Supplementary White, UV-A, and Far-Red Radiation Differentially Regulates Growth and Nutritional Qualities of Greenhouse Lettuce
Abstract
:1. Introduction
2. Results
2.1. Effects of Supplementary Light on Leaf Morphology of Greenhouse-Grown Lettuce
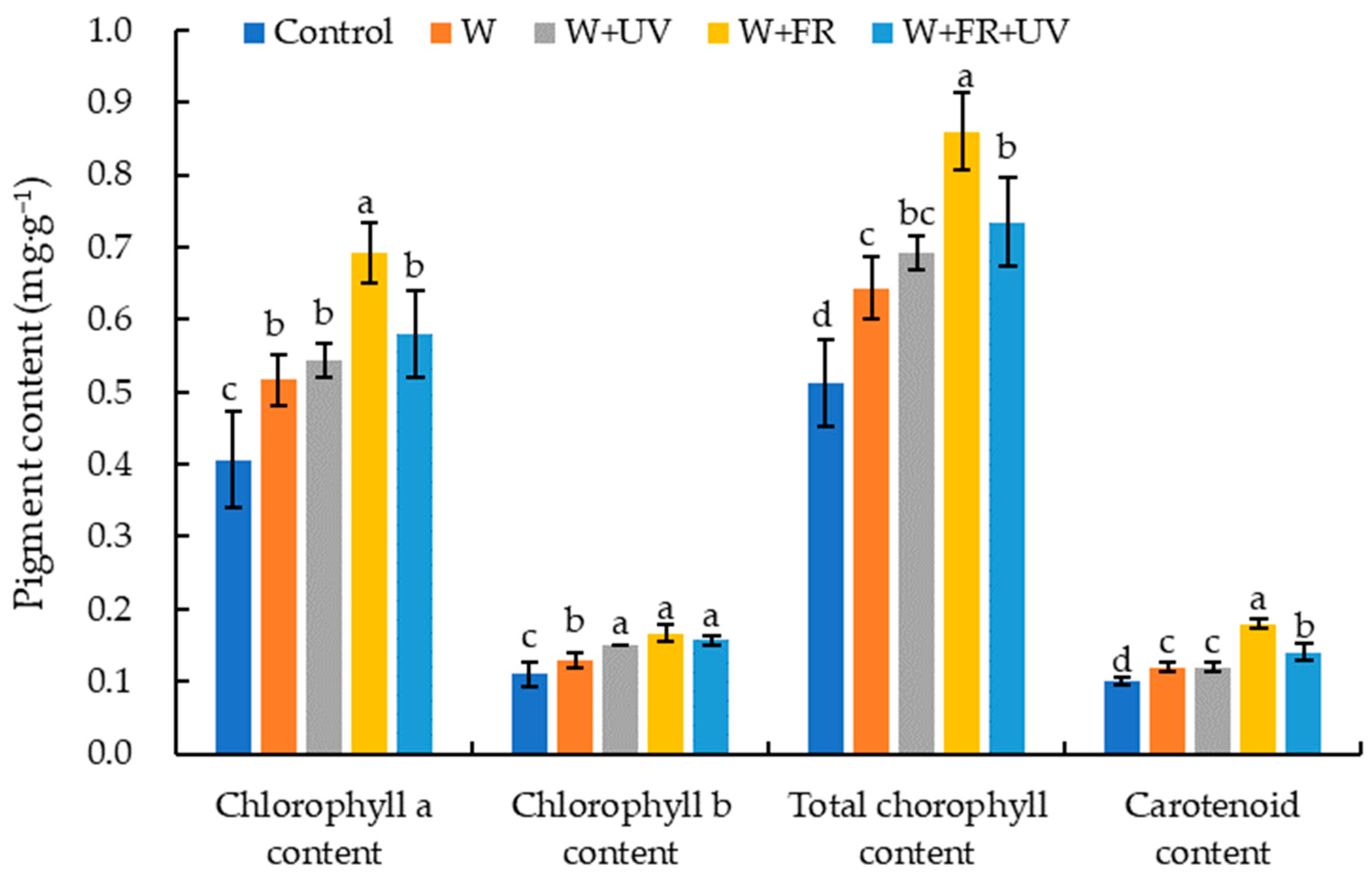
2.2. Impacts of Supplemental Light on Pigment Content of Greenhouse-Grown Lettuce
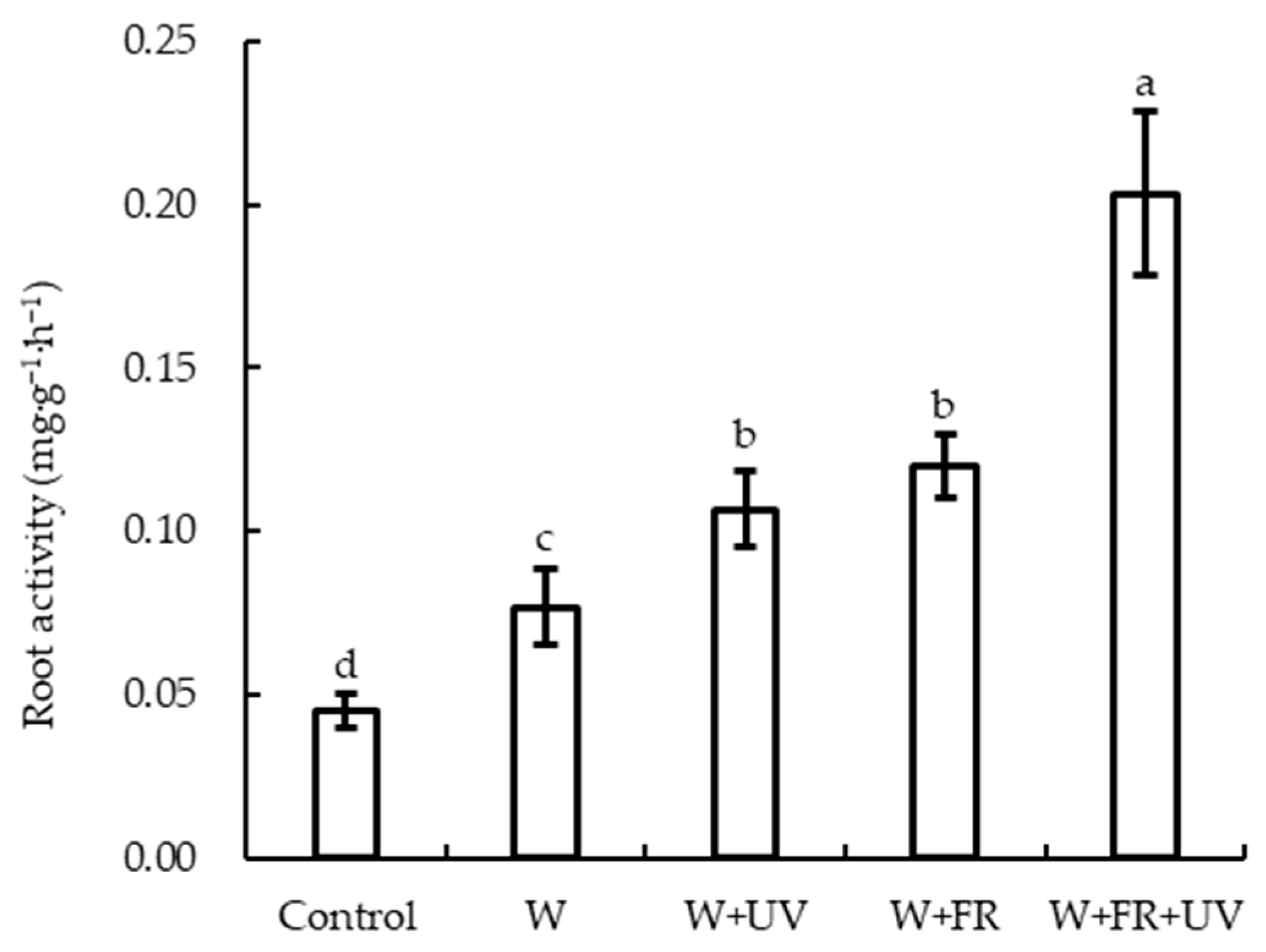
2.3. Effects of Supplementary Light on Biomass and Root Activity of Greenhouse-Grown Lettuce
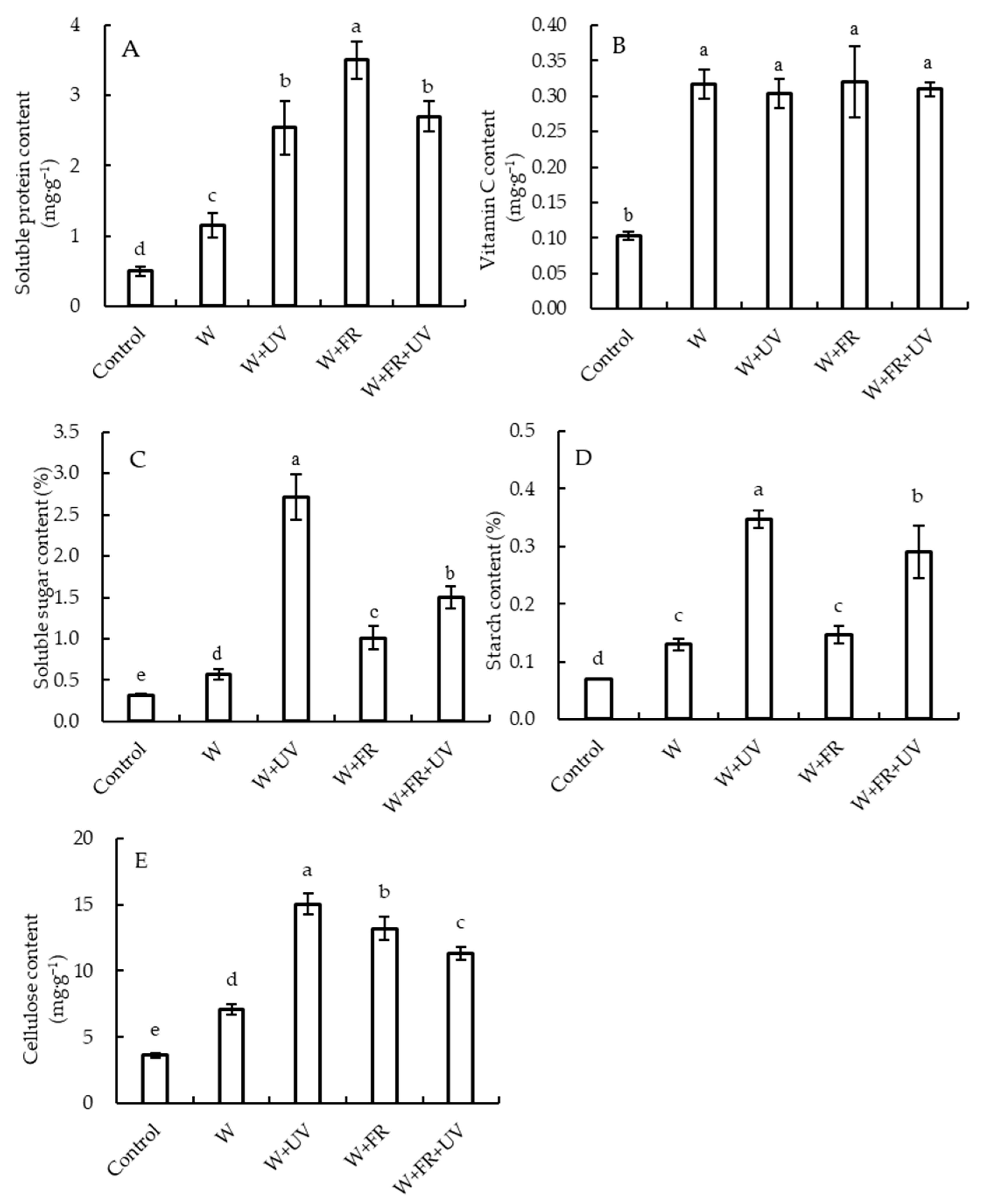
2.4. Impacts of Supplementary Light on Nutritional Quality of Greenhouse-Grown Lettuce
2.5. Correlation Analysis
2.6. Principal Component Analysis
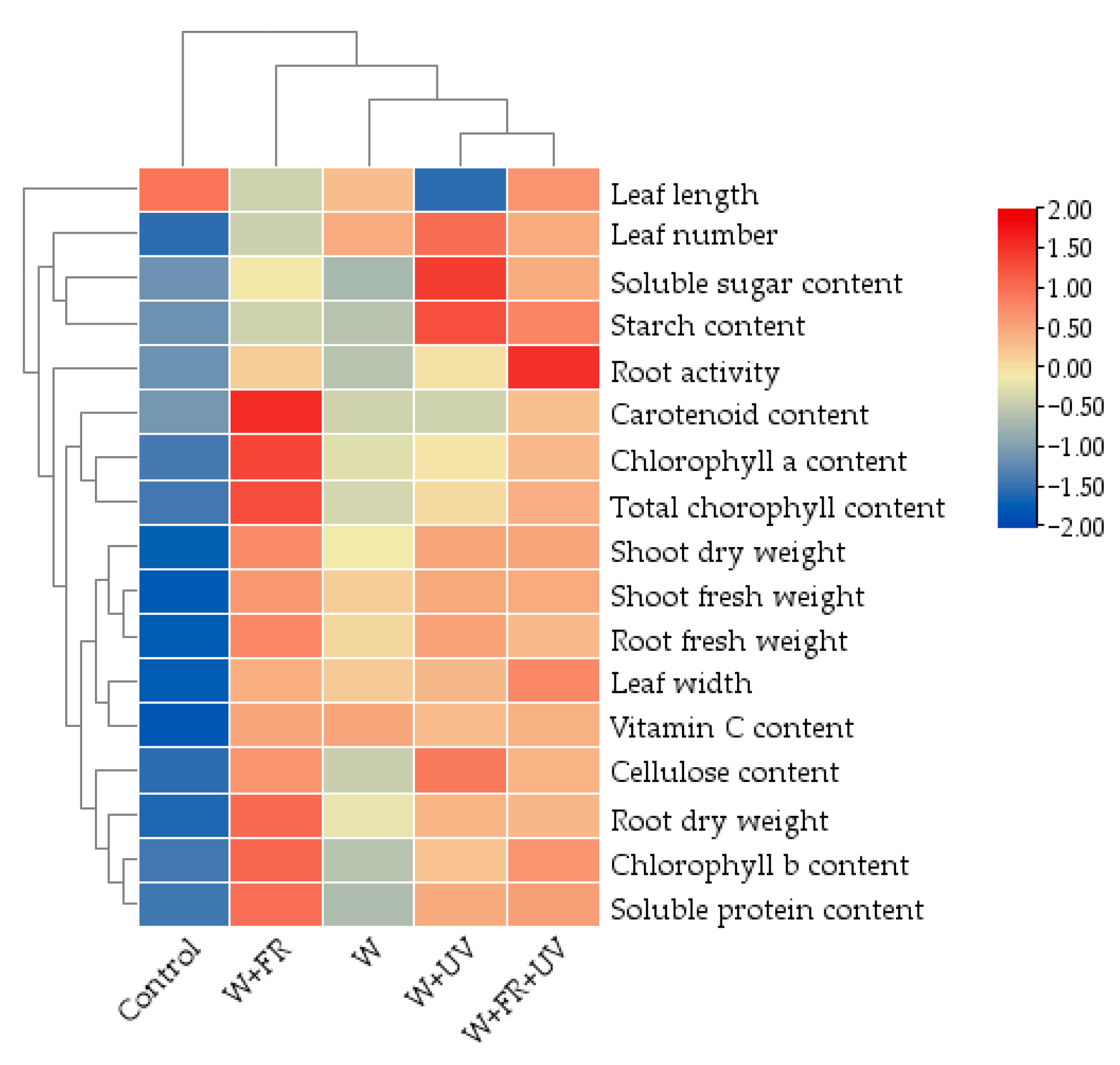
2.7. Heat Map Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
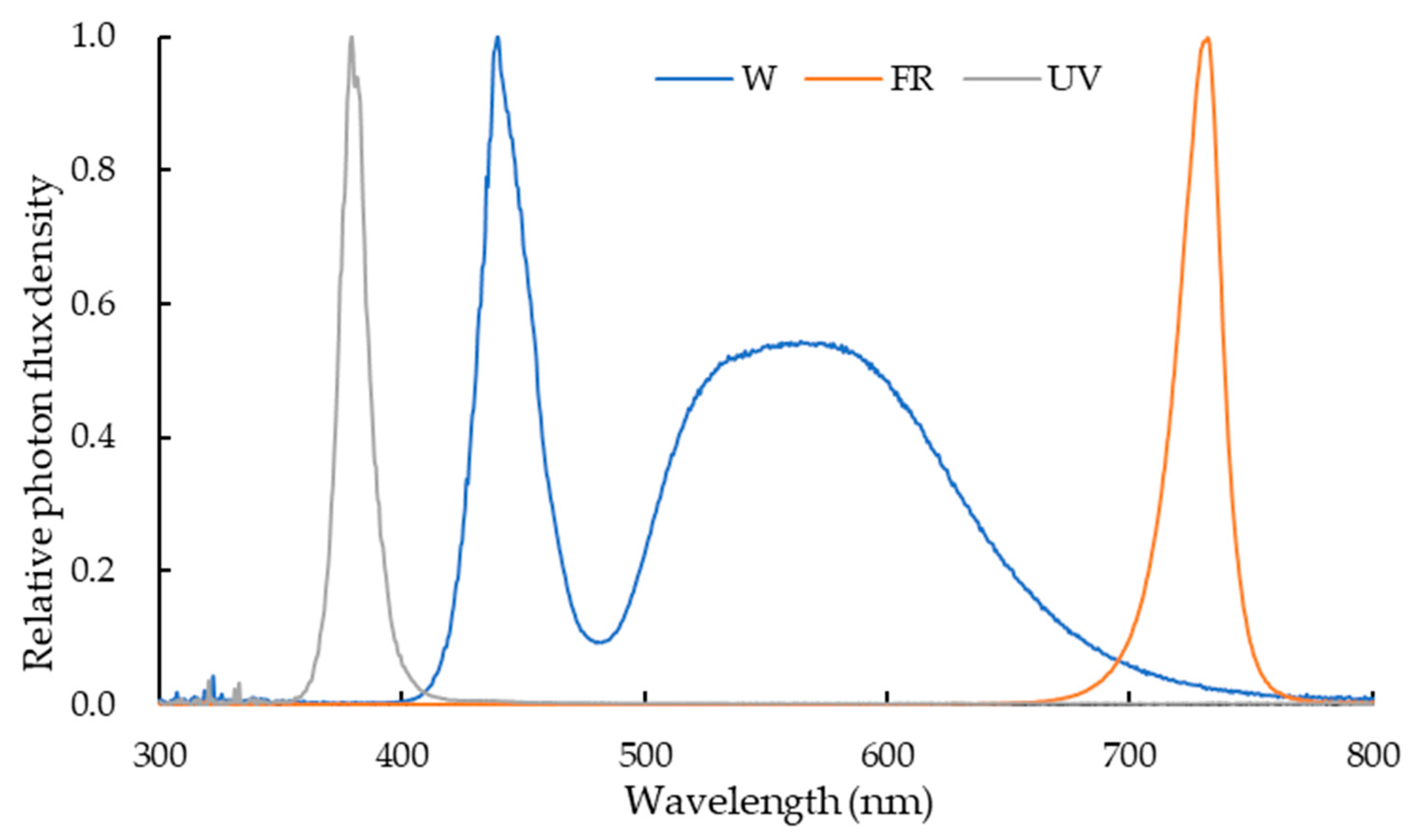
4.2. Treatment Design
4.3. Growth Indicator Measurement
4.3.1. Plant Morphology and Growth Characteristics
4.3.2. Pigment Contents
4.3.3. Root Activity
4.3.4. Starch, Cellulose, Vitamin C, Soluble Sugar, and Soluble Protein
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 June 2023).
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.Q.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cheng, R.F.; Wu, G.; Tong, Y.X.; Liu, Q.X. Effects of multi-layer three-dimensional cultivation combined with artificial lighting on lettuce yield and quality in greenhouse. J. China Agric. Univ. 2021, 26, 147–154. [Google Scholar]
- Yan, Z.; Wang, L.; Wang, Y.; Chu, Y.; Lin, D.; Yang, Y. Morphological and physiological properties of greenhouse-grown cucumber seedlings as influenced by supplementary light-emitting diodes with same daily light integral. Horticulturae 2021, 7, 361. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, W.K. Regulation of accumulation and metabolism circadian rhythms of starch and sucrose in two leaf-color lettuces by red:blue ratios of LED continuous light. Environ. Exp. Bot. 2022, 196, 104811. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond vegetables: Effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2021, 102, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés-Hernández, F. Amelioration effect of LED lighting in the bioactive compounds synthesis during carrot sprouting. Agronomy 2021, 11, 304. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; van Santen, E.; Gómez, C. Growth, Water-use efficiency, stomatal conductance, and nitrogen uptake of two lettuce cultivars grown under different percentages of blue and red light. Horticulturae 2018, 16, 3–13. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.H.; Zhou, Q.; Qu, Y.H. Growth, nutritional quality and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The evaluation of growth performance, photosynthetic capacity, and primary and secondary metabolite content of leaf lettuce grown under limited irradiation of blue and red LED light in an urban plant factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E. LEDs on Lettuce: White Light versus Red+Blue Light. Produce Grower. 25 June 2021. Available online: www.producegrower.com (accessed on 20 June 2023).
- Nájera, C.; Gallegos-Cedillo, V.M.; Ros, M.; Pascual, J.A. Role of Spectrum-Light on Productivity, and Plant Quality over Vertical Farming Systems: Bibliometric Analysis. Horticulturae 2023, 9, 63. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Galvao, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and light signal perception by plants-an emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Kim, D.; Moon, T.; Kwon, S.; Hwang, I.; Son, J.E. Supplemental inter-lighting with additional far-red to red and blue light increases the growth and yield of greenhouse sweet peppers (Capsicum annuum L.) in winter. Hortic. Environ. Biotechnol. 2022, 64, 83–95. [Google Scholar] [CrossRef]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Elevated UV photon fluxes minimally affected cannabinoid concentration in a high-CBD cultivar. Front. Plant Sci. 2023, 14, 1220585. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB radiation as innovative tools to biofortify horticultural crops with nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Lee, H.S.; Castle, W.S.; Coates, G.A. High-performance liquid chromatography for the characterization of carotenoids in the new sweet orange (Earlygold) grown in Florida, USA. J. Chromatogr. A 2001, 913, 371–377. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; de Vos, C.H.R.; Maas, F.M.; Jonker, H.H.; van den Broeck, H.C.; Jordi, W.; Pot, C.S.; Keizer, L.C.P.; Schapendonk, A.H.C.M. Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol. Plant. 2003, 117, 171–178. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.Y.; Oh, M.M. Supplemental radiation of ultraviolet-A light-emitting diode improves growth, antioxidant phenolics, and sugar alcohols of ice plant. Hortic. Environ. Biotechnol. 2021, 62, 559–570. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of pre-harvest supplemental UV-A/Blue and Red/Blue LED lighting on lettuce growth and nutritional quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulturae 2022, 8, 435. [Google Scholar] [CrossRef]
- Kang, S.Y.; Zhang, Y.T.; Zhang, Y.Q.; Zou, J.; Yang, Q.C.; Li, T. Ultraviolet-A radiation stimulates growth of indoor cultivated tomato (Solanum lycopersicum) seedlings. HortScience 2018, 53, 1429–1433. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Elias, K.; Zhang, Y.Q.; Zou, J.; Bian, Z.H.; Yang, Q.C.; Li, T. UVA radiation promotes tomato growth through morphological adaptation leading to increased light interception. Environ. Exp. Bot. 2020, 176, 104073. [Google Scholar] [CrossRef]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C.B. The effect of supplemental blue, red and far-red light on the growth and the nutritional quality of red and green leaf lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Kim, H.K.; Yang, T.; Choi, S.; Wang, Y.J.; Lin, M.Y.; Liceaga, A.M. Supplemental intracanopy far-red radiation to red LED light improves fruit quality attributes of greenhouse tomatoes. Sci. Hortic. 2020, 261, 108985. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Raza, M.A.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Kim, H.J.; Lin, M.-Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental far-red light stimulates lettuce growth: Disentangling morphological and physiological effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Mao, H.P.; Hang, T.; Zhang, X.D.; Lu, N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Liu, J.; Guo, Y.; Cheng, F.; Yang, Y.; Yan, Z. Long supplementary light duration under same daily light integral provided by white plus blue light-emitting diodes improves quality of greenhouse-grown tomato seedlings. Hortic. Environ. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Yu, D. The role of light quality in regulating early seedling development. Plants 2023, 12, 2746. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Growth and appearance quality of four microgreen species under light-emitting diode lights with different spectral combinations. HortScience 2020, 55, 1399–1405. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and shade-avoidance responses in plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaiepour, S.Z.; Tahmasebi, Z.; Taab, A.; Rashidi-Monfared, S. Effect of redroot pigweed interference on antioxidant enzyme and light response of common bean (Phaseolus vulgaris L.) depends on cultivars and growth stages. Sci. Rep. 2023, 13, 4289. [Google Scholar] [CrossRef]
- Yamori, W. Photosynthesis and respiration. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 197–206. [Google Scholar]
- Santos, E.F.; Zanchim, B.J.; de Campos, A.G.; Garrone, R.F.; Junior, J.L. Photosynthesis rate, chlorophyll content and initial development of physic nut without micronutrient fertilization. Rev. Bras. Cienc. 2013, 37, 1334–1342. [Google Scholar] [CrossRef]
- Appolloni, E.; Paucek, I.; Pennisi, G.; Manfrini, L.; Gabarrell, X.; Gianquinto, G.; Orsini, F. Winter greenhouse tomato cultivation: Matching leaf pruning and supplementary lighting for improved yield and precocity. Agronomy 2023, 13, 671. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Cheng, J.; Lin, D.; Yang, Y. Morphology, growth, and physiological traits of greenhouse cucumber seedlings as affected by supplementary white and blue LEDs. Int. J. Agric. Biol. Eng. 2022, 15, 60–66. [Google Scholar] [CrossRef]
- Oelze-Karow, H.; Mohr, H. Phytochrome action on chlorophyll synthesis—A study of the escape from photoreversibility. Plant Physiol. 1982, 70, 863–866. [Google Scholar] [CrossRef]
- He, J.; Jawahir Nur, K.B.; Lin, Q. Quantity of supplementary LED lightings regulates photosynthetic apparatus, improves photosynthetic capacity and enhances productivity of cos lettuce grown in a tropical greenhouse. Photosynth. Res. 2021, 149, 187–199. [Google Scholar] [CrossRef]
- Rahman, M.H.; Azad, M.O.K.; Islam, M.J.; Rana, M.S.; Li, K.-h.; Lim, Y.S. Production of potato (Solanum tuberosum L.) seed tuber under artificial LED light irradiation in plant factory. Plants 2021, 10, 297. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, X.Z.; Guo, W.Z.; Wang, L.C.; Qiao, X.J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Ji, F.; Gan, P.D.; Liu, N.; He, D.X.; Yang, P. Effects of LED spectrum and daily light integral on growth and energy use efficiency of tomato seedlings. Trans. CSAE 2020, 36, 231–238. [Google Scholar]
- Huber, B.M.; Louws, F.J.; Hernández, R. Impact of different daily light integrals and carbon dioxide concentrations on the growth, morphology, and production efficiency of tomato seedlings. Front. Plant Sci. 2021, 12, 615853. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA radiation is beneficial for yield and quality of indoor cultivated lettuce. Front. Plant Sci. 2019, 10, 1563–1572. [Google Scholar] [CrossRef]
- Lee, M.; Park, S.; Oh, M. Growth and cell division of lettuce plants under various ratios of red to far-red light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Lee, M.; Son, K.; Oh, M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Light. Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Murakami, K.; Matsuda, R.; Fujiwara, K. Quantification of excitation energy distribution between photosystems based on a mechanistic model of photosynthetic electron transport. Plant Cell Environ. 2018, 41, 148–159. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Liu, W.; Zha, L.; Shao, M.; Li, B. Light quality affected the growth and root organic carbon and autotoxin secretions of hydroponic lettuce. Plants 2020, 9, 1542. [Google Scholar] [CrossRef]
- Tezuka, T.; Yamaguchi, F.; Ando, Y. Physiological activation in radish plants by UV-A radiation. J. Photochem. Photobiol. B Biol. 1994, 24, 33–40. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, Y.Q.; Yang, Q.C.; Li, T. Overhead supplemental far-red light stimulates tomato growth under intra-canopy lighting with LEDs. J. Integr. Agric. 2019, 18, 62–69. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, W.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine improves the sucrose metabolism of lettuce to resist high-temperature stress. Plant Growth Regul. 2022, 96, 497–509. [Google Scholar] [CrossRef]
- Larsen, D.H.; Marcelis, L.F.M.; van Kempen, D.; Kohlen, W.; Nicole, C.C.S.; Woltering, E.J. Far-red light during cultivation improves postharvest chilling tolerance in basil. Postharvest Biol. Technol. 2023, 198, 112232. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Li, H.S. Experimental Principle and Technology of Plant Physiology and Biochemistry, 1st ed.; Higher Education Press: Beijing, China, 2000; pp. 119–120, 184–185, 195–197, 246–249. [Google Scholar]
- Takahashi, K.; Fujino, K.; Kikuta, Y.; Koda, Y. Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid. Plant Sci. 1995, 111, 11–18. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semi-micro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, D.X.; Niu, G.H.; Yan, Z.N.; Song, J.X. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential effects of low light intensity on broccoli microgreens growth and phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Treatment | Leaf Length (cm) | Leaf Width (cm) | Leaf Number | |||
|---|---|---|---|---|---|---|
| Control | 19.6 ± 1.0 | a | 9.0 ± 0.6 | c | 5.0 ± 1.0 | b |
| W | 17.4 ± 0.7 | b | 14.6 ± 0.3 | b | 6.3 ± 0.6 | a |
| W+UV | 14.7 ± 1.8 | c | 14.7 ± 0.2 | b | 6.7 ± 0.6 | a |
| W+FR | 17.4 ± 1.1 | b | 16.1 ± 0.5 | a | 5.7 ± 0.6 | ab |
| W+FR+UV | 17.8 ± 1.0 | ab | 16.2 ± 0.5 | a | 6.3 ± 0.6 | a |
| Treatment | Shoot Fresh Weight (g/Plant) | Root Fresh Weight (g/Plant) | Shoot Dry Weight (g/Plant) | Root Dry Weight (g/Plant) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | 9.40 ± 0.50 | d | 0.67 ± 0.12 | e | 0.64 ± 0.02 | d | 0.05 ± 0.01 | d |
| W | 34.53 ± 0.38 | c | 3.68 ± 0.10 | d | 2.61 ± 0.05 | c | 0.35 ± 0.01 | c |
| W+UV | 42.35 ± 0.43 | b | 5.18 ± 0.23 | b | 3.90 ± 0.06 | b | 0.49 ± 0.06 | b |
| W+FR | 47.33 ± 0.90 | a | 6.02 ± 0.33 | a | 4.50 ± 0.13 | a | 0.67 ± 0.01 | a |
| W+FR+UV | 42.63 ± 0.68 | b | 4.43 ± 0.16 | c | 3.90 ± 0.02 | b | 0.48 ± 0.02 | b |
| Principal Component | Eigenvalue | Contribution Rate | Cumulative Contribution Rate |
|---|---|---|---|
| (%) | (%) | ||
| 1 | 12.90 | 75.85 | 75.85 |
| 2 | 2.57 | 15.13 | 90.98 |
| Treatment | FAC1 | FAC2 | Composite Score |
|---|---|---|---|
| Control | −5.82 | 0.31 | −4.37 |
| W | −1.07 | −0.12 | −0.83 |
| W+UV | 2.05 | −2.31 | 1.21 |
| W+FR | 2.97 | 2.20 | 2.58 |
| W+FR+UV | 1.87 | −0.08 | 1.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Wang, C.; Li, Z.; Li, X.; Cheng, F.; Lin, D.; Yang, Y. Supplementary White, UV-A, and Far-Red Radiation Differentially Regulates Growth and Nutritional Qualities of Greenhouse Lettuce. Plants 2023, 12, 3234. https://doi.org/10.3390/plants12183234
Yan Z, Wang C, Li Z, Li X, Cheng F, Lin D, Yang Y. Supplementary White, UV-A, and Far-Red Radiation Differentially Regulates Growth and Nutritional Qualities of Greenhouse Lettuce. Plants. 2023; 12(18):3234. https://doi.org/10.3390/plants12183234
Chicago/Turabian StyleYan, Zhengnan, Chunling Wang, Zhixin Li, Xin Li, Fei Cheng, Duo Lin, and Yanjie Yang. 2023. "Supplementary White, UV-A, and Far-Red Radiation Differentially Regulates Growth and Nutritional Qualities of Greenhouse Lettuce" Plants 12, no. 18: 3234. https://doi.org/10.3390/plants12183234




