Selection of Soybean and Cowpea Cultivars with Superior Performance under Drought Using Growth and Biochemical Aspects
Abstract
:1. Introduction
2. Results
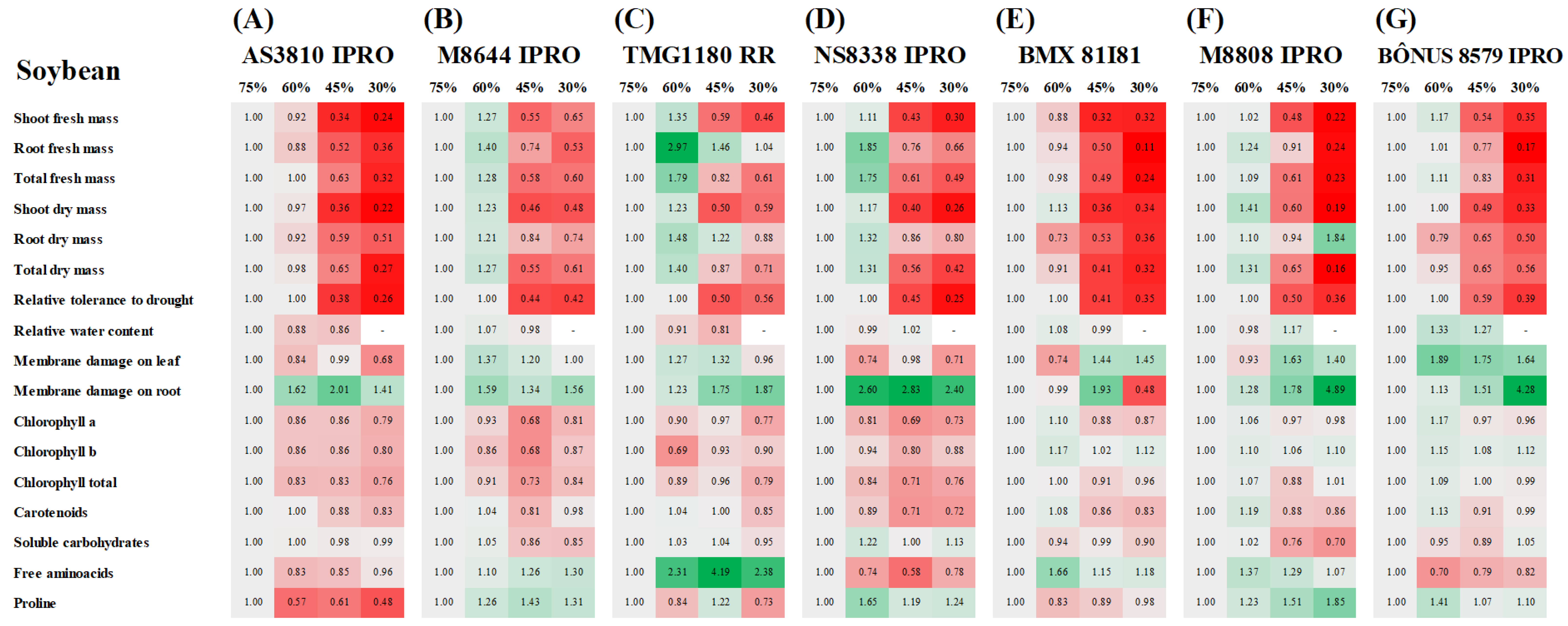
2.1. Plant Growth
2.2. Relative Water Content and Membrane Damage
2.3. Accumulation of Photosynthetic Pigments
2.4. Accumulation of Organic Compounds
2.5. Principal Component Analysis (PCA)
3. Discussion
3.1. Soybean Crop Has Low Water Requirements for Elevated Growth during the Vegetative Stage and Displays Drought Tolerance Higher Than Cowpea Crop
3.2. Cowpea and Soybean Cultivars Display Contrasting Responses to Water Deficit
3.3. Leguminous Crops Activate Specific Biochemical Mechanisms for Drought Tolerance
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Plant Growth
4.3. Relative Water Content and Membrane Damage
4.4. Photosynthetic Pigments
4.5. Soluble Carbohydrates, Free Amino Acids and Proline
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). The State of the World’s Land and Water Resources for Food and Agriculture: Systems at Breaking Point; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- UN (United Nations). The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; UNESCO: Paris, France, 2023; Available online: https://www.unesco.org/reports/wwdr/2023/en/download (accessed on 21 July 2023).
- Omomowo, O.I.; Babalola, O.O. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci. 2021, 12, 751731. [Google Scholar] [CrossRef] [PubMed]
- CONAB (Brazilian Supply Company). Monitoring of Brazilian Grain Harvest 2022/2023, 10th ed.; Tenth Survey; CONAB: Brasília, Brazil, 2023; Volume 1. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 15 July 2023).
- USDA (United States Department of Agriculture). World Agricultural Production. Circular Series. 2023. Available online: http://www.usda.gov (accessed on 21 July 2023).
- Wang, C.; Linderholm, H.W.; Song, Y.; Wang, F.; Liu, Y.; Tian, J.; Xu, J.; Song, Y.; Ren, G. Impacts of drought on maize and soybean production in Northeast China during the past five decades. Int. J. Environ. Res. Public Health 2020, 17, 2459. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.H.F.M.; Antolin, L.A.S.; Zanon, A.L.; Andrade-Junior, A.S.; Souza, H.A.; Carvalho, K.S.; Vieira-Junior, N.A.; Marin, F.R. Impact assessment of soybean yield and water productivity in Brazil due to climate change. Eur. J. Agron. 2021, 129, 126329. [Google Scholar] [CrossRef]
- Nguyen, H.; Thompson, A.; Costello, C. Impacts of historical droughts on maize and soybean production in the southeastern United States. Agric. Water Manag. 2023, 281, 108237. [Google Scholar] [CrossRef]
- FAOSTAT (Food and Agriculture Organization Statistics). Food and Agriculture Data. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 July 2023).
- CONAB (Brazilian Supply Company). Monitoring of Brazilian Grain Harvest 2020/2021, 8th ed.; Twelfth Survey; CONAB: Brasília, Brazil, 2021; Volume 12. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 15 July 2023).
- Souza, P.J.O.P.; Farias, V.D.S.; Pinto, J.V.N.; Nunes, H.G.G.C.; Souza, E.B.; Fraisse, C.W. Yield gap in cowpea plants as function of water deficits during reproductive stage. Rev. Bras. Eng. Agr. Amb. 2020, 24, 372–378. [Google Scholar] [CrossRef]
- Praxedes, S.C.; Gomes-Filho, E.; Damatta, F.M.; Lacerda, C.F.; Prisco, J.T. Salt stress tolerance in cowpea is poorly related to the ability to cope with oxidative stress. Acta Bot Croat. 2014, 73, 51–62. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Kausar, A.; Zeidi, M.A.; Asekova, S.; Siddique, K.H.M.; Farooq, M. Plant photosynthetic responses under drought stress: Effects and management. J. Agron. Crop Sci. 2023, 1, 1–22. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lazaridi, E.; Bebeli, P.J. Cowpea Constraints and Breeding in Europe. Plants 2023, 12, 1339. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A. Effect of salicylic acid and abscisic acid on morpho–physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. 2015, 6, 1771–1788. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Katam, R.; Shokri, S.; Murthy, N.; Singh, S.K.; Suravajhala, P.; Khan, M.N.; Bahmani, M.; Sakata, K.; Reddy, K.R. Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean. PLoS ONE 2022, 15, e0233905. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plant. 2018, 24, 37–50. [Google Scholar] [CrossRef]
- Mesquita, R.O.; Coutinho, F.S.; Vital, C.E.; Nepomuceno, A.L.; Williams, T.C.R.; Ramos, H.J.O.; Loureiro, M.E. Physiological approach to decipher the drought tolerance of a soybean genotype from Brazilian savana. Plant Physiol. Bioch. 2020, 151, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F.; Li, S.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant. Biol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Dutra, W.F.; Melo, A.S.; Suassuna, J.F.; Dutra, A.F.; Silva, D.C.; Maia, J.M. Antioxidative responses of cowpea cultivars to water deficit and salicylic acid treatment. Agron. J. 2017, 109, 895–905. [Google Scholar] [CrossRef]
- Cui, Q.; Xiong, H.; Yufeng, Y.; Eaton, S.; Imamura, S.; Santamaria, J.; Ravelombola, W.; Mason, R.E.; Wood, L.; Mozzoni, L.A.; et al. Evaluation of drought tolerance in Arkansas cowpea lines at seedling stage. HortScience 2020, 55, 1132–1143. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Ansari, W.A.; Atri, N.; Ahmad, J.; Qureshi, M.I.; Singh, B.; Kumar, R.; Rai, V.; Pandey, S. Drought mediated physiological and molecular changes in muskmelon (Cucumis melo L.). PLoS ONE 2019, 14, e0222647. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef]
- Pandey, K.; Kumar, R.S.; Prasad, P.; Pande, V.; Trivedi, P.K.; Shirke, P.A. Coordinated regulation of photosynthesis and sugar metabolism in guar increases tolerance to drought. Environ. Exp Bot. 2022, 194, 104701. [Google Scholar] [CrossRef]
- Miranda, R.S.; Mesquita, R.O.; Freitas, N.S.; Prisco, J.T.; Gomes-Filho, E. Nitrate: Ammonium nutrition alleviates detrimental effects of salinity by enhancing photosystem II efficiency in sorghum plants. Rev. Bras. Eng. Agr. Amb. 2014, 18, 8–12. [Google Scholar] [CrossRef]
- Araújo, G.S.; Miranda, R.S.; Mesquita, R.O.; Paula, S.O.; Prisco, J.T.; Gomes-Filho, E. Nitrogen assimilation pathways and ionic homeostasis are crucial for photosynthetic apparatus efficiency in salt-tolerant sunflower genotypes. Plant Growth Regul. 2018, 86, 375–388. [Google Scholar] [CrossRef]
- Anda, A.; Simon, B.; Soós, G.; Silva, J.A.T.; Menyhárt, L. Water stress modifies canopy light environment and qualitative and quantitative yield components in two soybean varieties. Irrig. Sci. 2021, 39, 549–566. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Bioch. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plantarum 2020, 172, 1321–1335. [Google Scholar] [CrossRef]
- Živanović, B.; Milić Komić, S.; Tosti, T.; Vidović, M.; Prokić, L.; Veljović Jovanović, S. Leaf soluble sugars and free amino acids as important components of abscisic acid-mediated drought response in tomato. Plants 2020, 9, 1147. [Google Scholar] [CrossRef]
- Freitas, P.A.F.; Carvalho, H.H.; Costa, J.H.; Miranda, R.S.; Saraiva, K.D.C.; Oliveira, F.D.B.; Coelho, D.G.; Prisco, J.T.; Gomes-Filho, E. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar] [CrossRef]
- Benitez, L.C.; Vighi, I.L.; Auler, P.A.; Amaral, M.N.; Moraes, G.P.; Rodrigues, G.S.; Maia, L.C.; Magalhães-Júnior, A.M.; Braga, E.J.B. Correlation of proline content and gene expression involved in the metabolism of this amino acid under abiotic stress. Acta Physiol. Plant. 2016, 38, 267. [Google Scholar] [CrossRef]
- Souza, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2014; 416p. [Google Scholar]
- Miranda, R.S.; Souza, F.I.L.; Alves, A.F.; Souza, R.R.; Mesquita, R.O.; Ribeiro, M.I.D.; Santana-Filho, J.A.; Gomes-Filho, E. Salt-acclimation physiological mechanisms at the vegetative stage of cowpea cultivars in soils from a semiarid region. J. Soil. Sci. Plant Nutr. 2021, 21, 3530–3543. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–314. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Cienc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org (accessed on 21 February 2022).




| Soybean Cultivars | 75% | 60% | 45% | 30% | 75% | 60% | 45% | 30% |
|---|---|---|---|---|---|---|---|---|
| Shoot fresh mass (g/plant) | Shoot dry mass (g/plant) | |||||||
| AS3810 IPRO | 8.26 Aa | 7.57 Aa | 2.80 Ba | 1.94 Ba | 1.71 Aa | 1.66 Aa | 0.61 Ba | 0.37 Ba |
| M8644 IPRO | 4.20 Ac | 5.32 Ab | 2.33 Ba | 2.72 Ba | 1.09 Ab | 1.35 Ab | 0.50 Ba | 0.53 Ba |
| TMG1180 RR | 5.61 Bb | 7.60 Aa | 3.30 Ca | 2.57 Ca | 1.33 Ab | 1.64 Aa | 0.67 Ba | 0.79 Ba |
| NS8338 IPRO | 7.29 Aa | 8.10 Aa | 3.11 Ba | 2.20 Ba | 1.65 Aa | 1.94 Aa | 0.66 Ba | 0.43 Ba |
| BMX81I81 IPRO | 7.20 Aa | 6.36 Ab | 2.31 Ba | 2.27 Ba | 1.29 Ab | 1.45 Ab | 0.46 Ba | 0.44 Ba |
| M8808 IPRO | 5.77 Ab | 5.89 Ab | 2.77 Ba | 1.24 Ca | 0.99 Bb | 1.40 Ab | 0.60 Ca | 0.18 Da |
| BÔNUS8579 IPRO | 7.89 Aa | 9.20 Aa | 4.27 Ba | 2.76 Ca | 1.85 Aa | 1.85 Aa | 0.91 Ba | 0.61 Ba |
| Root fresh mass (g/plant) | Root dry mass (g/plant) | |||||||
| AS3810 IPRO | 4.07 Ab | 3.58 Ab | 2.11 Ba | 1.45 Ba | 0.44 Ac | 0.40 Aa | 0.26 Bb | 0.23 Bb |
| M8644 IPRO | 3.47 Bb | 4.94 Aa | 2.34 Ca | 1.67 Ca | 0.39 Ac | 0.47 Aa | 0.30 Ba | 0.23 Bb |
| TMG1180 RR | 2.03 Cc | 6.03 Aa | 2.96 Ba | 2.10 Ca | 0.34 Bd | 0.46 Aa | 0.37 Bb | 0.27 Cb |
| NS8338 IPRO | 2.94 Bc | 4.92 Aa | 2.01 Ca | 1.74 Ca | 0.31 Bd | 0.41 Aa | 0.27 Bb | 0.25 Bb |
| BMX81I81 IPRO | 5.27 Aa | 4.95Aa | 2.65 Ba | 0.58 Cb | 0.70 Aa | 0.46 Ba | 0.33 Ca | 0.23 Db |
| M8808 IPRO | 2.47 Ac | 3.05 Ab | 2.23 Aa | 0.59 Bb | 0.25 Bd | 0.28 Bb | 0.24 Bb | 0.42 Aa |
| BÔNUS8579 IPRO | 3.97 Ab | 3.99 Ab | 3.05 Ba | 0.67 Cb | 0.54 Ab | 0.47 Aa | 0.35 Ba | 0.28 Bb |
| Total fresh mass (g/plant) | Total dry mass (g/plant) | |||||||
| AS3810 IPRO | 12.33 Aa | 11.15 Ac | 4.90 Bc | 3.40 Ba | 2.15 Aa | 2.27 Aa | 0.87 Ba | 0.60 Bb |
| M8644 IPRO | 7.68 Bc | 10.26 Ac | 4.67 Cc | 4.39 Ca | 1.49 Ab | 1.81 Ab | 0.80 Ba | 0.76 Bb |
| TMG1180 RR | 7.64 Bc | 13.62 Aa | 6.26 Bb | 4.27 Ca | 1.67 Bb | 2.10 Aa | 1.04 Ca | 1.17 Ca |
| NS8338 IPRO | 10.23 Bb | 13.02 Ab | 5.12 Cc | 3.94 Ca | 1.96 Ba | 2.35 Aa | 0.93 Ca | 0.60 Cb |
| BMX81I81 IPRO | 13.59 Aa | 12.14 Ab | 4.96 Bc | 2.85 Cb | 2.28 Aa | 1.91 Bb | 0.79 Ca | 0.67 Cb |
| M8808 IPRO | 7.23 Bc | 8.95 Ad | 5.00 Cc | 1.83 Db | 1.24 Bb | 1.68 Ab | 0.83 Ca | 0.60 Cb |
| BÔNUS8579 IPRO | 11.86 Ba | 14.47 Aa | 7.94 Ca | 3.43 Da | 2.39 Aa | 2.32 Aa | 1.26 Ba | 0.89 Ca |
| Relative tolerance to drought (%) * | Relative water content (%) ** | |||||||
| AS3810 IPRO | 100 Aa | 100 Aa | 38.39 Bc | 26.34 Cc | 52.6 Aa | 46.3 Aa | 45.4 Ab | - |
| M8644 IPRO | 100 Aa | 100 Aa | 44.06 Bc | 41.76 Bb | 44.9 Aa | 48.2 Aa | 44.2 Ab | - |
| TMG1180 RR | 100 Aa | 100 Aa | 49.64 Bb | 55.86 Ca | 49.5 Aa | 45.2 Aa | 40.3 Ab | - |
| NS8338 IPRO | 100 Aa | 100 Aa | 44.64 Bc | 25.43 Cc | 56.9 Aa | 56.1 Aa | 58.3 Aa | - |
| BMX81I81 IPRO | 100 Aa | 100 Aa | 41.41 Bc | 35.07 Cb | 49.3 Aa | 53.5 Aa | 49.0 Ab | - |
| M8808 IPRO | 100 Aa | 100 Aa | 49.65 Bb | 35.80 Cb | 52.0 Aa | 50.7 Aa | 61.1 Aa | - |
| BÔNUS8579 IPRO | 100 Aa | 100 Aa | 58.75 Ba | 38.54 Cb | 42.5 Ba | 56.6 Aa | 53.9 Aa | - |
| Membrane damage in leaves (%) | Membrane damage in roots (%) | |||||||
| AS3810 IPRO | 30.4 Aa | 25.7 Aa | 30.1 Ba | 20.6 Ba | 35.9 Ba | 58.1 Aa | 72.1 Aa | 50.6 Bb |
| M8644 IPRO | 22.8 Bb | 31.1 Aa | 27.4 Aa | 22.8 Ba | 32.2 Aa | 51.1 Aa | 43.2 Aa | 50.3 Ab |
| TMG1180 RR | 23.7 Bb | 30.1 Aa | 31.3Aa | 22.8 Ba | 31.5 Ba | 38.1 Ba | 55.2 Aa | 58.9 Ab |
| NS8338 IPRO | 28.4 Aa | 21.1 Bb | 27.7 Aa | 20.3 Ba | 19.4 Ba | 50.5 Aa | 55.0 Aa | 46.6 Ab |
| BMX81I81 IPRO | 17.6 Bc | 13.1 Bc | 25.4 Aa | 25.5 Aa | 34.7 Ba | 34.3 Ba | 66.9Aa | 16.5 Bc |
| M8808 IPRO | 16.3 Bc | 15.2 Bc | 26.6 Aa | 22.9 Aa | 34.6 Ca | 44.3 Ca | 61.8 Ba | 169.3 Aa |
| BÔNUS8579 IPRO | 16.4 Bc | 31.1 Aa | 28.7 Aa | 26.9 Aa | 36.5 Ba | 41.1 Ba | 54.9 Ba | 156.2 Aa |
| Chl a (μg g−1 DM) | Chl b (μg g−1 DM) | |||||||
| AS3810 IPRO | 4419 Aa | 3801 Bb | 3821 Ba | 3478 Ba | 1376 Aa | 1182 Ba | 1180 Ba | 1095 Ba |
| M8644 IPRO | 3556 Ab | 3289 Ac | 2425 Bb | 2896 Bb | 1108 Ab | 952 Bb | 758 Bb | 959 Bb |
| TMG1180 RR | 3990 Aa | 3603 Ac | 3875 Aa | 3059 Bb | 1263 Aa | 873 Bb | 1169 Aa | 1132 Aa |
| NS8338 IPRO | 4179 Aa | 3399 Bc | 2883 Bb | 3043 Bb | 1106 Ab | 1035 Ab | 886 Ab | 975 Ab |
| BMX81I81 IPRO | 3888 Ab | 4289 Aa | 3436 Ba | 3368 Ba | 1036 Ab | 1215 Aa | 1057 Aa | 1156 Aa |
| M8808 IPRO | 3658 Ab | 3881 Ab | 3557 Aa | 3596 Aa | 1045 Ab | 1145 Aa | 1112 Aa | 1153 Aa |
| BÔNUS8579 IPRO | 3657 Bb | 4279 Aa | 3565 Ba | 3499 Ba | 1013 Ab | 1163 Aa | 1097 Aa | 1134 Aa |
| Chl total (μg g−1 DM) | Carotenoids (μg g−1 DM) | |||||||
| AS3810 IPRO | 6039 Aa | 5016 Ba | 5035 Ba | 4602 Ba | 951 Aa | 950 Aa | 837 Aa | 787 Aa |
| M8644 IPRO | 4695 Ab | 4278 Aa | 3408 Bb | 3934 Ba | 740 Aa | 772 Ab | 603 Ab | 725 Aa |
| TMG1180 RR | 5286 Aa | 4722 Aa | 5082 Aa | 4200 Aa | 836 Aa | 870 Ab | 835 Aa | 712 Aa |
| NS8338 IPRO | 5343 Aa | 4466 Ba | 3795 Bb | 4042 Ba | 942 Aa | 836 Ab | 665 Bb | 676 Ba |
| BMX81I81 IPRO | 4977 Ab | 4960 Aa | 4525 Aa | 4780 Aa | 882 Aa | 955 Aa | 759 Ba | 735 Ba |
| M8808 IPRO | 4744 Ab | 5067 Aa | 4177 Aa | 4778 Aa | 893 Ba | 1060 Aa | 784 Ba | 772 Ba |
| BÔNUS8579 IPRO | 4716 Ab | 5154 Aa | 4695 Aa | 4660 Aa | 853 Ba | 962 Aa | 774 Aa | 845 Aa |
| Soluble carbohydrates (μmol g−1 DM) | Free amino acids (μmol g−1 DM) | |||||||
| AS3810 IPRO | 1287 Aa | 1283 Aa | 1266 Aa | 1275 Aa | 217.8 Ab | 180.1 Ab | 184.1 Ac | 210.1 Aa |
| M8644 IPRO | 1228 Aa | 1287 Aa | 1058 Ba | 1048 Bb | 154.2 Ac | 169.0 Ab | 194.5 Ab | 200.5 Aa |
| TMG1180 RR | 1163 Aa | 1199 Aa | 1206 Aa | 1100 Ab | 77.4 Cb | 178.6 Bb | 324.1 Aa | 183.9 Ba |
| NS8338 IPRO | 863 Ab | 1050 Ab | 863 Ab | 973 Ab | 263.2 Aa | 195.9 Bb | 153.0 Cc | 205.5 Ba |
| BMX81I81 IPRO | 1173 Aa | 1101 Ab | 1157 Aa | 1057 Ab | 196.6 Bb | 326.5 Aa | 225.5 Bb | 232.1 Ba |
| M8808 IPRO | 1106 Aa | 1124 Ab | 845 Bb | 774 Bc | 163.9 Bc | 224.4 Ab | 211.2 Ab | 175.9 Ba |
| BÔNUS8579 IPRO | 1101 Aa | 1042 Ab | 984 Ab | 1152 Aa | 214.8 Ab | 150.5 Bb | 169.4 Bc | 177.1 Ba |
| Proline (μmol g−1 DM) | ||||||||
| AS3810 IPRO | 6.69 Aa | 3.79 Bb | 4.07 Ba | 3.24 Bb | ||||
| M8644 IPRO | 2.11 Ac | 2.67 Ab | 3.02 Ab | 2.76 Ab | ||||
| TMG1180RR | 2.65 Ac | 2.22 Ab | 3.23 Ab | 1.95 Ab | ||||
| NS8338 IPRO | 3.75 Bb | 6.17 Aa | 4.46 Ba | 4.66 Ba | ||||
| BMX81I81 IPRO | 3.37 Ab | 2.82 Ab | 3.00 Ab | 3.30 Ab | ||||
| M8808 IPRO | 2.48 Bc | 3.05 Bb | 3.74 Aa | 4.58 Aa | ||||
| BÔNUS8579 IPRO | 2.36 Ac | 3.33 Ab | 2.53 Ab | 2.61 Ab | ||||
| Cowpea Cultivars | 75% | 60% | 45% | 30% | 75% | 60% | 45% | 30% |
|---|---|---|---|---|---|---|---|---|
| Shoot fresh mass (g/plant) | Shoot dry mass (g/plant) | |||||||
| Aracê | 64.83 Aa | 8.83 Ba | 5.96 Ba | - | 7.18 Aa | 1.18 Ba | 0.75 Ba | - |
| Novaera | 38.20 Ab | 10.20 Ba | 7.02 Ba | - | 6.19 Aa | 1.79 Ba | 0.98 Ba | - |
| Pajeú | 39.32 Ab | 6.29 Ba | 4.90 Ba | - | 5.09 Aa | 1.61 Ba | 0.68 Ba | - |
| Pitiúba | 46.48 Ab | 5.69 Ba | 5.55 Ba | - | 5.54 Aa | 1.20 Ba | 0.81 Ba | - |
| Tumucumaque | 43.41 Ab | 7.683 Ba | 4.30 Ba | - | 5.42 Aa | 1.07 Ba | 0.60 Ba | - |
| TVU | 42.02 Ab | 6.04 Ba | 4.07 Ba | - | 6.21 Aa | 1.15 Ba | 0.45 Ba | - |
| Xique-xique | 55.22 Aa | 15.37 Ba | 5.76 Ba | - | 6.56 Aa | 2.24 Ba | 0.93 Ba | - |
| Root fresh mass (g/plant) | Root dry mass (g/plant) | |||||||
| Aracê | 13.91 Aa | 2.41 Ba | 1.02 Ba | - | 1.14 Ab | 0.29 Ba | 0.08 Ba | - |
| Novaera | 12.35 Aa | 2.51 Ba | 1.25 Ba | - | 1.45 Aa | 0.25 Ba | 0.13 Ba | - |
| Pajeú | 7.93 Ab | 2.40 Ba | 1.13 Ba | - | 1.16 Ab | 0.28 Ba | 0.09 Ba | - |
| Pitiúba | 11.26 Ab | 2.08 Ba | 0.95 Ba | - | 1.13 Ab | 0.15 Ba | 0.09 Ba | - |
| Tumucumaque | 14.88 Aa | 3.08 Ba | 1.38 Ba | - | 1.33 Aa | 0.37 Ba | 0.08 Ca | - |
| TVU | 9.86 Ab | 1.94 Ba | 0.82 Ba | - | 1.12 Ab | 0.20 Ba | 0.09 Ba | - |
| Xique-xique | 10.55 Ab | 3.12 Ba | 1.16 Ba | - | 0.96 Ab | 0.36 Ba | 0.12 Ba | - |
| Total fresh mass (g/plant) | Total dry mass (g/plant) | |||||||
| Aracê | 78.75 Aa | 11.23 Ba | 6.97 Ba | - | 8.33 Aa | 1.46 Bb | 0.82 Bb | - |
| Novaera | 50.55 Ac | 12.70 Ba | 8.27 Ba | - | 7.65 Aa | 2.04 Ba | 1.11 Ba | - |
| Pajeú | 47.25 Ac | 8.69 Ba | 6.03 Ba | - | 6.25 Aa | 1.88 Ba | 0.77 Bb | - |
| Pitiúba | 57.74 Ac | 7.766 Ba | 6.50 Ba | - | 6.67 Aa | 1.36 Bb | 0.89 Bb | - |
| Tumucumaque | 58.29 Ac | 10.76 Ba | 5.69 Ba | - | 6.75 Aa | 1.44 Bb | 0.68 Bb | - |
| TVU | 51.88 Ac | 7.97 Ba | 4.89 Ba | - | 7.33 Aa | 1.35 Bb | 0.54 Bb | - |
| Xique-xique | 65.77 Ab | 18.49 Ba | 6.93 Ca | - | 7.53 Aa | 2.60 Ba | 1.05 Ba | - |
| Relative tolerance to drought (%) * | Relative water content (%) | |||||||
| Aracê | 100 Aa | 10.1 Bb | 8.3 Bb | - | 84.5% Ba | 95.2% Aa | 80.2% Ba | - |
| Novaera | 100 Aa | 28.7 Ba | 15.8 Ba | - | 78.5% Aa | 81.5% Ab | 72.7% Aa | - |
| Pajeú | 100 Aa | 32.4 Ba | 13.8 Cb | - | 77.2% Aa | 81.7% Ab | 55.7% Bb | - |
| Pitiúba | 100 Aa | 13.7 Bb | 21.2 Ba | - | 84.2% Aa | 82.5% Ab | 75.5% Aa | - |
| Tumucumaque | 100 Aa | 12.8 Bb | 9.0 Bb | - | 83.0% Aa | 82.7% Ab | 63.0% Bb | - |
| TVU | 100 Aa | 18.9 Bb | 7.6 Bb | - | 82.5% Aa | 92.7% Aa | 65.2% Bb | - |
| Xique-xique | 100 Aa | 36.7 Ba | 15.5 Ca | - | 89.2% Aa | 79.2% Bb | 72.2% Ba | - |
| Membrane damage in leaves (%) | Membrane damage in roots (%) | |||||||
| Aracê | 82.5% Aa | 69.0% Ba | 61.5% Ba | - | 60.5% Aa | 48.7% Ba | 49.5% Ba | - |
| Novaera | 70.0% Ab | 72.0% Aa | 59.5% Ba | - | 68.0% Aa | 42.0% Ba | 41.2% Ba | - |
| Pajeú | 74.2% Aa | 76.2% Aa | 39.7% Bb | - | 47.0% Ab | 36.2% Aa | 43.0% Aa | - |
| Pitiúba | 80.5% Aa | 76.2% Aa | 47.0% Bb | - | 64.5% Aa | 45.0% Ba | 48.0% Ba | - |
| Tumucumaque | 62.0% Ab | 64.2% Aa | 53.0% Ab | - | 51.7% Ab | 44.5% Aa | 53.5% Aa | - |
| TVU | 77.7% Aa | 72.2% Aa | 61.7% Ba | - | 44.0% Ab | 39.7% Aa | 49.2% Aa | - |
| Xique-xique | 70.7% Ab | 73.7% Aa | 66.0% Aa | - | 70.5% Aa | 44.2% Ba | 50.0% Ba | - |
| Chl a (μg g−1 DM) | Chl b (μg g−1 DM) | |||||||
| Aracê | 3353 Ab | 2535 Ab | 1366 Bb | - | 1084 Ab | 1181 Aa | 847 Aa | - |
| Novaera | 2858 Ab | 2959 Aa | 764 Bc | - | 892 Bb | 1351 Aa | 252 Cb | - |
| Pajeú | 2785 Ab | 2461 Ab | 2324 Aa | - | 841 Ab | 883 Ab | 921 Aa | - |
| Pitiúba | 3144 Aa | 1769 Bc | 2030 Ba | - | 1101 Ab | 670 Bb | 925 Aa | - |
| Tumucumaque | 3335 Aa | 3256 Aa | 1273 Bb | - | 1459 Aa | 1427 Aa | 532 Bb | - |
| TVU | 3353 Aa | 1958 Bc | 1559 Bb | - | 1504 Aa | 858 Bb | 501 Cb | - |
| Xique-xique | 2777 Ab | 2095 Bc | 1893 Ba | - | 974 Ab | 984 Ab | 849 Aa | - |
| Chl total (μg g−1 DM) | Carotenoids (μg g−1 DM) | |||||||
| Aracê | 3744 Ab | 3699 Ab | 2375 Ba | - | 492 Aa | 414 Ab | 332 Ba | - |
| Novaera | 3775 Ab | 4292 Aa | 1022 Bc | - | 542 Aa | 479 Aa | 185 Bc | - |
| Pajeú | 3573 Ab | 3464 Ab | 3245 Aa | - | 543 Aa | 393 Bb | 379 Ba | - |
| Pitiúba | 4261 Aa | 2442 Bc | 2845 Ba | - | 541 Aa | 313 Bb | 396 Ba | - |
| Tumucumaque | 4780 Aa | 4669 Aa | 1803 Bb | - | 510 Aa | 489 Aa | 282 Bb | - |
| TVU | 4839 Aa | 2808 Bc | 1922 Bb | - | 587 Aa | 375 Bb | 365 Ba | - |
| Xique-xique | 3632 Ab | 3404 Ab | 2732 Aa | - | 552 Aa | 415 Bb | 344 Ba | - |
| Soluble carbohydrates (μmol g−1 DM) | Free amino acids (μmol g−1 DM) | |||||||
| Aracê | 194 Ab | 204 Ab | 209 Ab | - | 535 Cb | 843 Ba | 1504 Aa | - |
| Novaera | 267 Aa | 238 Aa | 204 Bb | - | 514 Bb | 753 Aa | 949 Ac | - |
| Pajeú | 268 Aa | 264 Aa | 295 Aa | - | 703 Ab | 592 Aa | 466 Bd | - |
| Pitiúba | 224 Ab | 280 Aa | 250 Aa | - | 583 Ab | 310 Bb | 119 Be | - |
| Tumucumaque | 256 Aa | 233 Aa | 175 Bb | - | 861 Ba | 429 Cb | 1160 Ab | - |
| TVU | 229 Ab | 187 Ab | 192 Ab | - | 959 Aa | 496 Bb | 477 Bd | - |
| Xique-xique | 229 Ab | 263 Aa | 273 Aa | - | 767 Aa | 643 Ba | 478 Bd | - |
| Proline (μmol g−1 DM) | ||||||||
| Aracê | 65 Ba | 72 Ba | 131 Ab | - | ||||
| Novaera | 64 Aa | 97 Aa | 93 Ac | - | ||||
| Pajeú | 75 Aa | 61 Aa | 84 Ac | - | ||||
| Pitiúba | 77 Ba | 69 Ba | 158 Ab | - | ||||
| Tumucumaque | 115 Ba | 62 Ca | 177 Ab | - | ||||
| TVU | 62 Ca | 105 Ba | 341 Aa | - | ||||
| Xique-xique | 87 Ba | 92 Ba | 145 Ab | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, R.d.S.; Fonseca, B.S.F.d.; Pinho, D.S.; Batista, J.Y.N.; Brito, R.R.d.; Silva, E.M.d.; Ferreira, W.S.; Costa, J.H.; Lopes, M.d.S.; Sousa, R.H.B.d.; et al. Selection of Soybean and Cowpea Cultivars with Superior Performance under Drought Using Growth and Biochemical Aspects. Plants 2023, 12, 3134. https://doi.org/10.3390/plants12173134
Miranda RdS, Fonseca BSFd, Pinho DS, Batista JYN, Brito RRd, Silva EMd, Ferreira WS, Costa JH, Lopes MdS, Sousa RHBd, et al. Selection of Soybean and Cowpea Cultivars with Superior Performance under Drought Using Growth and Biochemical Aspects. Plants. 2023; 12(17):3134. https://doi.org/10.3390/plants12173134
Chicago/Turabian StyleMiranda, Rafael de Souza, Bruno Sousa Figueiredo da Fonseca, Davielson Silva Pinho, Jennyfer Yara Nunes Batista, Ramilos Rodrigues de Brito, Everaldo Moreira da Silva, Wesley Santos Ferreira, José Hélio Costa, Marcos dos Santos Lopes, Renan Henrique Beserra de Sousa, and et al. 2023. "Selection of Soybean and Cowpea Cultivars with Superior Performance under Drought Using Growth and Biochemical Aspects" Plants 12, no. 17: 3134. https://doi.org/10.3390/plants12173134
APA StyleMiranda, R. d. S., Fonseca, B. S. F. d., Pinho, D. S., Batista, J. Y. N., Brito, R. R. d., Silva, E. M. d., Ferreira, W. S., Costa, J. H., Lopes, M. d. S., Sousa, R. H. B. d., Neves, L. F., Penha, J. A. F., Santos, A. S., Lima, J. J. P., Paula-Marinho, S. d. O., Neto, F. d. A., Aguiar, É. S. d., Santos, C. P. d., & Gomes-Filho, E. (2023). Selection of Soybean and Cowpea Cultivars with Superior Performance under Drought Using Growth and Biochemical Aspects. Plants, 12(17), 3134. https://doi.org/10.3390/plants12173134










