Increased Tolerance of Massion’s pine to Multiple-Toxic-Metal Stress Mediated by Ectomycorrhizal Fungi
Abstract
:1. Introduction
2. Results
2.1. The Effects of C. geophilum on Biomass, Root Morphology, and Element Absorption of Massion’s pine Seedlings in Mine Soil
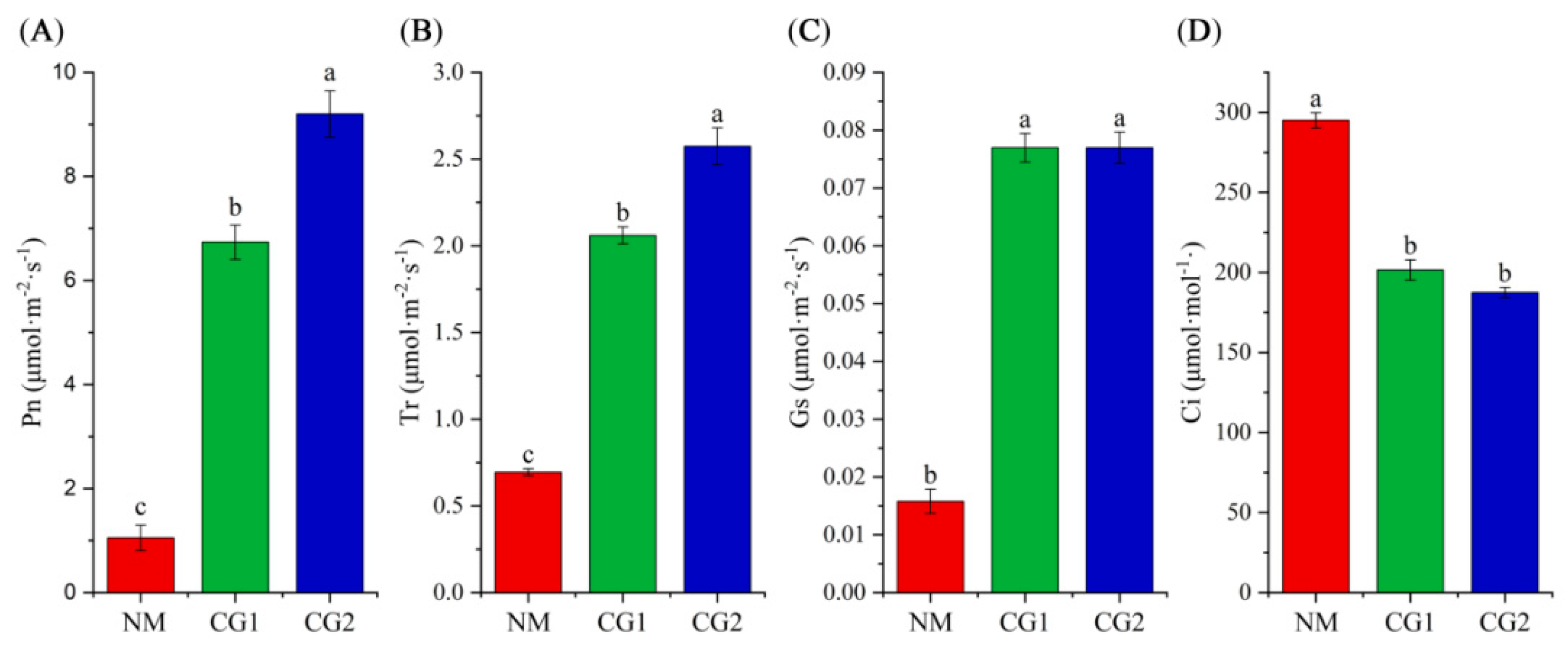
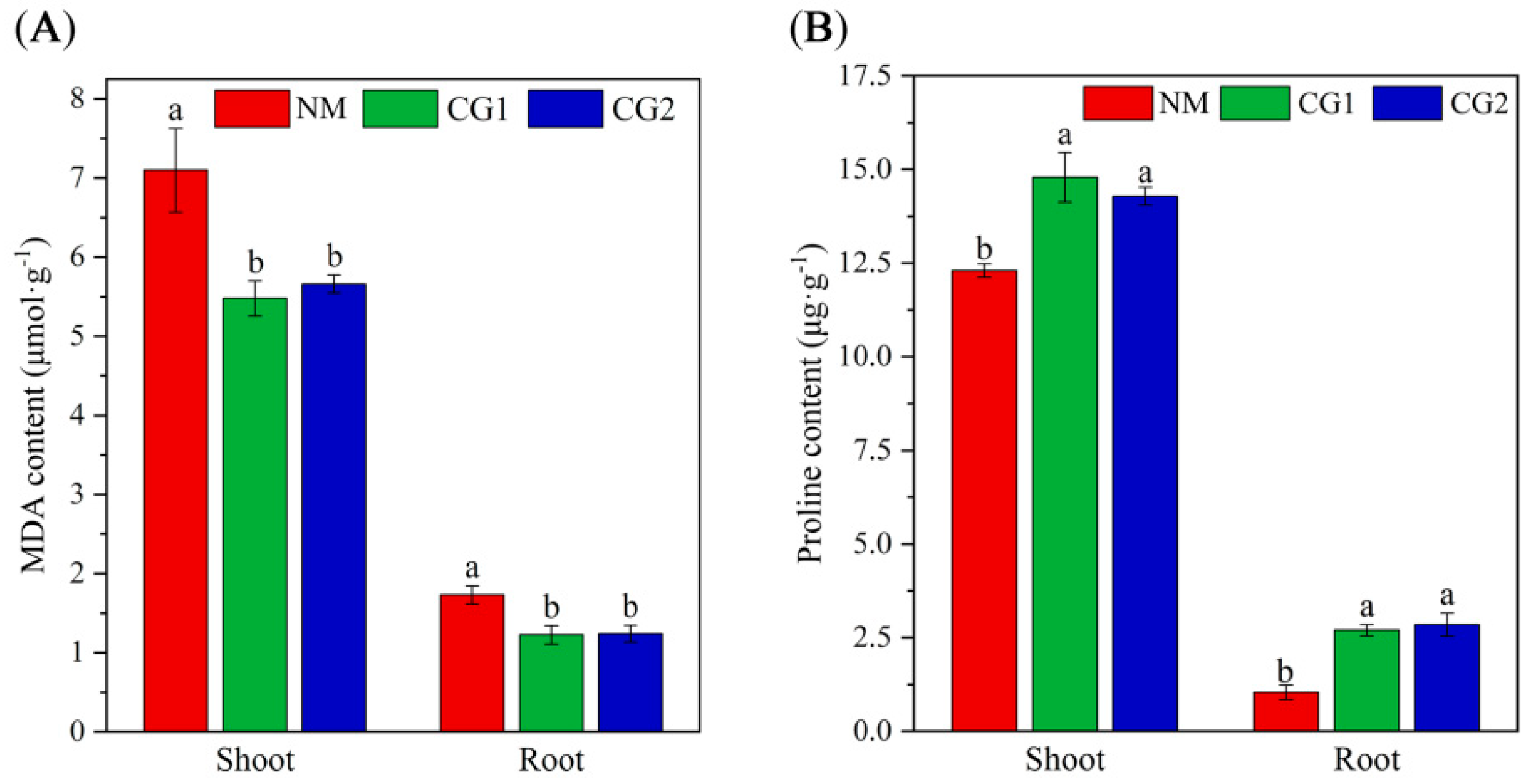
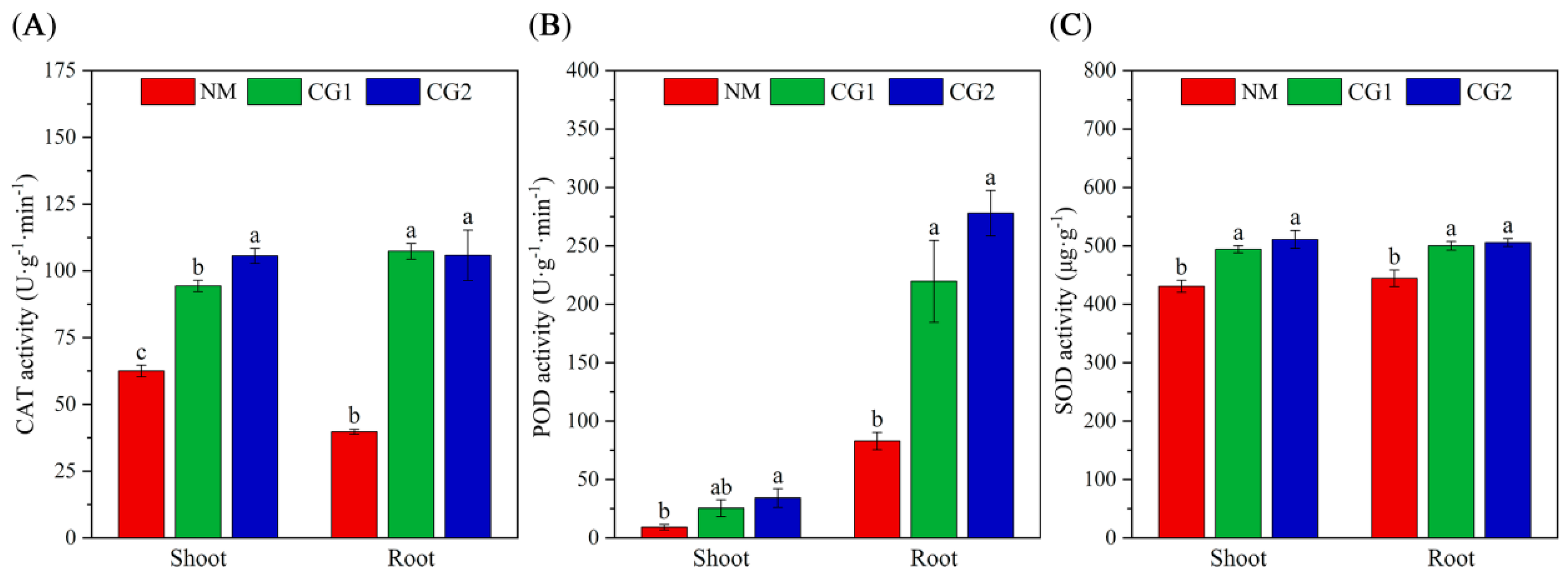
2.2. Effects of C. geophilum on Photosynthetic Characteristics and Antioxidant Enzyme Activities of Massion’s pine Seedlings in Mining Soil
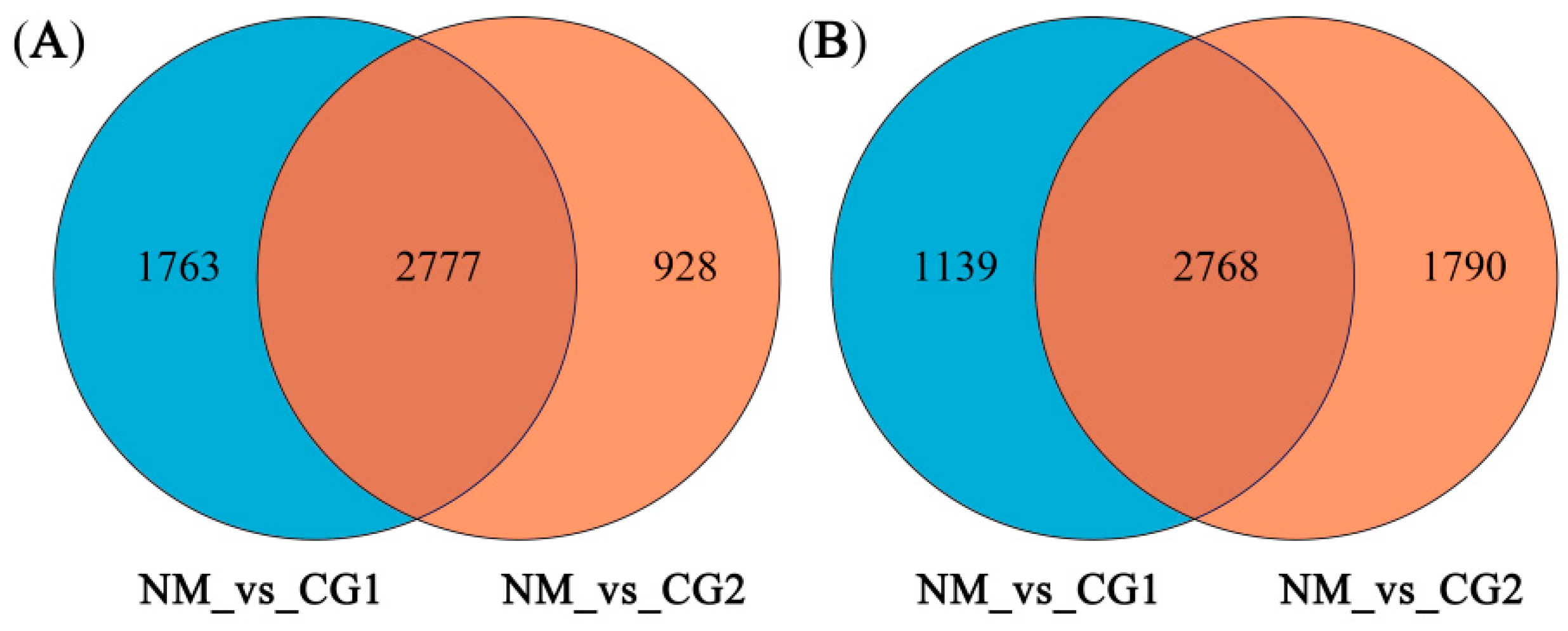
2.3. Validation of Differentially Expressed Genes (DEGs)
2.4. RT-qPCR Verification of DEGs
3. Discussion
4. Materials and Methods
4.1. Preparation of Massion’s pine Seedlings Cultivation
4.2. Inoculation of Massion’s pine Seedlings
4.3. Soil Collection and Characterization
4.4. Measurement of Morphological Parameters
4.5. Determination of Photosynthetic Characteristics, Antioxidant Systems, and Osmotic Regulating Substances in Massion’s pine Seedlings
4.6. Determination of Elemental Content
4.7. RNA-Seq Analysis
4.8. Validation by RT-qPCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayansina, A.; Olubukola, B. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar]
- Sousa, N.R.; Ramos, M.A.; Marques, A.P.; Castro, P.M. The effect of ectomycorrhizal fungi forming symbiosis with pinus pinaster seedlings exposed to cadmium. Sci. Total Environ. 2012, 414, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Singh, S.; Nazar, R. Activities of Antioxidative Enzymes, Sulphur Assimilation, Photosynthetic Activity and Growth of Wheat (Triticum aestivum) Cultivars Differing in Yield Potential Under Cadmium Stress. J. Agron. Crop Sci. 2007, 193, 435–444. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Jeddi, K.; Siddique, K.H.; Chaieb, M.; Hessini, K. Physiological and biochemical responses of Lawsonia inermis L. to heavy metal pollution in arid environments. S. Afr. J. Bot. 2021, 143, 7–16. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Ding, K.; Liu, W.S.; Baker, A.J.M.; Fei, Y.H.; He, H.; Wang, Y.; Jin, C.; Wang, S.; et al. Inoculation of ectomycorrhizal fungi contributes to the survival of tree seedlings in a copper mine tailing. J. For. Res. 2015, 20, 493–500. [Google Scholar] [CrossRef]
- Huang, J.; Nara, K.; Lian, C.; Zong, K.; Peng, K.; Xue, S.; Shen, Z. Ectomycorrhizal fungal communities associated with masson pine (pinus massoniana lamb.) in Pb–Zn mine sites of central south china. Mycorrhiza 2012, 22, 589–602. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees--a review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Chot, E.; Reddy, M. Role of ectomycorrhizal symbiosis behind the host plants ameliorated tolerance against heavy metal stress. Front. Microbiol. 2022, 13, 855473. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kuek, C.; Chaudhry, T.; Khoo, C.; Hayes, W. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Sebastiana, M.; Pereira, V.T.; Alcântara, A.; Pais, M.S.; Silva, A.B. Ectomycorrhizal inoculation with pisolithus tinctorius increases the performance of Quercus suber L. (cork oak) nursery and field seedlings. New For. 2013, 44, 937–949. [Google Scholar] [CrossRef]
- Agnieszka, S.; Leszek, K.; Magdalena, K.; Magdalena, K.; Teresa, H.P. Inoculation with a Pb-tolerant strain of Paxillus involutus improves growth and Pb tolerance of Populus × canescens under in vitro conditions. Plant Soil 2017, 412, 253–266. [Google Scholar]
- Fernández-Fuego, D.; Keunen, E.; Cuypers, A.; Bertrand, A.; González, A. Mycorrhization protects Betula pubescens Ehr. from metal-induced oxidative stress increasing its tolerance to grow in an industrial polluted soil. J. Hazard. Mater. 2017, 336, 119–127. [Google Scholar] [CrossRef]
- Martins, L.L.; Mourato, M.P.; Cardoso, A.I.; Pinto, A.P.; Mota, A.; Mota, A.M.; Gonçalves, M.L.; Varennes, V. Oxidative stress induced by cadmium in Nicotiana tabacum L.: Effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiol. Plant. 2018, 33, 1375–1383. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, J.; Zhang, X.; Shen, Z.; Shi, L.; Chen, Y. The great potential for phytoremediation of abandoned tailings pond using ectomycorrhizal Pinus sylvestris. Sci. Total Environ. 2020, 719, 137475. [Google Scholar] [CrossRef]
- Frey, B.; Zierold, K.; Brunner, I. Extracellular complexation of cd in the hartig net and cytosolic zn sequestration in the fungal mantle of picea abies-hebeloma crustuliniforme ectomycorrhizas. Plant Cell Environ. 2010, 23, 1257–1265. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Léger, J.C.; Morabito, D. Assisted phytostabilization of a multicontaminated mine technosol using biochar amendment: Early stage evaluation of biochar feedstock and particle size effects on as and Pb accumulation of two Salicaceae species (Salix viminalis and Populus euramericana). Chemosphere 2017, 194, 316–326. [Google Scholar] [CrossRef]
- Wang, L.; Otgonsuren, B.; Godbold, D. Mycorrhizas and soil ecosystem function of co-existing woody vegetation islands at the alpine tree line. Plant Soil 2017, 411, 467–481. [Google Scholar] [CrossRef]
- Sun, F.; Wen, D.; Kuang, Y.; Li, J.; Li, J.; Zuo, W. Concentrations of heavy metals and polycyclic aromatic hydrocarbons in needles of Masson pine (Pinus massoniana L.) growing nearby different industrial sources. J. Environ. Sci. 2010, 22, 1006–1013. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, X.P.; Ding, G.J. Ectomycorrhizal symbiosis enhances tolerance to low phosphorous through expression of phosphate transporter genes in masson pine (Pinus massoniana). Acta Physiol. Plant. 2017, 39, 101. [Google Scholar] [CrossRef]
- Yu, P.; Sun, Y.; Huang, Z.; Zhu, F.; Sun, Y.; Jiang, L. The effects of ectomycorrhizal fungi on heavy metals’ transport in pinus massoniana and bacteria community in rhizosphere soil in mine tailing area. J. Hazard. Mater. 2020, 381, 121203. [Google Scholar] [CrossRef]
- Tang, Y.T.; Chao, Y.; He, Z.; Qiu, R. Heavy metal contamination affects the core microbiome and assembly processes in metal mine soils across eastern china. J. Hazard. Mater. 2023, 443, 130241. [Google Scholar]
- Li, M. Ecological restoration of mine land with particular reference to the metalliferous mine wasteland in china: A review of research and practice. Sci. Total Environ. 2006, 357, 38–53. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, Y.; Tian, K.; He, X.; Jia, Y.; He, Z.; Wang, W.; Xiang, C.; Tian, X. Insight into nitrogen and phosphorus enrichment on cadmium phytoextraction of hydroponically grown salix matsudana koidz cuttings. Environ. Sci. Pollut. Res. Int. 2020, 27, 8406–8417. [Google Scholar] [CrossRef]
- Adriaensen, K.; Vangronsveld, J.; Colpaert, J. Zinc-tolerant Suillus bovinus improves growth of Zn-exposed Pinus sylvestris seedlings. Mycorrhiza 2006, 16, 553–558. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Wevers, J.H.L.; Krznaric, E.; Adriaensen, K. How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann. For. Sci. 2011, 68, 17–24. [Google Scholar] [CrossRef]
- Ahonen-Jonnarth, U.; Finlay, R. Effects of elevated nickel and cadmium concentrations on growth and nutrient uptake of mycorrhizal and non-mycorrhizal pinus sylvestris seedlings. Plant Soil 2001, 236, 129–138. [Google Scholar] [CrossRef]
- Dagher, D.J.; Pitre, F.E.; Hijri, M. Ectomycorrhizal fungal inoculation of sphaerosporella brunnea significantly increased stem biomass of salix miyabeana and decreased lead, tin, and zinc, soil concentrations during the phytoremediation of an industrial landfill. J. Fungi 2020, 6, 87. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, C.; Hu, N.; Wang, B.; Zheng, K.; Zhao, Z.; Li, T. Contributions of ectomycorrhizal fungi in a reclaimed poplar forest (Populus yunnanensis) in an abandoned metal mine tailings pond, southwest China. J. Hazard. Mater. 2023, 448, 130962. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Li, J.; Mei, H.; Hu, S.; Han, R.; Zhao, Z.; Chen, X.; Li, X.; Kamatchi Reddiar, D. Mycorrhizal colonization enhanced sorghum bicolor tolerance under soil water deficit conditions by coordination of proline and reduced glutathione (GSH). J. Agric. Food Chem. 2022, 70, 4243–4255. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, C.; Zhang, C.; Li, H.; Lipka, U.; Polle, A. The role of ectomycorrhizas in heavy metal stress tolerance of host plants. Environ. Exp. Bot. 2014, 108, 47–62. [Google Scholar] [CrossRef]
- De Oliveira, V.H.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of Cd and Zn in populus trichocarpa. Environ. Exp. Bot. 2020, 171, 103925. [Google Scholar] [CrossRef]
- Sun, W.; Yang, B.; Zhu, Y.; Wang, H.; Qin, G.; Yang, H. Ectomycorrhizal fungi enhance the tolerance of phytotoxicity and cadmium accumulation in oak (Quercus acutissima Carruth.) seedlings: Modulation of growth properties and the antioxidant defense responses. Environ. Sci. Pollut. Res. Int. 2021, 29, 6526–6537. [Google Scholar] [CrossRef]
- Canton, G.; Bertolazi, A.; Cogo, A.; Eutrópio, F.J.; Melo, J.; de Souza, S.B.; Krohling, C.A.; Campostrini, E.; da Silva, A.G.; Façanha, A.R.; et al. Biochemical and ecophysiological responses to manganese stress by ectomycorrhizal fungus pisolithus tinctorius and in association with eucalyptus grandis. Mycorrhiza 2016, 26, 475–487. [Google Scholar] [CrossRef]
- Mohammadhasani, F.; Ahmadimoghadam, A.; Asrar, Z.; Mohammadi, S.Z. Effect of zn toxicity on the level of lipid peroxidation and oxidative enzymes activity in badami cultivar of pistachio (Pistacia vera L.) colonized by ectomycorrhizal fungus. Indian J. Plant Physiol. 2017, 22, 206–212. [Google Scholar] [CrossRef]
- Heinonsalo, J.; Juurola, E.; Linden, A.; Pumpanen, J. Ectomycorrhizal fungi affect scots pine photosynthesis through nitrogen and water economy, not only through increased carbon demand. Environ. Exp. Bot. 2015, 109, 103–112. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Q.; Yang, J.; Chen, T.; Zhu, G.; Peters, M.; Wei, R.; Tian, L.; Wang, C.; Tan, D.; et al. Cadmium accumulation and tolerance of two castor cultivars in relation to antioxidant systems. J. Environ. Sci. 2014, 26, 2048–2055. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationship in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, M.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Effects of low nitrogen supply on relationships between photosynthesis and nitrogen status at different leaf position in wheat seedlings. Plant Growth Regul. 2013, 70, 257–263. [Google Scholar] [CrossRef]
- Amna; Ali, N.; Masood, S.; Mukhtar, M.; Kamran, M.A.; Rafique, M.; Munis, M.F.; Chaudhary, H.J. Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of linum usitatissimum in association with glomus intraradices. Environ. Monit. Assess. 2015, 187, 311. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Y.; Ding, S.; Li, Z.; Shi, W.G.; Zhang, Y.; Luo, Z.B. Sulfur nutrition stimulates lead accumulation and alleviates its toxicity in populus deltoides. Tree Physiol. 2018, 38, 1724–1741. [Google Scholar] [CrossRef]
- Khator, K.; Saxena, I.; Shekhawat, G. Nitric oxide induced Cd tolerance and phytoremediation potential of B. juncea by the modulation of antioxidant defense system and ROS detoxification. Biometals 2021, 34, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Sun, C.; Guo, Q.; Zeeshan, M.; Milham, P.; Qin, S.; Ma, J.; Yang, Y.; Lai, H.; Huang, J. Dual RNA and 16S ribosomal DNA sequencing reveal arbuscular mycorrhizal fungi-mediated mitigation of selenate stress in Zea mays L. and reshaping of soil microbiota. Ecotoxicol. Environ. Saf. 2022, 247, 114217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.S.; Jiang, W.S.; Liu, D.H. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ. Sci. Pollut. Res. Int. 2013, 20, 1117–1123. [Google Scholar] [CrossRef]
- Rozpadek, P.; Wezowicz, K.; Stojakowska, A.; Malarz, J.; Surówka, E.; Sobczyk, L.; Ważny, R.; Miszalski, Z.; Turnau, K. Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 2014, 112, 217–224. [Google Scholar] [CrossRef]
- Wen, Z.; Shi, L.; Tang, Y.; Shen, Z.; Chen, Y. Effects of pisolithus tinctorius and Cenococcum geophilum inoculation on pine in copper-contaminated soil to enhance phytoremediation. Int. J. Phytoremediation 2016, 19, 387–394. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce Nacl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soil and Agro-Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Vicia faba L. Environ. Exp. Bot. 2019, 161, 290–302. [Google Scholar] [CrossRef]
- Wang, S.C.; Liang, D.; Li, C.; Hao, Y.L.; Ma, F.W.; Shu, H.R. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Abdelkrim, S.; Jebara, S.H.; Jebara, M. Antioxidant systems responses and the compatible solutes as contributing factors to lead accumulation and tolerance in Lathyrus sativus inoculated by plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2018, 166, 427–436. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Zhou, F.; Qin, J.; Li, H. Biochar and crushed straw additions affect cadmium absorption in cassava-peanut intercropping system. Ecotoxicol. Environ. Saf. 2019, 167, 520–530. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F.; You, Y.; Wang, Y.; Yang, D. Integration of earthworms and arbuscular mycorrhizal fungi into phytoremediation of cadmium-contaminated soil by Solanum nigrum L. J. Hazard. Mater. 2020, 389, 121873. [Google Scholar] [CrossRef] [PubMed]








| Treatment | Length | SurfArea | AvgDiam | RootVolume | Tips | Forks |
|---|---|---|---|---|---|---|
| NM | 174.1 ± 8.88 a | 24.91 ± 1.51 b | 0.46 ± 0.02 b | 0.28 ± 0.03 b | 323 ± 5.13 a | 831.33 ± 21.46 b |
| CG1 | 197.72 ± 20.48 a | 40.22 ± 4.91 a | 0.65 ± 0.02 a | 0.66 ± 0.11 a | 359.33 ± 69.29 a | 1353.67 ± 83.56 a |
| CG2 | 208.68 ± 28.84 a | 38.63 ± 3.06 a | 0.69 ± 0.09 a | 0.62 ± 0.08 a | 337.33 ± 26.49 a | 989.33 ± 96.53 b |
| Treatment | pH | TK (g·kg−1) | TP (g·kg−1) | SOM (g·kg−1) | Cr (g·kg−1) | Pb (g·kg−1) | Mn (g·kg−1) | Cd (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| OS | 6.21 ± 0.17 | 5.22 ± 0.29 | 0.51 ± 0.02 | 26.73 ± 0.76 | 0.37 ± 0.02 | 0.78 ± 0.01 | 22.76 ± 1.38 | 12.90 ± 0.26 |
| NM | 5.27 ± 0.03 a | 4.22 ± 0.05 a | 0.61 ± 0.01 a | 27.87 ± 1.37 a | 0.35 ± 0.01 a | 0.74 ± 0.02 a | 20.4 ± 1.37 a | 2.87 ± 0.42 b |
| CG1 | 5.48 ± 0.09 a | 4.17 ± 0.09 a | 0.55 ± 0.07 a | 26.12 ± 2.24 a | 0.34 ± 0.01 a | 0.63 ± 0.02 b | 18.73 ± 0.65 a | 3.65 ± 0.77 b |
| CG2 | 5.49 ± 0.1 a | 4.44 ± 0.42 a | 0.58 ± 0.08 a | 29.09 ± 1.06 a | 0.32 ± 0.02 a | 0.70 ± 0.01 b | 20.8 ± 2.03 a | 5.76 ± 0.38 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhang, P.; Pang, W.; Zhang, Y.; Alwathnani, H.A.; Rensing, C.; Yang, W. Increased Tolerance of Massion’s pine to Multiple-Toxic-Metal Stress Mediated by Ectomycorrhizal Fungi. Plants 2023, 12, 3179. https://doi.org/10.3390/plants12183179
Zhang T, Zhang P, Pang W, Zhang Y, Alwathnani HA, Rensing C, Yang W. Increased Tolerance of Massion’s pine to Multiple-Toxic-Metal Stress Mediated by Ectomycorrhizal Fungi. Plants. 2023; 12(18):3179. https://doi.org/10.3390/plants12183179
Chicago/Turabian StyleZhang, Taoxiang, Panpan Zhang, Wenbo Pang, Yuhu Zhang, Hend. A. Alwathnani, Christopher Rensing, and Wenhao Yang. 2023. "Increased Tolerance of Massion’s pine to Multiple-Toxic-Metal Stress Mediated by Ectomycorrhizal Fungi" Plants 12, no. 18: 3179. https://doi.org/10.3390/plants12183179
APA StyleZhang, T., Zhang, P., Pang, W., Zhang, Y., Alwathnani, H. A., Rensing, C., & Yang, W. (2023). Increased Tolerance of Massion’s pine to Multiple-Toxic-Metal Stress Mediated by Ectomycorrhizal Fungi. Plants, 12(18), 3179. https://doi.org/10.3390/plants12183179






