; Hamza, A.; Virk, N. Growth response of wheat to titania nanoparticles application. NUST J. Engin. Sci. 2014, 7, 42–46. [Google Scholar]
- Yamamoto, A.; Nakamura, T.; Adu-Gyamfi, J.J.; Saigusa, M. Relationship between chlorophyll content in leaves of sorghum and pigeonpea determined by extraction method and by chlorophyll meter (SPAD-502). J. Plant Nutr. 2002, 25, 2295–2301. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.Q.; Zhang, H.; Zhu, J.H.; Yao, X.G.; Zhang, X.B.; Zheng, K.F. Assessment of chlorophyll content based on image color analysis, comparison with SPAD-502. In Proceedings of the 2010 2nd International Conference on Information Engineering and Computer Science, Wuhan, China, 25–26 December 2010. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Dashti, S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta. Agric. Slov. 2016, 107, 265–276. [Google Scholar] [CrossRef]
- Liu, L.I.; Cao, Y.; Guo, Q.; Zhu, Z. Nanosized Titanium dioxide seed priming enhances salt tolerance of an ornamental and medicinal plant Paeonia Suffruticosa. Pak. J. Bot. 2021, 53, 1167–1175. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Binary mixture of nanoparticles in sewage sludge: Impact on spinach growth. Chemosphere 2020, 254, 126794. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace. Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef]
- Mingyu, S.; Fashui, H.; Chao, L.; Xiao, W.; Xiaoqing, L.; Liang, C.; Fengqing, G.; Fan, Y.; Zhongrui, L. Effects of nano-anatase TiO2 on absorption, distribution of light, and photoreduction activities of chloroplast membrane of spinach. Biol. Trace. Elem. Res. 2007, 118, 120–130. [Google Scholar] [CrossRef]
- Lei, Z.; Mingyu, S.; Chao, L.; Liang, C.; Hao, H.; Xiao, W.; Xiaoqing, L.; Fan, Y.; Fengqing, G.; Fashui, H. Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol. Trace. Elem. Res. 2007, 119, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Gao, J.; Xu, G.; Qian, H.; Liu, P.; Zhao, P.; Hu, Y. Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ. Pollut. 2013, 176, 63–70. [Google Scholar] [CrossRef]
- Alharby, H.F.; Rizwan, M.; Iftikhar, A.; Hussaini, K.M.; ur Rehman, M.Z.; Bamagoos, A.A.; Alharbi, B.M.; Asrar, M.; Yasmeen, T.; Ali, S. Effect of gibberellic acid and titanium dioxide nanoparticles on growth, antioxidant defense system and mineral nutrient uptake in wheat. Ecotoxicol. Environ. Saf. 2021, 221, 112436. [Google Scholar] [CrossRef]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef]
- Missaoui, T.; Smiri, M.; Chemingui, H.; Alhalili, Z.; Hafiane, A. Disturbance in mineral nutrition of fenugreek grown in water polluted with nanosized titanium dioxide. Bull. Environ. Contam. Toxicol. 2021, 106, 327–333. [Google Scholar] [CrossRef]
- Hrubý, M.; Cígler, P.; Kuzel, S. Contribution to understanding the mechanism of titanium action in plant. J. Plant. Nutr. 2002, 25, 577–598. [Google Scholar] [CrossRef]
- Pošćić, F.; Mattiello, A.; Fellet, G.; Miceli, F.; Marchiol, L. Effects of cerium and titanium oxide nanoparticles in soil on the nutrient composition of barley (Hordeum vulgare L.) kernels. Int. J. Environ. Public Health 2016, 13, 577. [Google Scholar] [CrossRef]
- Kelemen, G.; Keresztes, A.; Bacsy, E.; Feher, M.; Fodor, P.; Pais, I. Distribution and intracellular localization of titanium in plants after titanium treatment. Food Struct. 1993, 12, 67–72. [Google Scholar]
- Cai, F.; Wu, X.; Zhang, H.; Shen, X.; Zhang, M.; Chen, W.; Gao, Q.; White, J.C.; Tao, S.; Wang, X. Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). NanoImpact 2017, 5, 101–108. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Hsieh, C.H. Spherical zinc oxide nano particles from zinc acetate in the precipitation method. J. Chin. Chem. Soc. 2007, 54, 31–34. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis, Part 3: Chemical Methods; Book Series No. 5; ASA and SSSA: Madison, WI, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Johnson, G.W.; Workman, S.M.; Jones, J.B., Jr.; Miller, R.O. Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectrometry. In Methods of Soil Analysis, Part 3: Chemical Methods; Book Series No. 5; ASA and SSSA: Madison, WI, USA, 1996; Volume 5, pp. 91–139. [Google Scholar] [CrossRef]
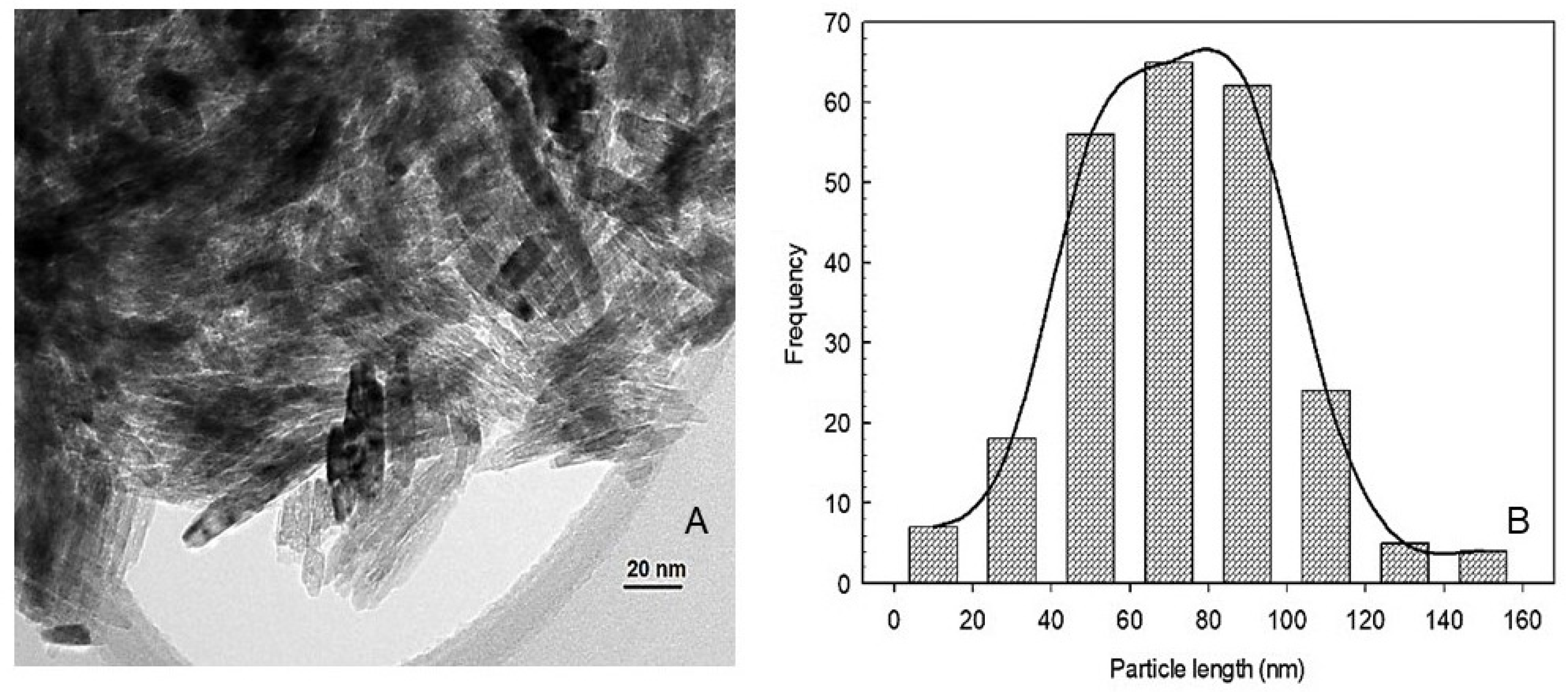
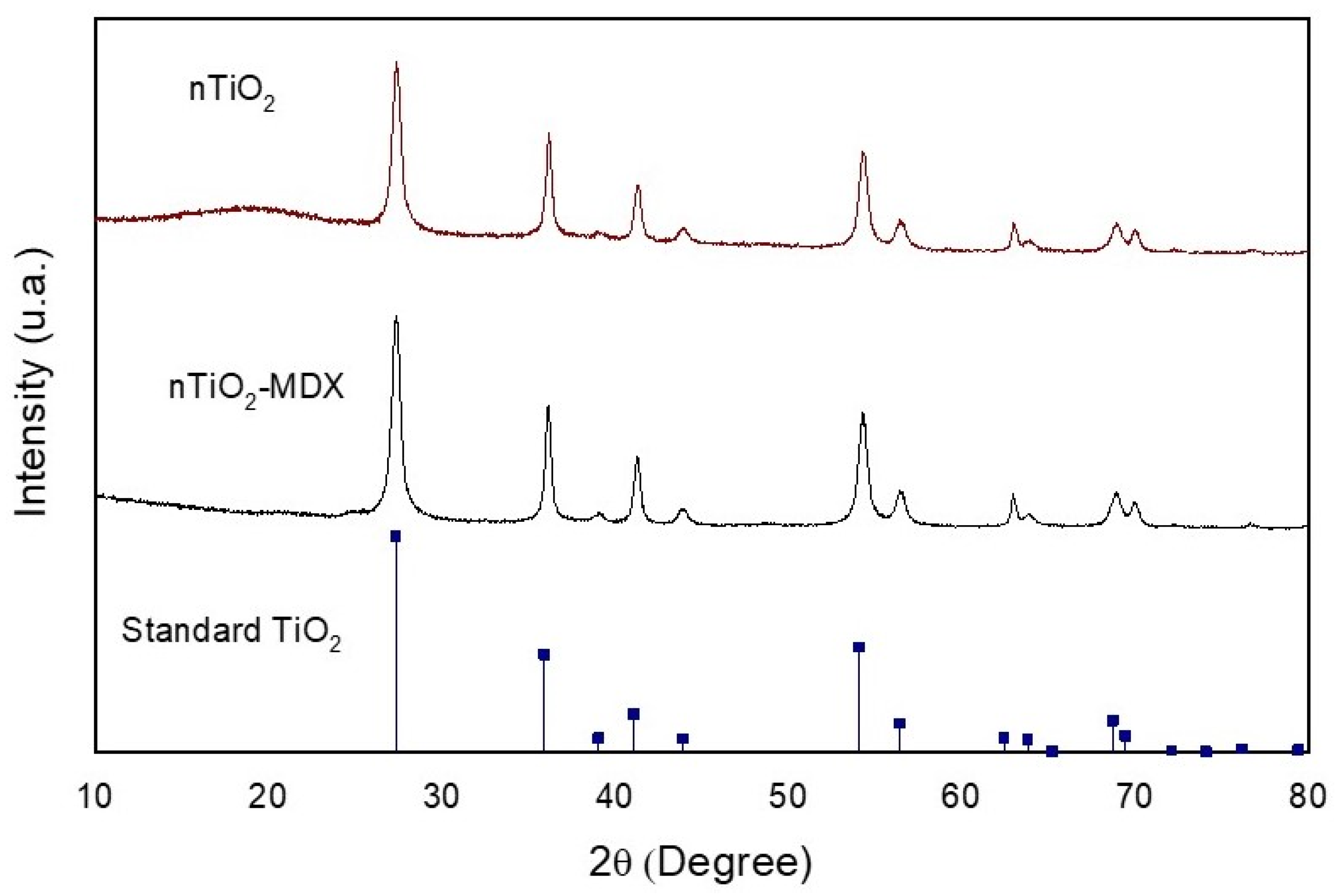
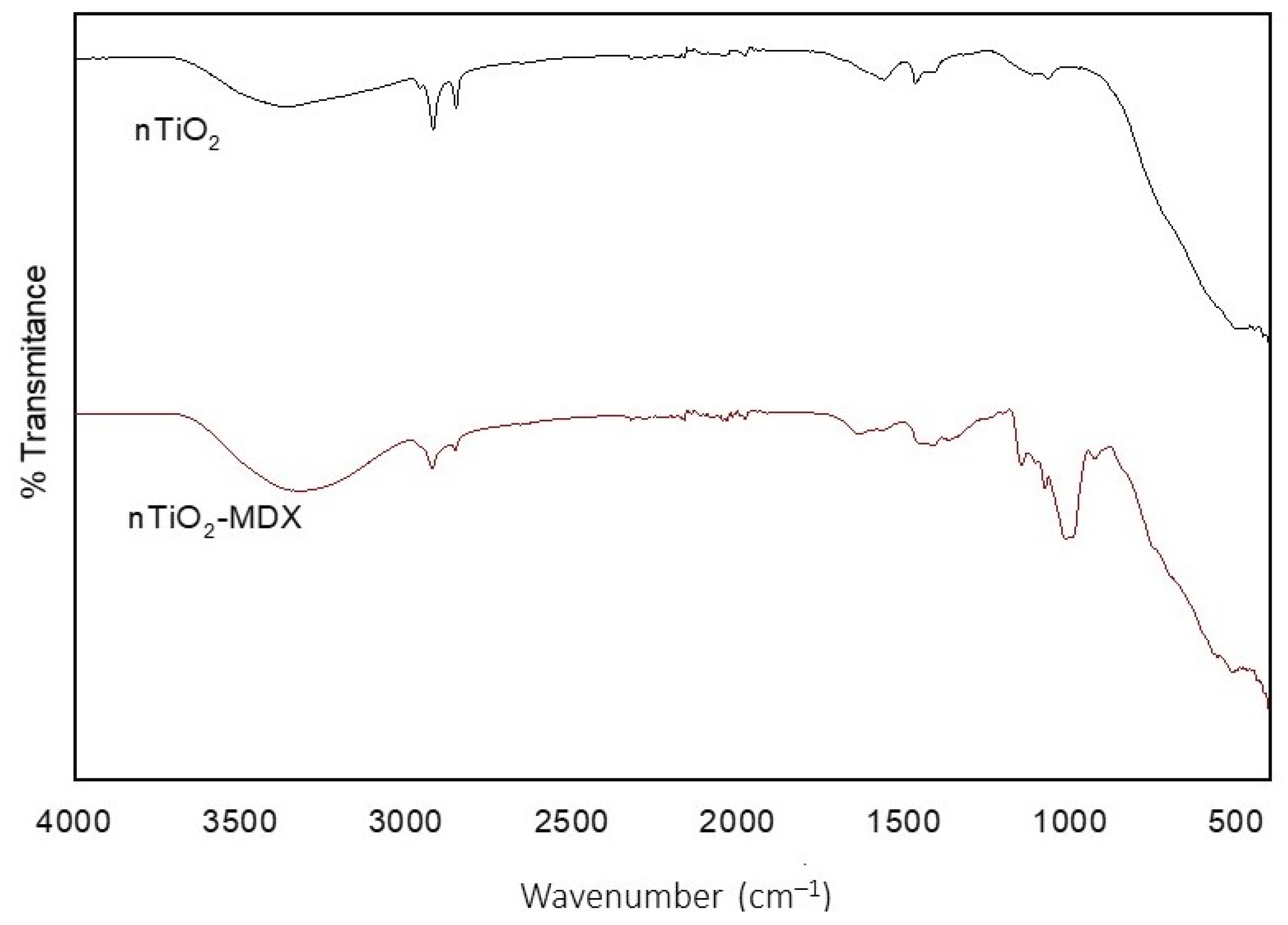



| Treatment | Application Method | Yield kg/Plant | Fruit Weight g | PD mm | ED mm | Firmness kg m−2 | TSS °Brix |
|---|---|---|---|---|---|---|---|
| Control | 6.40 c | 128.0 c | 52.6 c | 63.8 c | 2.96 b | 5.09 b | |
| nTiO2 | Foliar | 7.57 b | 151.4 b | 55.0 ab | 66.6 ab | 3.25 b | 5.40 b |
| Drench | 7.41 b | 148.2 b | 54.1 b | 66.1 b | 3.28 b | 6.66 a | |
| nTiO2 + MDX | Foliar | 7.77 b | 155.5 b | 54.9 ab | 67.9 a | 3.94 a | 6.70 a |
| Drench | 8.14 a | 162.8 a | 55.6 a | 67.6 a | 3.21 b | 6.94 a | |
| ANOVA | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | |
| Orthogonal contrasts | |||||||
| Control vs. nTiO2 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.025 | p = 0.001 | |
| Control vs. nTiO2-MDX | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.001 | |
| nTiO2 vs. nTiO2-MDX | p = 0.001 | p = 0.002 | ns | p = 0.003 | p = 0.025 | p = 0.001 | |
| Treatment | Application Method | Plant Height m | Stem Diameter Mm | Leaf DW g | Root DW g | Stem DW g | Total DW g | SPAD |
|---|---|---|---|---|---|---|---|---|
| Control | 2.10 b | 20.3 c | 162 b | 22.3 b | 89.7 c | 274 c | 56.8 b | |
| nTiO2 | Foliar | 2.25 ab | 21.8 b | 193 b | 26.0 b | 109.2 ab | 328 b | 63.6 a |
| Drench | 2.25 ab | 24.5 a | 178 b | 25.6 b | 96.6 ab | 300 bc | 62.6 a | |
| nTiO2- + MDX | Foliar | 2.34 a | 22.8 b | 165 b | 21.8 b | 95.4 bc | 282 c | 63.7 a |
| Drench | 2.25 ab | 25.4 a | 226 a | 36.4 a | 111.8 a | 374 a | 64.2 a | |
| ANOVA | p = 0.031 | p = 0.001 | p = 0.001 | p = 0.001 | p = 0.009 | p = 0.001 | p = 0.003 | |
| Orthogonal contrasts | ||||||||
| Control vs. nTiO2 | p = 0.017 | p = 0.001 | p = 0.047 | p = 0.037 | p = 0.017 | p = 0.001 | p = 0.001 | |
| Control vs. nTiO2-MDX | p = 0.003 | p = 0.001 | p = 0.006 | p = 0.001 | p = 0.013 | p = 0.001 | p = 0.001 | |
| nTiO2 vs. nTiO2-MDX | ns | p = 0.049 | ns | p = 0.048 | ns | ns | ns | |
| Treatment | Application Method | Photosynthesis Rate µmol CO2 m−2s−1 | Stomatic Conductance mol H2O m−2s−1 | Internal Concentration μmol CO2 mol air−1 | Transpiration Rate mmol H2O m−2s−1 |
|---|---|---|---|---|---|
| Control | 7.74 b | 0.390 b | 323.0 a | 7.59 b | |
| nTiO2 | Foliar | 17.05 a | 0.461 b | 296.0 ab | 8.21 b |
| Drench | 20.11 a | 0.450 b | 278.2 b | 8.94 ab | |
| nTiO2 + MDX | Foliar | 18.40 a | 0.439 b | 276.7 b | 9.43 ab |
| Drench | 20.81 a | 0.600 a | 293.1 ab | 10.40 a | |
| ANOVA | p = 0.001 | p = 0.003 | p = 0.037 | p=0.013 | |
| Orthogonal contrasts | |||||
| Control vs. nTiO2 | p = 0.001 | p = 0.045 | p = 0.011 | p = 0.050 | |
| Control vs. nTiO2-MDX | p = 0.001 | p = 0.017 | p = 0.008 | p = 0.001 | |
| nTiO2 vs. nTiO2-MDX | ns | p = 0.049 | ns | p = 0.015 | |
| Treatment | Application Method | NO3− mg L−1 | K mg L−1 | Ca mg L−1 |
|---|---|---|---|---|
| Control | 836 c | 1208 b | 581 c | |
| nTiO2 | Foliar | 1098 b | 2640 a | 844 ab |
| Drench | 988 bc | 2260 a | 942 ab | |
| nTiO2 + MDX | Foliar | 1212 b | 2100 a | 744 bc |
| Drench | 1680 a | 2460 a | 1038 a | |
| ANOVA | p = 0.001 | p = 0.004 | p = 0.001 | |
| Orthogonal contrasts | ||||
| Control vs. nTiO2 | p = 0.009 | p = 0.003 | p = 0.001 | |
| Control vs. nTiO2-MDX | p = 0.001 | p = 0.001 | p = 0.001 | |
| nTiO2 vs. nTiO2-MDX | p = 0.001 | ns | ns | |
| Treatment | Application Method | N % | P mg kg−1 | K mg kg−1 | Ca mg kg−1 | Mg mg kg−1 |
|---|---|---|---|---|---|---|
| Control | 2.12 a | 3875.6 bc | 24280.4 bc | 1398.8 a | 1233.8 a | |
| nTiO2 | Foliar | 2.09 a | 3752.9 c | 22569.3 c | 1144.0 b | 1192.3 a |
| Drench | 2.32 a | 4158.9 ab | 25489.1 ab | 1519.2 a | 1283.1 a | |
| nTiO2 + MDX | Foliar | 1.99 a | 4116.0 abc | 26880.3 a | 1378.4 a | 1289.4 a |
| Drench | 2.35 a | 4315.5 a | 23516.7 c | 1524.8 a | 1209.8 a | |
| ANOVA | p = 0.200 | p = 0.028 | p = 0.003 | p = 0.002 | p = 0.325 | |
| Orthogonal contrasts | ||||||
| Control vs. nTiO2 | ns | p = 0.002 | ns | ns | ns | |
| Control vs. nTiO2-MDX | ns | p = 0.003 | ns | ns | ns | |
| nTiO2 vs. nTiO2-MDX | ns | p = 0.006 | ns | ns | ns | |
| Treatment | Application Method | Fe mg kg−1 | Cu mg kg−1 | Mn mg kg−1 | Zn mg kg−1 | B mg kg−1 | Ti mg kg−1 |
|---|---|---|---|---|---|---|---|
| Control | 168.9 a | 4.98 ab | 18.2 ab | 28.7 a | 21.5 a | 0.847 a | |
| nTiO2 | Foliar | 133.2 ab | 4.83 b | 16.5 b | 19.3 b | 20.4 ab | 0.677 a |
| Drench | 161.2 a | 5.53 a | 19.8 a | 18.5 b | 20.3 ab | 0.778 a | |
| nTiO2 + MDX | Foliar | 138.2 ab | 4.99 ab | 18.8 ab | 22.2 ab | 21.9 a | 0.799 a |
| Drench | 112.0 b | 4.68 b | 18.8 ab | 20.2 b | 18.1 b | 0.743 a | |
| ANOVA | p = 0.028 | p = 0.151 | p = 0.257 | p = 0.014 | p = 0.005 | p = 0.942 | |
| Orthogonal contrasts | |||||||
| Control vs. nTiO2 | ns | ns | p = 0.019 | ns | ns | p = 0.042 | |
| Control vs. nTiO2-MDX | p = 0.002 | ns | p = 0.039 | p = 0.001 | ns | p = 0.050 | |
| nTiO2 vs. nTiO2-MDX | p = 0.049 | ns | ns | p = 0.025 | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Velasco, E.A.; Valdez-Aguilar, L.A.; Betancourt-Galindo, R.; González-Fuentes, J.A.; Baylón-Palomino, A. Covered Rutile-TiO2 Nanoparticles Enhance Tomato Yield and Growth by Modulating Gas Exchange and Nutrient Status. Plants 2023, 12, 3099. https://doi.org/10.3390/plants12173099
Pérez-Velasco EA, Valdez-Aguilar LA, Betancourt-Galindo R, González-Fuentes JA, Baylón-Palomino A. Covered Rutile-TiO2 Nanoparticles Enhance Tomato Yield and Growth by Modulating Gas Exchange and Nutrient Status. Plants. 2023; 12(17):3099. https://doi.org/10.3390/plants12173099
Chicago/Turabian StylePérez-Velasco, Eneida A., Luis A. Valdez-Aguilar, Rebeca Betancourt-Galindo, José Antonio González-Fuentes, and Adolfo Baylón-Palomino. 2023. "Covered Rutile-TiO2 Nanoparticles Enhance Tomato Yield and Growth by Modulating Gas Exchange and Nutrient Status" Plants 12, no. 17: 3099. https://doi.org/10.3390/plants12173099






