Effect of Co-Application of Azospirillum brasilense and Rhizobium pisi on Wheat Performance and Soil Nutrient Status under Deficit and Partial Root Drying Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Unit
2.2. Plant Analysis: Morpho-Biochemical Traits
2.3. Soil Fertility Analysis
2.4. Data Interpretation
3. Results
3.1. Wheat Growth Traits
3.2. Wheat Yield Traits
3.3. Relative Growth Rate of Wheat
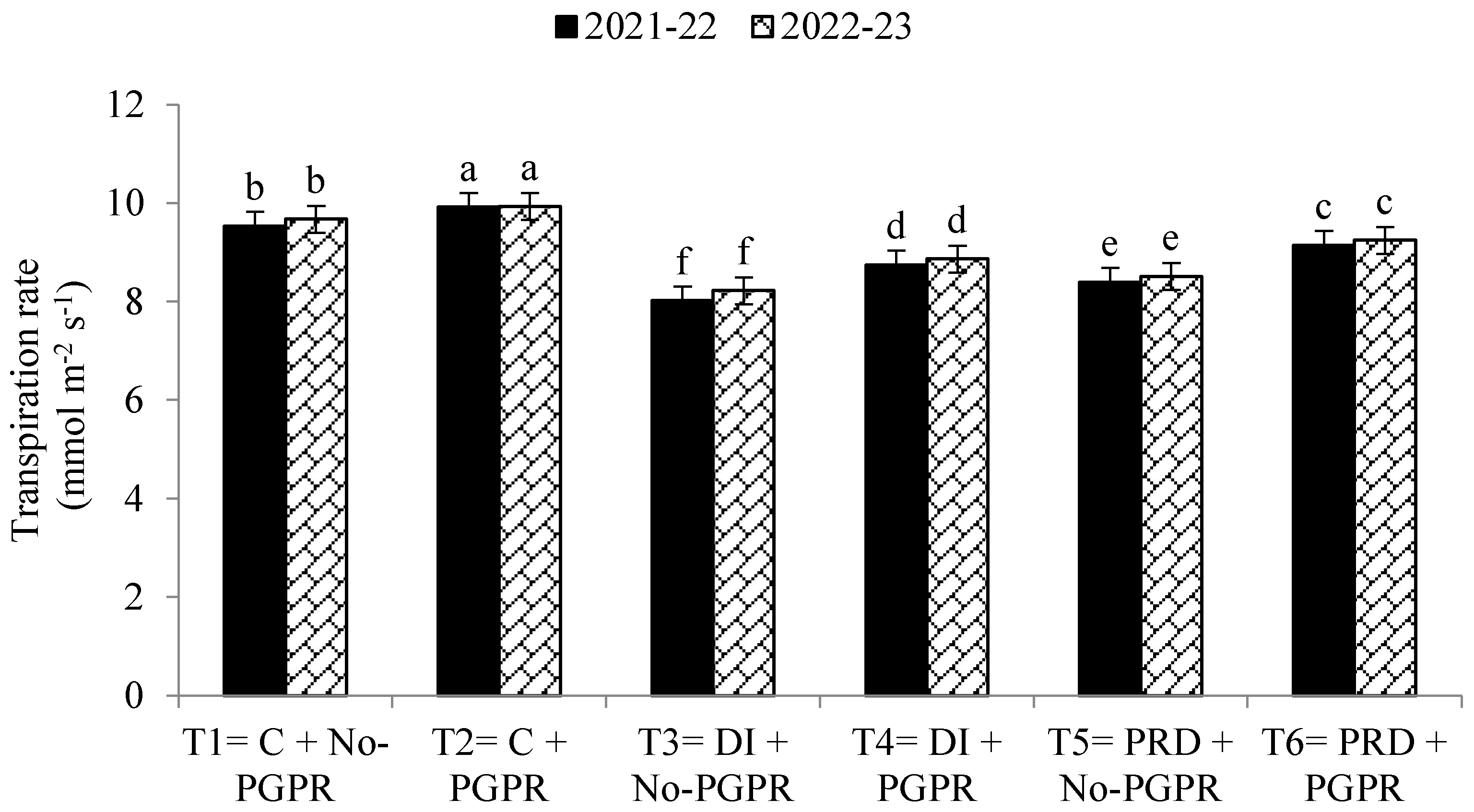
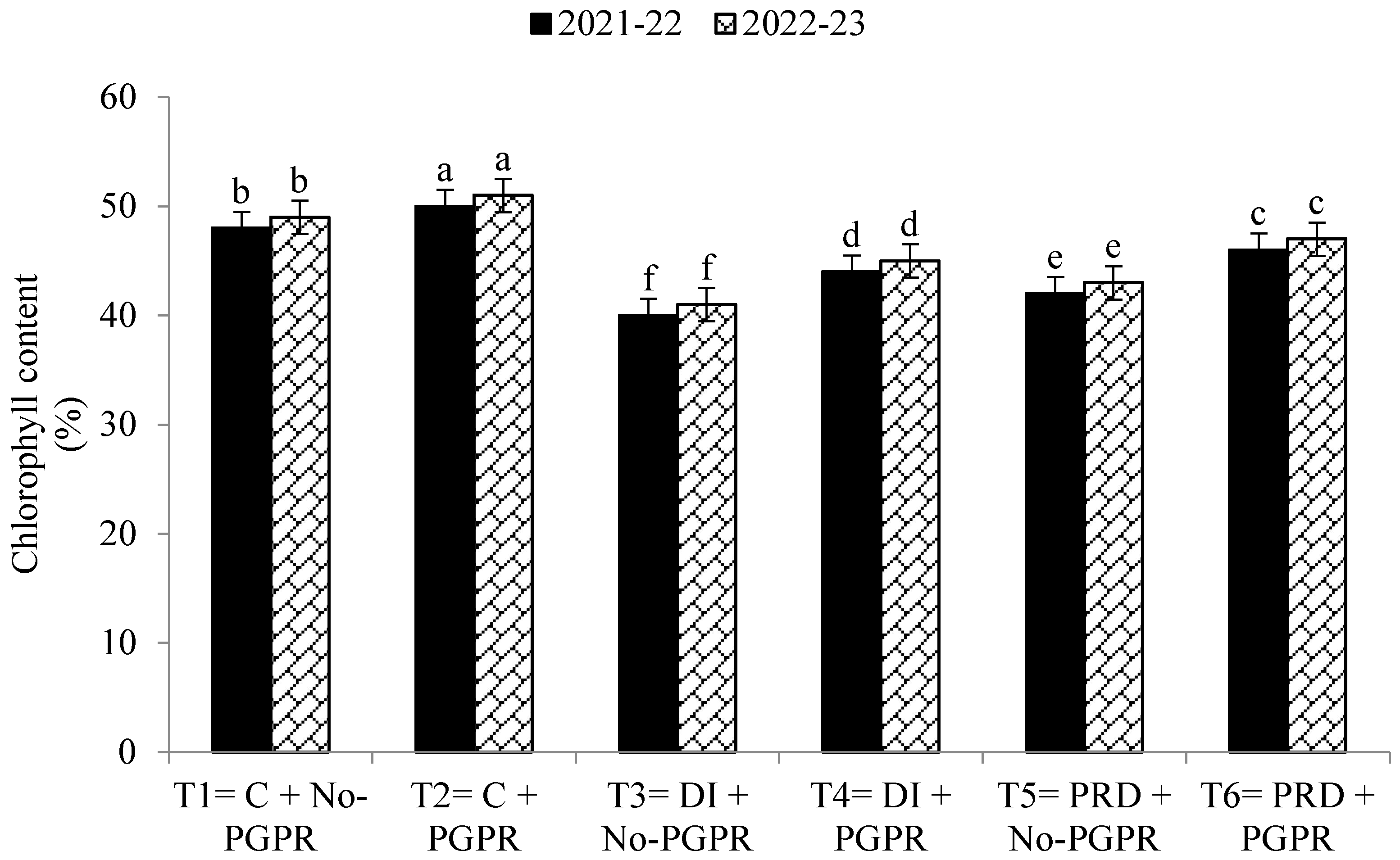
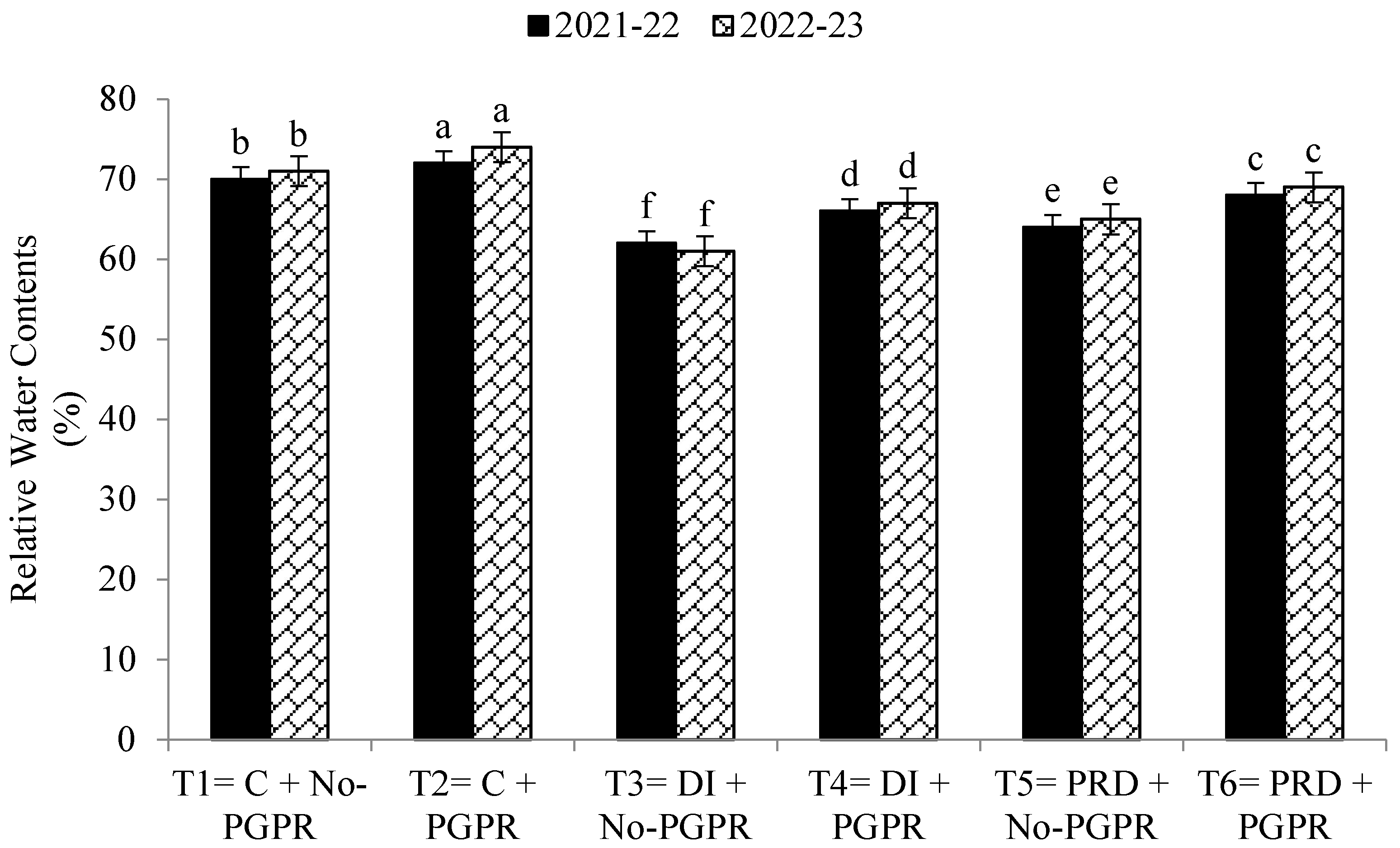
3.4. Gas Exchange Rate and Photosynthetic Capacity
3.5. Soil Fertility Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Akram, M. Growth and Yield Components Of Wheat Under Water Stress Of Different Growth Stages. Bangladesh J. Agric. Res. 2011, 36, 455–468. [Google Scholar] [CrossRef]
- Shahid, R.; Ren, M.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; Sohail, H.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2020, 172, 820–846. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Andersen, M.N.; Plauborg, F.; Poulsen, R.T.; Jensen, C.R.; Sepaskhah, A.R.; Hansen, S. Effects of irrigation strategies and soils on field-grown potatoes: Gas exchange and xylem [ABA]. Agric. Water Manag. 2010, 97, 1486–1494. [Google Scholar] [CrossRef]
- Hamdy, A.; Ragab, R.; Scarascia-Mugnozza, E. Coping with water scarcity: Water saving and increasing water productivity. Irrig. Drain. 2003, 52, 3–20. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Zhu, G.; Lin, X.; Jiao, Y.; Lu, S.; Liu, J.; Liu, J.; Zhang, W.; Ye, L.; Li, R.; et al. Dissipation and movement of soil water in artificial forest in arid oasis areas: Cognition based on stable isotopes. CATENA 2023, 228, 107178. [Google Scholar] [CrossRef]
- Tanji, K.K. Agricultural Salinity Assessment and Management; Scientific Publishers: New York, NY, USA, 2012; ISBN 9388812832. [Google Scholar]
- Ahmed, A.; Hussain, I.; Khalil, I.A.; Subhanulla, S.; Ahmed, G.; Ahmad, I.; Imtiaz, M. Effect of Bed Planting and Zero Tillage on Productivity and Water Use of Irrigated Maize Wheat Cropping System in Khyber Pakhtunkhwa Province of Pakistan. Sarhad J. Agric. 2018, 34, 696–704. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Kheir, A.M.S.; Al-Dhumri, S.; Alghamdi, A.G.; Omar, E.S.H.; Aboelsoud, H.M.; Abdella, K.A.; Abou El Hassan, W.H. Exploring optimal tillage improved soil characteristics and productivity of wheat irrigated with different water qualities. Agronomy 2019, 9, 233. [Google Scholar] [CrossRef]
- Chen, X.; Feng, S.; Qi, Z.; Sima, M.W.; Zeng, F.; Li, L.; Cheng, H.; Wu, H. Optimizing Irrigation Strategies to Improve Water Use Efficiency of Cotton in Northwest China Using RZWQM2. Agriculture 2022, 12, 383. [Google Scholar] [CrossRef]
- Elhani, S.; Haddadi, M.; Csákvári, E.; Zantar, S.; Hamim, A.; Villányi, V.; Douaik, A.; Bánfalvi, Z. Effects of partial root-zone drying and deficit irrigation on yield, irrigation water-use efficiency and some potato (Solanum tuberosum L.) quality traits under glasshouse conditions. Agric. Water Manag. 2019, 224, 105745. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Toleikiene, M.; Ayaz, M.; Hashemi, F.; Habib-Ur-Rahman, M.; Zaheer, M.S.; Ahmad, S.; Riaz, U.; Ali, M.; et al. Partial root-zone drying (PRD), its effects and agricultural significance: A review. Bull. Natl. Res. Cent. 2020, 44, 159. [Google Scholar] [CrossRef]
- Yactayo, W.; Ramírez, D.A.; Gutiérrez, R.; Mares, V.; Posadas, A.; Quiroz, R. Effect of partial root-zone drying irrigation timing on potato tuber yield and water use efficiency. Agric. Water Manag. 2013, 123, 65–70. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Roy, R.; Seleiman, M.F.; Ozlu, E.; Battaglia, M.L.; Uslu, Ö.S. Relationship between organic matter and microbial biomass in different vegetation types. In Microbial Syntrophy-Mediated Eco-Enterprising; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–245. [Google Scholar]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Sang, Y.; Mo, S.; Rehman, S.U.; Khan, S.; Khan, M.R.; He, S.; Jiang, C. Deciphering the biodesulfurization pathway employing marine mangrove Bacillus aryabhattai strain NM1-A2 according to whole genome sequencing and transcriptome analyses. Genomics 2023, 115, 110635. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Abdelaal, M.S. Effect of organic, inorganic and bio-fertilization on growth, yield and quality traits of some chickpea (Cicer arietinum L.) varieties. Egypt. J. Agron. 2018, 40, 105–117. [Google Scholar] [CrossRef]
- McGuire, A.V.; Northfield, T.D. Tropical Occurrence and Agricultural Importance of Beauveria bassiana and Metarhizium anisopliae. Front. Sustain. Food Syst. 2020, 4, 6. [Google Scholar] [CrossRef]
- Siddhartha, P.S.; Debashish, B.; Adrita, G.; Kalpataru, D.M.; Animesh, G. A review on the role of Azospirillum in the yield improvement of non leguminous crops. Afr. J. Microbiol. Res. 2012, 6, 1085–1102. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2014, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak-Wróbel, S.; Marek-Kozaczuk, M.; Kalita, M.; Karaś, M.; Wójcik, M.; Małek, W. Diversity and plant growth promoting properties of rhizobia isolated from root nodules of Ononis arvensis. Antonie Leeuwenhoek 2017, 110, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Iqbal, R.; Manevski, K. Investigating the effect of Azospirillum brasilense and Rhizobium pisi on agronomic traits of wheat (Triticum aestivum L.). Arch. Agron. Soil Sci. 2019, 65, 1554–1564. [Google Scholar] [CrossRef]
- Yan, Y.; Jarvie, S.; Liu, Q.; Zhang, Q. Effects of fragmentation on grassland plant diversity depend on the habitat specialization of species. Biol. Conserv. 2022, 275, 109773. [Google Scholar] [CrossRef]
- Nie, S.; Mo, S.; Gao, T.; Yan, B.; Shen, P.; Kashif, M.; Zhang, Z.; Li, J.; Jiang, C. Coupling effects of nitrate reduction and sulfur oxidation in a subtropical marine mangrove ecosystem with Spartina alterniflora invasion. Sci. Total. Environ. 2023, 862, 160930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liu, Y.; Shi, P.; Jia, W.; Zhou, J.; Liu, Y.; Ma, X.; Pan, H.; Zhang, Y.; Zhang, Z.; et al. Stable water isotope monitoring network of different water bodies in Shiyang River basin, a typical arid river in China. Earth Syst. Sci. Data 2022, 14, 3773–3789. [Google Scholar] [CrossRef]
- Zhang, T.; Song, B.; Han, G.; Zhao, H.; Hu, Q.; Zhao, Y.; Liu, H. Effects of coastal wetland reclamation on soil organic carbon, total nitrogen, and total phosphorus in China: A meta-analysis. Land Degrad. Dev. 2023, 34, 3340–3349. [Google Scholar] [CrossRef]
- Ran, C.; Bai, X.; Tan, Q.; Luo, G.; Cao, Y.; Wu, L.; Chen, F.; Li, C.; Luo, X.; Liu, M.; et al. Threat of soil formation rate to health of karst ecosystem. Sci. Total. Environ. 2023, 887, 163911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Z.; Yin, X.-A.; Zhu, Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total. Environ. 2021, 771, 144751. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Zhu, D.; Wang, W.; Liu, H.; Li, J.; Shi, N.; Ma, L.; Fu, S. Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indic. 2021, 130, 108031. [Google Scholar] [CrossRef]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2010, 27, 197–205. [Google Scholar] [CrossRef]
- Pereyra, M.A.; Ballesteros, F.M.; Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Seedlings growth promotion by Azospirillum brasilense under normal and drought conditions remains unaltered in Tebuconazole-treated wheat seeds. Eur. J. Soil Biol. 2009, 45, 20–27. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Spaepen, S.; du Jardin, P.; Delaplace, P. Biostimulant effects of rhizobacteria on wheat growth and nutrient uptake depend on nitrogen application and plant development. Arch. Agron. Soil Sci. 2018, 65, 58–73. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; El-Mageed, T.A.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- El-Mageed, T.A.A.; Gyushi, M.A.H.; Hemida, K.A.; El-Saadony, M.T.; El-Mageed, S.A.A.; Abdalla, H.; AbuQamar, S.F.; El-Tarabily, K.A.; Abdelkhalik, A. Coapplication of Effective Microorganisms and Nanomagnesium Boosts the Agronomic, Physio-Biochemical, Osmolytes, and Antioxidants Defenses Against Salt Stress in Ipomoea batatas. Front. Plant Sci. 2022, 13, 883274. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Imran, M.; Shahzad, S.M.; Arif, M.S.; Yasmeen, T.; Ali, S.; Ali, B.; Tanveer, A.; Ghani, M.A.; Nadeem, M.; Javed, M.A. Inoculation of Potassium Solubilizing Bacteria with Different Potassium Fertilization Sources Mediates Maize Growth and Productivity. Pak. J. Agric. Sci. 2020, 57, 1045–1055. [Google Scholar]
- Fukami, J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef]
- Liu, D.; Hu, L.Y.; Ali, B.; Yang, A.G.; Wan, G.L.; Xu, L.; Zhou, W.J. Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci. Plant Nutr. 2016, 62, 254–262. [Google Scholar] [CrossRef]
- Bhutta, S.K.; Bhutta, K.N.; Aslam, M.N.; Nasir, I.R.; Ali, M.A. Evaluation of Growth and Yield Attributes of Some Wheat Varieties under Local Conditions of Southern Punjab, Pakistan. J. Plant Breed. Genet. 2019, 7, 19–25. [Google Scholar] [CrossRef]
- Gardner, F.P.; Pearce, R.B.; Mitchell, R.L. Physiology of Crop Plants; Iowa State University Press: Ames, IA, USA, 1985. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbaiah, V.V.; Asija, G.K. A Rapid Procedure for Utilization of Available Nitrogen in Soil. Curr. Sci. 1956, 26, 258–260. [Google Scholar]
- Nelson, L.B.; Heidel, H. Soil Analysis Methods as Used in the Iowa State College Soil Testing Laboratory; Science and Education Publishing: Cagayan de Oro City, Philippines, 1952. [Google Scholar]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Srinivasan, V.; Bini, Y.K.; Subila, K.P.; Aravind, R.; Hamza, S. Effects of Plant Growth-Promoting Rhizobacteria and NPK Fertilizers on Biochemical and Microbial Properties of Soils Under Ginger (Zingiber officinale) Cultivation. Agric. Res. 2013, 2, 346–353. [Google Scholar] [CrossRef]
- Fathian, F.; Morid, S.; Kahya, E. Identification of trends in hydrological and climatic variables in Urmia Lake basin, Iran. Theor. Appl. Clim. 2015, 119, 443–464. [Google Scholar] [CrossRef]
- Chalmers, D.J.; Mitchell, P.D.; van Heek, L. Control of Peach Tree Growth and Productivity by Regulated Water Supply, Tree Density, and Summer Pruning1. J. Am. Soc. Hortic. Sci. 1981, 106, 307–312. [Google Scholar] [CrossRef]
- English, M.; Raja, S.N. Perspectives on deficit irrigation. Agric. Water Manag. 1996, 32, 1–14. [Google Scholar] [CrossRef]
- Wakchaure, G.; Minhas, P.; Meena, K.K.; Kumar, S.; Rane, J. Effect of plant growth regulators and deficit irrigation on canopy traits, yield, water productivity and fruit quality of eggplant (Solanum melongena L.) grown in the water scarce environment. J. Environ. Manag. 2020, 262, 110320. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alshahrani, T.S. Integrative Effects of Zinc Nanoparticle and PGRs to Mitigate Salt Stress in Maize. Agronomy 2023, 13, 1655. [Google Scholar] [CrossRef]
- Ahmad, S.; Raza, M.A.S.; Saleem, M.F.; Zahra, S.S.; Khan, I.H.; Ali, M.; Shahid, A.M.; Iqbal, R.; Zaheer, M.S. Mulching Strategies for Weeds Control and Water Conservation in Cotton. J. Agric. Biol. Sci. 2015, 8, 299–306. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Cheema, Z.A.; Cheema, M.A.; Khaliq, A. Physiological Role of Exogenously Applied Glycinebetaine to Improve Drought Tolerance in Fine Grain Aromatic Rice (Oryza sativa L.). J. Agron. Crop. Sci. 2008, 194, 325–333. [Google Scholar] [CrossRef]
- Stikić, R.; Popović, S.; Srdić, M.; Savić, D.; Jovanović, Z.; Prokić, L.; Zdravković, J. Partial Root Drying (PRD): A New Technique for Growing Plants That Saves Water and Improves the Quality of Fruit. Bulg. J. Plant Physiol. 2003, 29, 3–4. [Google Scholar]
- Posadas, A.; Rojas, G.; LMalaga, M.; Mares, V.; Quiroz, R.A. Partial Root-Zone Drying: An Alternative Irrigation Management to Improve the Water Use Efficiency of Potato Crops; International Potato Center: Lima, Peru, 2008. [Google Scholar]
- Iqbal, R.; Raza, M.A.S.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Zaheer, M.S.; Aslam, M.U.; Haider, I. Physiological and biochemical appraisal for mulching and partial rhizosphere drying of cotton. J. Arid. Land 2019, 11, 785–794. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Ahmad, S.; Saleem, M.F.; Khan, I.H.; Iqbal, R.; Zaheer, M.S.; Haider, I.; Ali, M. Physiological and biochemical assisted screening of wheat varieties under partial rhizosphere drying. Plant Physiol. Biochem. 2017, 116, 150–166. [Google Scholar] [CrossRef]
- Ahmad, S.; Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Iqbal, R.; Okone, O.G.; Raza, A.; Waqas, M.M.; Nawaz, M. Physiological and biochemical properties of wheat (Triticum aestivum L.) under different mulching and water management systems in the semi-arid region of Punjab, Pakistan. Arid. Land Res. Manag. 2022, 36, 181–196. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Xie, H.; Pasternak, J.J.; Glick, B.R. Isolation and Characterization of Mutants of the Plant Growth-Promoting Rhizobacterium Pseudomonas putida GR12-2 That Overproduce Indoleacetic Acid. Curr. Microbiol. 1996, 32, 67–71. [Google Scholar] [CrossRef]
- Mustafa, A.; Naveed, M.; Saeed, Q.; Nadeem Ashraf, M.; Hussain, A.; Abbas, T.; Kamran, M.; Minggang, X. Application potentials of plant growth promoting rhizobacteria and fungi as an alternative to conventional weed control methods. In Sustainable Crop Production; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/67546 (accessed on 1 January 2023).
- Zaheer, M.S.; Ali, H.H.; Soufan, W.; Iqbal, R.; Habib-ur-Rahman, M.; Iqbal, J.; Israr, M.; El Sabagh, A. Potential Effects of Biochar Application for Improving Wheat (Triticum aestivum L.) Growth and Soil Biochemical Properties under Drought Stress Conditions. Land 2021, 10, 1125. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, K.; Fan, Y.; Fang, H.; Nie, Y.; Wu, X.-L. The Bacterial MtrAB Two-Component System Regulates the Cell Wall Homeostasis Responding to Environmental Alkaline Stress. Microbiol. Spectr. 2022, 10, e0231122. [Google Scholar] [CrossRef]
- Bashan, Y.; Bustillos, J.J.; Leyva, L.A.; Hernandez, J.-P.; Bacilio, M. Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol. Fertil. Soils 2006, 42, 279–285. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdalla, A.M.; Elhamid, E.M.A.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Okon, O.G.; Nadeem, M.A.; Nawaz, M.; Bodlah, M.A.; Waqas, M.M.; Iqbal, J.; et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 33909–33919. [Google Scholar] [CrossRef] [PubMed]
- Wakrim, R.; Wahbi, S.; Tahi, H.; Aganchich, B.; Serraj, R. Comparative effects of partial root drying (PRD) and regulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agric. Ecosyst. Environ. 2005, 106, 275–287. [Google Scholar] [CrossRef]
- Lazauskas, S.; Povilaitis, V.; Antanaitis, Š.; Sakalauskaite, J.; Sakalauskiene, S.; Pšibišauskiene, G.; Auškalniene, O.; Raudonius, S.; Duchovskis, P. Winter Wheat Leaf Area Index under Low and Moderate Input Management and Climate Change. J. Food Agric. Environ. 2012, 10, 588–593. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Szepesi, Á.; Szőllősi, R. Mechanism of Proline Biosynthesis and Role of Proline Metabolism Enzymes Under Environmental Stress in Plants. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 337–353. [Google Scholar] [CrossRef]
- Peng, C.; Hong-Yan, W.; Zheng, R.-L.; Zhang, B.; Guo-Xin, S.U.N. Long-Term Effects of Biochar on Rice Production and Stabilisation of Cadmium and Arsenic Levels in Contaminated Paddy Soils. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 109, 415–420. [Google Scholar]
- Skopp, J.; Jawson, M.D.; Doran, J.W. Steady-State Aerobic Microbial Activity as a Function of Soil Water Content. Soil Sci. Soc. Am. J. 1990, 54, 1619–1625. [Google Scholar] [CrossRef]
- Marschner, P.; Hatam, Z.; Cavagnaro, T. Soil respiration, microbial biomass and nutrient availability after the second amendment are influenced by legacy effects of prior residue addition. Soil Biol. Biochem. 2015, 88, 169–177. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bolton, H.; Fansler, S.; Heredia-Langner, A.; Liu, C.; McCue, L.A.; Smith, J.; Bailey, V. Soil Respiration and Bacterial Structure and Function after 17 Years of a Reciprocal Soil Transplant Experiment. PLoS ONE 2016, 11, e0150599. [Google Scholar] [CrossRef] [PubMed]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual Relationships of Biochar and Soil PH, CEC, and Exchangeable Base Cations in a Model Laboratory Experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Elkhlifi, Z.; Kamran, M.; Maqbool, A.; El-Naggar, A.; Ifthikar, J.; Parveen, A.; Bashir, S.; Rizwan, M.; Mustafa, A.; Irshad, S.; et al. Phosphate-lanthanum coated sewage sludge biochar improved the soil properties and growth of ryegrass in an alkaline soil. Ecotoxicol. Environ. Saf. 2021, 216, 112173. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]



| Parameters | 2021-22 | 2022-23 |
|---|---|---|
| Organic matter (%) | 0.98 | 0.71 |
| pH | 7.42 | 7.57 |
| EC (µS/cm) | 226 | 231 |
| T.S.S. (%) | 0.47 | 0.57 |
| Available-P (ppm) | 6.09 | 5.21 |
| Available-P (ppm) | 116 | 111 |
| Saturation percentage | 37 | 32 |
| Soil separates | ||
| Sand (%) | 35 | 36 |
| Sand (%) | 41 | 39 |
| Sand (%) | 24 | 25 |
| Texture of Soil | Loam Soil | Loam Soil |
| Treatment | Plant Height (cm) | Spike Length (cm) | Number of Spikelets per Spike | |||
|---|---|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 95.07 b | 95.55 b | 19.41 b | 20.03 b | 28.54 b | 28.95 b |
| T2 = C + PGPR | 97.48 a | 98.11 a | 21.41 a | 21.89 a | 30.19 a | 30.65 a |
| T3 = DI + No PGPR | 84.10 f | 83.38 f | 12.45 f | 12.96 f | 20.22 e | 20.73 f |
| T4 = DI + PGPR | 90.74 d | 91.78 d | 16.45 d | 16.90 d | 24.32 c | 24.77 d |
| T5 = PRD + No PGPR | 87.70 e | 89.00 e | 14.52 e | 15.00 e | 22.23 d | 22.66 e |
| T6 = PRD + PGPR | 92.78 c | 93.48 c | 17.11 c | 17.53 c | 26.23 c | 26.70 c |
| Treatment | 1000 GW | Yield per Plant | ||
|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 42.78 b | 43.81 b | 1.183 b | 1.316 b |
| T2 = C + PGPR | 44.77 a | 45.92 a | 1.256 a | 1.393 a |
| T3 = DI + No PGPR | 31.82 f | 32.90 f | 0.693 f | 0.490 f |
| T4 = DI + PGPR | 37.66 d | 38.84 d | 0.913 d | 0.890 d |
| T5 = PRD + No PGPR | 34.32 e | 35.25 e | 0.816 e | 0.753 e |
| T6 = PRD + PGPR | 39.74 c | 40.66 c | 1.030 c | 1.090 c |
| Treatment | Crop Growth Rate at Tillering (g m−2 day−1) | Crop Growth Rate at the Flag Leaf Stage (g m−2 day−1) | ||
|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 2.14 b | 2.17 b | 9.19 b | 9.22 b |
| T2 = C + PGPR | 2.29 a | 2.32 a | 9.75 a | 9.71 a |
| T3 = DI + No PGPR | 1.64 f | 1.13 f | 7.09 f | 7.12 f |
| T4 = DI + PGPR | 1.84 d | 1.15 d | 8.19 d | 8.22 d |
| T5 = PRD + No PGPR | 1.74 e | 1.77 e | 7.69 e | 7.71 e |
| T6 = PRD + PGPR | 1.94 c | 1.97 c | 8.69 c | 8.72 c |
| Treatment | Relative Growth Rate at Tillering (g m−2 day−1) | Relative Growth Rate at the Flag Leaf Stage (g m−2 day−1) | ||
|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 0.134 b | 0.139 b | 4.236 b | 4.263 b |
| T2 = C + PGPR | 0.143 a | 0.148 a | 4.580 a | 4.610 a |
| T3 = DI + No PGPR | 0.076 f | 0.074 f | 3.543 f | 3.483 f |
| T4 = DI + PGPR | 0.104 d | 0.108 d | 3.856 d | 3.826 d |
| T5 = PRD + No PGPR | 0.086 e | 0.085 e | 3.676 e | 3.680 e |
| T6 = PRD + PGPR | 0.122 c | 0.127 c | 4.016 c | 4.076 c |
| Treatments | NAR at Tillering (g m−2 day−1) | NAR at the Flag Leaf Stage (g m−2 day−1) | ||
|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 1.423 b | 1.431 b | 4.433 b | 4.446 b |
| T2 = C + PGPR | 1.530 a | 1.527 a | 4.620 a | 4.643 a |
| T3 = DI + No PGPR | 1.042 f | 1.020 f | 3.633 f | 3.640 f |
| T4 = DI + PGPR | 1.234 d | 1.224 d | 4.023 d | 4.036 d |
| T5 = PRD + No PGPR | 1.125 e | 1.129 e | 3.830 e | 3.846 e |
| T6 = PRD + PGPR | 1.320 c | 1.318 c | 4.243 c | 4.263 c |
| Treatment | Leaf Area Index at Tillering | Leaf Area Index at the Flag Leaf Stage | ||
|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 2.32 b | 2.41 b | 7.06 b | 7.12 b |
| T2 = C + PGPR | 2.51 a | 2.63 a | 7.35 a | 7.43 a |
| T3 = DI + No PGPR | 1.70 f | 1.83 f | 6.01 f | 6.14 f |
| T4 = DI + PGPR | 1.98 d | 1.99 d | 6.57 d | 6.65 d |
| T5 = PRD + No PGPR | 1.81 e | 1.89 e | 6.31 e | 6.47 e |
| T6 = PRD + PGPR | 2.12 c | 2.21 c | 6.86 c | 6.95 c |
| Treatment | Available Soil Nitrogen (mg kg−1) | Available Soil Phosphorus (mg kg−1) | Available Soil Potassium (mg kg−1) | Soil Organic Matter (%) | Soil Respiration (SR) (µg CO2-C g−1 day−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2021-22 | 2022-23 | 2021-22 | 2022-23 | 2021-22 | 2022-23 | 2021-22 | 2022-23 | 2021-22 | 2022-23 | |
| T1 = Control + No PGPR | 30.14 b | 29.15 b | 14.30 b | 13.21 b | 60.37 b | 58.26 b | 1.31 b | 1.12 b | 24.11 b | 25.21 b |
| T2 = C + PGPR | 32.33 a | 31.35 a | 15.42 a | 14.33 a | 62.57 a | 60.46 a | 1.42 a | 1.23 a | 25.18 a | 26.34 a |
| T3 = DI + No PGPR | 22.21 f | 21.23 f | 10.42 f | 09.33 f | 52.45 f | 50.34 f | 0.98 f | 0.71 f | 20.14 f | 21.34 f |
| T4 = DI + PGPR | 26.32 d | 25.36 d | 12.31 d | 11.22 d | 56.58 d | 54.47 d | 1.17 d | 0.94 d | 22.16 d | 23.34 d |
| T5 = PRD + No PGPR | 24.12 e | 23.15 e | 11.41 e | 10.32 e | 54.37 e | 52.26 e | 1.09 e | 0.83 e | 21.47 e | 22.31 e |
| T6 = PRD + PGPR | 28.21 c | 27.26 c | 13.33 c | 12.24 c | 58.48 c | 56.37 c | 1.25 c | 1.02 c | 23.34 c | 24.45 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammad, B.A.; Zaheer, M.S.; Ali, H.H.; Hameed, A.; Ghanem, K.Z.; Seleiman, M.F. Effect of Co-Application of Azospirillum brasilense and Rhizobium pisi on Wheat Performance and Soil Nutrient Status under Deficit and Partial Root Drying Stress. Plants 2023, 12, 3141. https://doi.org/10.3390/plants12173141
Alhammad BA, Zaheer MS, Ali HH, Hameed A, Ghanem KZ, Seleiman MF. Effect of Co-Application of Azospirillum brasilense and Rhizobium pisi on Wheat Performance and Soil Nutrient Status under Deficit and Partial Root Drying Stress. Plants. 2023; 12(17):3141. https://doi.org/10.3390/plants12173141
Chicago/Turabian StyleAlhammad, Bushra Ahmed, Muhammad Saqlain Zaheer, Hafiz Haider Ali, Akhtar Hameed, Kholoud Z. Ghanem, and Mahmoud F. Seleiman. 2023. "Effect of Co-Application of Azospirillum brasilense and Rhizobium pisi on Wheat Performance and Soil Nutrient Status under Deficit and Partial Root Drying Stress" Plants 12, no. 17: 3141. https://doi.org/10.3390/plants12173141
APA StyleAlhammad, B. A., Zaheer, M. S., Ali, H. H., Hameed, A., Ghanem, K. Z., & Seleiman, M. F. (2023). Effect of Co-Application of Azospirillum brasilense and Rhizobium pisi on Wheat Performance and Soil Nutrient Status under Deficit and Partial Root Drying Stress. Plants, 12(17), 3141. https://doi.org/10.3390/plants12173141








