Seasonal Differences in Leaf Photoprotective Potential between Adults and Juveniles of Two Mediterranean Perennials with Distinct Growth Forms: A Comparative Field Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Site, and Sampling
2.2. Chlorophyll Fluorescence Measurements in the Light Acclimated State
2.3. Rapid Light Curve Fitting
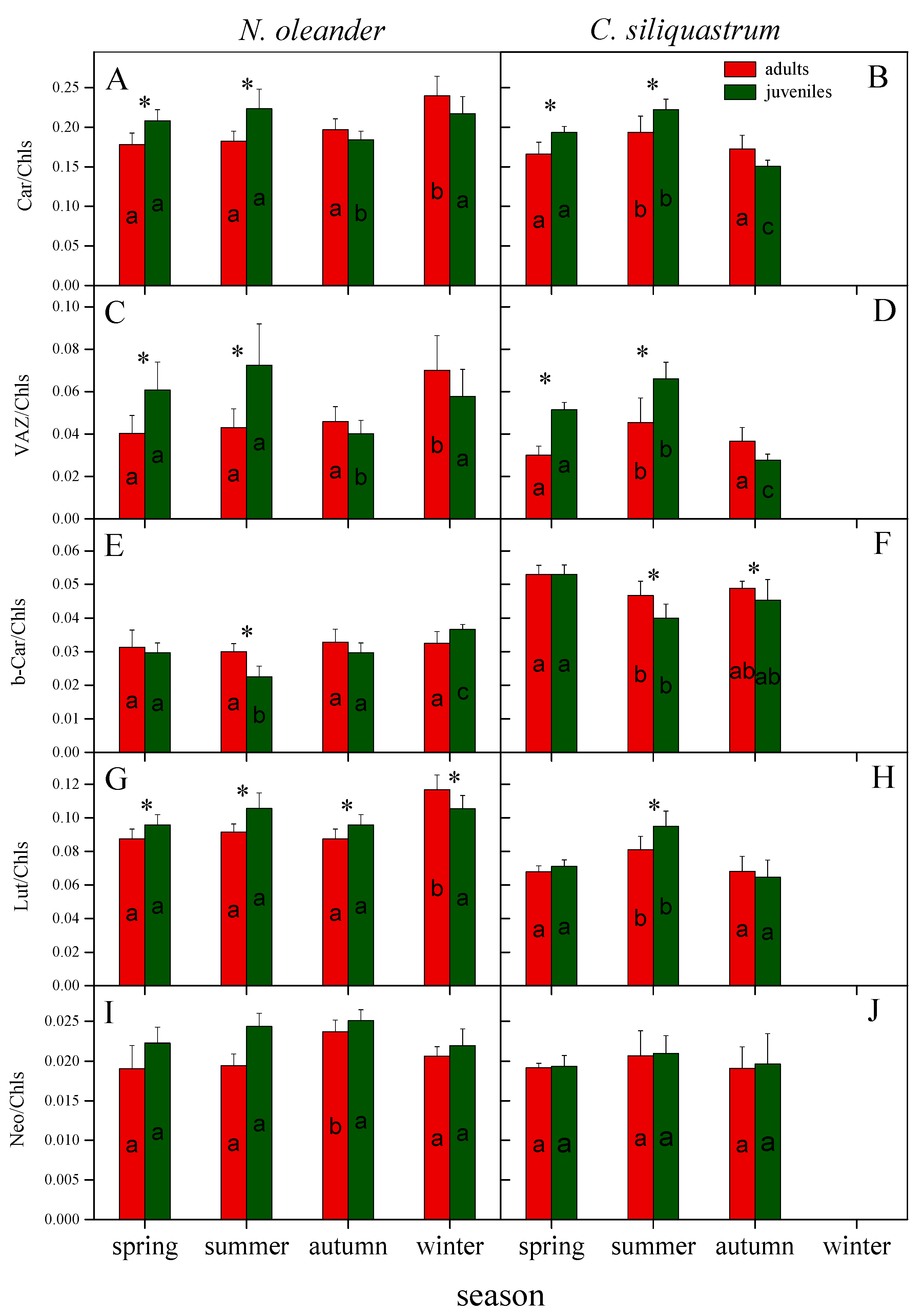
2.4. Photosynthetic Pigments
2.5. Statistical Analysis
3. Results
3.1. Chlorophyll Fluorescence Measurements
3.2. Photosynthetic Parameters after RLC Fitting
3.3. Photosynthetic Pigments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Werner, C.; Correia, O.; Beyschlag, W. Characteristic patterns of chronic and dynamic photoinhibition of different functional groups in a Mediterranean ecosystem. Funct. Plant Biol. 2002, 29, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Vitale, L.; Virzo de Santo, A. Photosynthesis and photoprotective strategies in Laurus nobilis L. and Quercus ilex L. under summer drought and winter cold. Plant Biosyst. 2008, 142, 472–479. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Catoni, R.; Gratani, L. Variations in leaf respiration and photosynthesis ratio in response to air temperature and water availability among Mediterranean evergreen species. J. Arid Environ. 2014, 102, 82–88. [Google Scholar] [CrossRef]
- Chondrogiannis, C.; Grammatikopoulos, G. Transition from juvenility to maturity strengthens photosynthesis in sclerophyllous and deciduous but not in semi-deciduous Mediterranean shrubs. Environ. Exp. Bot. 2021, 181, 104265. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoinhibition. Encycl. Appl. Plant Sci. 2017, 1, 78–85. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plant. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig-Adams, B. Regulation of Photosynthetic Light Energy Capture, Conversion, and Dissipation in Leaves of Higher Plants. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 17–47. [Google Scholar] [CrossRef]
- Kyparissis, A.; Manetas, Y. Seasonal leaf dimorphism in a semi-deciduous Mediterranean shrub: Ecophysiological comparisons between winter and summer leaves. Acta Oecol. 1993, 14, 23–32. [Google Scholar]
- Kytridis, V.-P.; Manetas, Y. Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: Evidence linking the putative antioxidant role to the proximity of oxy-radical source. J. Exp. Bot. 2006, 57, 2203–2210. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Olkawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Kotakis, C.; Petropoulou, Y.; Stamatakis, K.; Yiotis, C.; Manetas, Y. Evidence for active cyclic electron flow in twig chlorenchyma in the presence of an extremely deficient linear electron transport activity. Planta 2006, 225, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, J. State transitions-the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Niinemets, Ü.; García-Plazaola, J.I.; Tosens, T. Photosynthesis during leaf development and ageing. In Terrestrial Photosynthesis in a Changing Environment, 1st ed.; Flexas, J., Loreto, F., Medrano, H., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 353–372. [Google Scholar]
- Chondrogiannis, C.; Grammatikopoulos, G. Photosynthesis in developing leaf of juveniles and adults of three Mediterranean species with different growth forms. Photosynth. Res. 2016, 130, 427–444. [Google Scholar] [CrossRef]
- Niinemets, Ü. Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 2002, 22, 515–535. [Google Scholar] [CrossRef]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between saplings and adult trees: An integration of field results by meta-analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Tenchunen, J.D.; Beyschlag, W. Spatial and age-dependent modifications of photosynthetic capacity in four Mediterranean oak species. Funct. Plant Biol. 2004, 31, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Niinemets, Ü.; Teskey, R.O. Tree size-and age-related changes in leaf physiology and their influence on carbon gain. In Size-and Age-Related Changes in Tree Structure and Function; Tree Physiology; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 4, pp. 235–253. [Google Scholar] [CrossRef]
- Bond, B.J. Age-related changes in photosynthesis of woody plants. Trends Plant Sci. 2000, 5, 349–353. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Stomatal responses to drought of mature trees and seedlings of two co-occurring Mediterranean oaks. For. Ecol. Manag. 2004, 187, 281–294. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Kuusk, V.; Niinemets, Ü.; Valladares, F. Structural controls on photosynthetic capacity through juvenile-to-adult transition and needle ageing in Mediterranean pines. Funct. Ecol. 2018, 32, 1479–1491. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T. Photosynthetic responses to stress in Mediterranean evergreens: Mechanisms and models. Environ. Exp. Bot. 2014, 103, 24–41. [Google Scholar] [CrossRef]
- Kyparissis, A.; Petropoulou, Y.; Manetas, Y. Summer survival of leaves in a softleaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: Avoidance of photoinhibitory damage through decreased chlorophyll contents. J. Exp. Bot. 1995, 46, 1825–1831. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Casadesús, A.; Müller, M.; Munné-Bosch, S. Leaf orientation as part of the leaf developmental program in the semi-deciduous shrub, Cistus albidus L.: Diurnal, positional, and photoprotective effects during winter. Front. Plant Sci. 2019, 10, 767. [Google Scholar] [CrossRef]
- Valladares, F.; Dobarro, I.; Sánchez-Gómez, D.; Pearcy, R.W. Photoinhibition and drought in Mediterranean woody saplings: Scaling effects and interactions in sun and shade phenotypes. J. Exp. Bot. 2005, 56, 483–494. [Google Scholar] [CrossRef]
- Baraldi, R.; Canaccini, F.; Cortes, S.; Magnani, F.; Rapparini, F.; Zamboni, A.; Raddi, S. Role of xanthophyll cycle-mediated photoprotection in Arbutus unedo plants exposed to water stress during the Mediterranean summer. Photosynthetica 2008, 46, 378–386. [Google Scholar] [CrossRef]
- Thomas, S.C. Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiol. 2010, 30, 555–573. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kyzeridou, A.; Stamatakis, K.; Petropoulou, Y. The non-foliar hypoxic photosynthetic syndrome: Evidence or enhanced pools and functionality of xanthophyll cycle components and active cyclic electron flow in fruit chlorenchyma. Planta 2015, 241, 1051–1059. [Google Scholar] [CrossRef]
- Thayer, S.S.; Björkman, O. Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 1990, 23, 331–343. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Chotikarn, P.; Pramneechote, P.; Sinutok, S. Photosynthetic Responses of Freshwater Macrophytes to the Daily Light Cycle in Songkhla Lagoon. Plants 2022, 11, 2806. [Google Scholar] [CrossRef]
- Di Castri, F. Climatographical comparisons between Chile and the western coast of North America. In Mediterranean Type Ecosystems; Springer: Berlin/Heidelberg, Germany, 1973; pp. 21–36. [Google Scholar]
- Karavatas, S.; Manetas, Y. Seasonal patterns of photosystem 2 photochemical efficiency in evergreen sclerophylls and drought semi-deciduous shrubs under Mediterranean field conditions. Photosynthetica 1999, 36, 41–49. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Barradas, M.C.D.; Ain-Lhout, F.; Alvarez-Cansino, L.; Esquivias, M.P.; Novo, F.G. Seasonal physiological plasticity and recovery capacity after summer stress in Mediterranean scrub communities. Plant Ecol. 2011, 212, 127–142. [Google Scholar] [CrossRef]
- Foyer, C.H.; Harbinson, J. Photosynthetic regulation. In Terrestrial Photosynthesis in a Changing Environment: A Molecular, Physiological and Ecological Approach; Cambridge University Press: Cambridge, UK, 2011; pp. 20–40. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.I.; Olano, J.M.; Hernández, A.; Becerril, J.M. Photoprotection in evergreen Mediterranean plants during sudden periods of intense cold weather. Trees 2003, 17, 285–291. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in drought response strategies with ontogeny in Quercus rubra: Implications for scaling from seedlings to mature trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, B.; Hernández, A.; Garcia-Plazaola, J.I.; Esteban, R.; Míguez, F.; Artetxe, U.; Gómez-Sagast, M.T. Photoprotective strategies of Mediterranean plants in relation to morphological traits and natural environmental pressure: A meta-analytical approach. Front. Plant Sci. 2017, 8, 1051. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Faria, T.; Abadia, J.; Chaves, M.M.; Pereira, J.S. Seasonal changes in xanthophyll composition and photosynthesis of cork oak (Quercus suber L.) leaves under Mediterranean climate. J. Exp. Bot. 1997, 48, 1667–1674. [Google Scholar] [CrossRef]
- Kyparissis, A.; Drilias, P.; Manetas, Y. Seasonal fluctuations in photoprotective (xanthophyll cycle) and photoselective (chlorophylls) capacity in eight Mediterranean plant species belonging to two different growth forms. Func. Plant Biol. 2000, 27, 265–272. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.C. Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and water stress. Plant Physiol. 1988, 87, 17–24. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F.; Ravenel, J.; Chanu, D.; Parot, P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: Influence of the xanthophyll content. Plant Cell Environ. 1996, 19, 1359–1368. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Bota, J.; Abadía, A.; Sampol, B.; Escalona, J.M.; Flexas, J. Effects of drought on light-energy dissipation mechanisms in high-light-acclimated, field-grown grapevines. Funct. Plant Biol. 2002, 29, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Muller, O.; Cohu, C.M.; Demmig-Adams, B. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth. Res. 2013, 117, 31–44. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Roach, T.; Verhoeven, A.; García-Plazaola, J.I. Shedding light on the dark side of xanthophyll cycles. New Phytol. 2021, 230, 1336–1344. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Ball, M.C. Protection and storage of chlorophyll in overwintering evergreens. Proc. Natl. Acad. Sci. USA 2000, 97, 11098–11101. [Google Scholar] [CrossRef]
- Corcuera, L.; Morales, F.; Abadía, A.; Gil-Pelegrín, E. Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. Tree Physiol. 2005, 25, 599–608. [Google Scholar] [CrossRef]
- Verhoeven, A. Sustained energy dissipation in winter evergreens. New Phytol. 2014, 201, 57–65. [Google Scholar] [CrossRef]
- Li, X.-P.; Björkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Govindjee. The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: Definitions, timelines, viewpoints, open questions. In Nonphotochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Advances in Photosynthesis and Respiration Series; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 40, pp. 1–44. [Google Scholar]




| ETRmax | Ek | Em | |||||
| Adults | Juveniles | Adults | Juveniles | Adults | Juveniles | ||
| N. oleander | Spring | 174.2 ± 12.8 a | 99.8 ± 7.2 a* | 660 ± 45 a | 373 ± 22 a* | 1930 ± 120 a | 1500 ± 85 a* |
| Summer | 136.5 ± 13.9 b | 45.6 ± 6.1 b* | 515 ± 33 b | 375 ± 39 a* | 1820 ± 110 a | 950 ± 60 b* | |
| Autumn | 154.5 ± 14.5 ab | 159.4 ± 16.7 c | 582 ± 31 b | 568 ± 35 b | 1820 ± 130 a | 1940 ± 75 c | |
| Winter | 92.1 ± 6.4 c | 34.5 ± 4.4 b* | 646 ± 39 a | 377 ± 32 a* | 1540 ± 90 b | 950 ± 50 b* | |
| C. siliquastrum | Spring | 151.4 ± 16.7 a | 114.1 ± 11.2 a* | 539 ± 24 a | 427 ± 26 a* | 1400 ± 120 ab | 1200 ± 80 a |
| Summer | 148.8 ± 17.3 a | 95.4 ± 6.4 a* | 528 ± 27 a | 365 ± 35 b* | 1500 ± 95 a | 1400 ± 75 b | |
| Autumn | 140.3 ± 19.2 a | 102 ± 13.7 a* | 501 ± 29 a | 393 ± 22 b* | 1350 ± 70 b | 1000 ± 55 c* | |
| Chls (μg/cm2) | Car (μg/cm2) | ||||
| Adults | Juveniles | Adults | Juveniles | ||
| N. oleander | Spring | 45.5 ± 7.1 a | 40.3 ± 6.7 a | 8.8 ± 1.8 a | 8.4 ± 1.3 a |
| Summer | 46.6 ± 12.4 a | 42.2 ± 5.9 a | 8.4 ± 1.9 a | 8.7 ± 1.5 a | |
| Autumn | 57.7 ± 5.3 a | 61.5 ± 9.1 b | 11.3 ± 0.9 b | 11.3 ± 1.8 b | |
| Winter | 50.8 ± 8.4 a | 58.1 ± 9.1 b | 12.1 ± 1.7 b | 13.2 ± 1.7 b | |
| C. siliquastrum | Spring | 39.3 ± 5.2 a | 21.0 ± 0.8 a* | 6.5 ± 0.7 a | 3.9 ± 0.1 a* |
| Summer | 37.9 ± 5.1 a | 24.9 ± 3.7 a* | 7.3 ± 1.0 a | 5.3 ± 1.2 b* | |
| Autumn | 40.9 ± 5.8 a | 24.7 ± 3.5 a* | 7.0 ± 0.6 a | 3.9 ± 0.5 a* | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chondrogiannis, C.; Kotsi, K.; Grammatikopoulos, G.; Petropoulou, Y. Seasonal Differences in Leaf Photoprotective Potential between Adults and Juveniles of Two Mediterranean Perennials with Distinct Growth Forms: A Comparative Field Study. Plants 2023, 12, 3110. https://doi.org/10.3390/plants12173110
Chondrogiannis C, Kotsi K, Grammatikopoulos G, Petropoulou Y. Seasonal Differences in Leaf Photoprotective Potential between Adults and Juveniles of Two Mediterranean Perennials with Distinct Growth Forms: A Comparative Field Study. Plants. 2023; 12(17):3110. https://doi.org/10.3390/plants12173110
Chicago/Turabian StyleChondrogiannis, Christos, Kassiani Kotsi, George Grammatikopoulos, and Yiola Petropoulou. 2023. "Seasonal Differences in Leaf Photoprotective Potential between Adults and Juveniles of Two Mediterranean Perennials with Distinct Growth Forms: A Comparative Field Study" Plants 12, no. 17: 3110. https://doi.org/10.3390/plants12173110





