Abstract
This study was conducted to determine the differences in the chemical composition of the essential oils and hydrosols of six different Veronica species (V. agrestis, V. anagalloides, V. austriaca ssp. jacquinii, V. beccabunga, Veronica cymbalaria, and V. officinalis) and to test their antiproliferative and apoptotic activities, according to the authors’ knowledge, because of insufficient research and lack of information. Also, the goal was to determine which obtained samples were better in achieving antiproliferative and apoptotic activities and due to which volatile components. Therefore, essential oils (EOs) and hydrosols (HYs) were isolated from the above-mentioned Veronica species by microwave-assisted extraction (MAE). Phytochemical identification of the free volatile compounds was performed using a GC equipped with a flame ionization detector and a mass spectrometer. Their antiproliferative and apoptotic activities against two human cancer cell lines, breast cancer cell line MDA-MB-231 and bladder cancer cell line T24, were determined. The main compounds identified in the studied Veronica EOs and HYs were terpinen-4-ol (0.34–6.49%), linalool (0.34–6.61%), (E)-caryophyllene (0.97–7.55%), allo-aromadendrene (0.18–2.21%), caryophyllene oxide (1.42–23.83%), benzene acetaldehyde (0.26–13.34%), and β-ionone (1.08–16.53%). In general, HYs of the tested Veronica species showed higher antiproliferative activity (IC50 13.41–42.05%) compared to EOs (IC50 158.1–970.4 µg/mL) on MDA-MB-231 and T24 cancer cell lines after 48 and 72 h. V. agrestis EO showed the best apoptotic effect among the EOs on the MDA-MB-231 cancer cell line (10.47 ± 0.53% and 9.06 ± 0.74% of early/late apoptosis, compared with control 3.61 ± 0.62% and 0.80 ± 0.17% of early/late apoptosis, respectively) and among the HYs V. cymbalaria showed 9.95 ± 1.05% and 3.06 ± 0.28% of early/late apoptosis and V. anagalloides 8.29 ± 1.09% and 1.95 ± 0.36% of early/late apoptosis compared with control (for EO was 7.45 ± 1.01% and 0.54 ± 0.25%, and for HY was 4.91 ± 1.97% and 0.70 ± 0.09% of early/late apoptosis, respectively) on the T24 cancer cell line. Future research will include other Croatian species of the genus Veronica to gain a more complete insight into the biological activity of the volatile products of this genus for potential discovery of drugs based on natural plant extracts.
1. Introduction
Throughout the long development of civilization, people have selected plants as food or/and medicine, primarily based on organoleptic evaluations. Scientific confirmation of the use of medicinal plants began with the development of analytical methods, especially chromatography. Chromatographic techniques enabled the identification and quantification of components of specialized metabolites from plant extracts and stimulated interest in studying the effects of these natural products on long-term health and preventive treatment [1]. Prior to the identification and quantification of interesting and potentially biologically active compounds, they must first be isolated from plant material. In their study, Dunkić et al. [2] reported the results of isolation of volatile compounds (VCs) by classical hydrodistillation and microwave-assisted extraction (MAE). Some VCs were only isolated with either hydrodistillation or MAE. The results obtained can be explained by the fact that hydrodistillation method is known to have some disadvantages, since the same compounds are decomposed because of the high temperatures and long extraction times. On the other hand, microwave distillation can sometimes lead to the isolation of few components, as stated in the study of Wu et al. [3]. However, modern techniques such as MAE are much faster, easier to use, and environmentally acceptable and enable extraction of bioactive components with less energy than conventional extraction methods [4,5]. Two types of samples can be obtained using the MAE technique, essential oil (EO), and hydrosol (HY). Essential oils are lipophilic, aromatized liquids with volatile constituents, obtained from plant material by steam distillation and named after the plant from which they originate [6]. Hydrosols are actually flavored waters obtained by condensation of water vapor in distillation processes [7] and contain a small quantity of water-soluble volatiles. Therefore, unlike EOs, HYs are safer for human use [8,9]. The general study of specialized plant metabolites contributes to the development of different areas of phytochemistry [10]. Specialized metabolites are very specific to certain plant families, genera, and species and contain an incredibly large library of bioactive compounds. Depending on the concentration, these compounds can also be toxic, and in adjusted doses, they represent a broad spectrum of phytochemical effects on human cells, bacteria, fungi, and parasites [10]. Genus Veronica, which has recently been studied because of its characteristic specialized metabolites, formerly a member of the Scrophulariaceae, was subsequently transferred to the Plantaginaceae family after phylogenetic and chemotaxonomic studies [11]. To date, research on specialized metabolites of the genus Veronica has mainly focused on iridoid glycosides, flavonoids, and saponins [12,13,14], while the free volatile compounds, which also constitute an important part of the specialized metabolites of this genus, have only recently begun to be studied in more detail [15,16,17]. Some Veronica species are used in traditional medicine worldwide for the treatment of different disorders such as the following: as an antiscorbutic, for wound healing, in respiratory diseases for cough, or as an expectorant [18]. Previous research on the Veronica species has shown that because of their specialized metabolites, they possess antioxidant, antimicrobial, cytotoxic, and antitumor activities [19,20,21]. Harput et al. [22] demonstrated the antiproliferative activity of investigated methanolic extracts from five Veronica species against two tumor cell lines, KB and B16 cells. Their results showed that the MeOH extracts possessed anti-inflammatory and cytotoxic activities. EOs have been demonstrated to possess anticancer properties through different mechanisms, including cancer prevention mechanisms, as well as the impact of the established tumor cell itself and the interaction with the microenvironment [23]. Key features of cancer include resistance to cell death. Therefore, therapeutic strategies are aimed to induce apoptosis [24]. In medical treatment, EOs and raw natural extracts are generally well accepted by patients, although their good reputation (because of the widespread belief that their naturalness is a guarantee of safety) may hide occasional toxicity problems because of the presence of specific components [25]. Despite different Veronica extracts having been used in traditional medicine for cancer treatment, only a few species have been studied for their cytotoxic and anticancer activity [14]. Apoptosis, programmed cell death, is a process that includes cell changes such as cell contraction, blebbing, DNA fragmentation, nuclear fragmentation, chromatin condensation, and mRNA decay [26]. Defects in apoptotic processes are associated with various diseases, including cancer. Uncontrolled cell proliferation is associated with insufficient apoptosis [26]. Therefore, scientific research is increasingly focused on medicinal plant research and the elucidation of signaling pathways that control cell cycle arrest and apoptosis. Because of all mentioned above, six species of the genus Veronica were selected for this study.
Consequently, the aim of this study was the phytochemical identification of FVCs in EOs and HYs isolated by microwave-assisted extraction (MAE) from six different Veronica species distributed in Croatia, V. agrestis L., V. anagalloides Guss., V. austriaca ssp. jacquinii L., V. beccabunga L., V. cymbalaria Bodard, and V. officinalis L., and determination of their antiproliferative and apoptotic activities against two human cancer cell lines: breast cancer cell line MDA-MB-231, and bladder cancer cell line T24.
2. Results
2.1. Extraction of Volatile Components from Six Veronica Species
Extraction of volatiles from the six Croatian Veronica species collected in 2022 (Table 1) was performed by microwave-assisted extraction (MAE). Each extract consists of two parts, lipid and water, and both parts of the extracts of all studied species were analyzed by gas chromatography–mass spectrometry (GC-MS). The results of the composition of the lipid part (essential oil, EO) and the water part (hydrosol, HY) are presented in Table 2 and Table 3.

Table 1.
Details on collection data and origin of investigated Veronica species.

Table 2.
Constituents of the essential oils (EOs, %) obtained by microwave extraction of six Veronica species.

Table 3.
Constituents of the hydrosols (HYs, %) obtained by microwave extraction of six Veronica species.
2.1.1. Composition of Essential Oil
The compounds linalool, (E)-caryophyllene, allo-aromadendrene, caryophyllene oxide, hexahydrofarnesyl acetone, phytol, β-ionone, hexadecanoic acid, docosane, tricosane, tetracosane, and octacosane were detected in six studied Veronica EOs (Table 2).
Peculiarities in the EO composition for each species were investigated. In the composition of V. agrestis, the dominant compound is phytol (56.57%); in V. anagalloides, the dominant compounds are hexahydrofarnesyl acetone (16.17%) and β-ionone (13.13%). In addition, in the composition of V. austriaca ssp. jacquinii EO, hexadecanoic acid (27.66%) and phytol (13.02%) are the predominant compounds. Phytol and hexadecanoic acid are also the predominant constituents in the species V. beccabunga, with 28.08% and 17.06%, respectively. In addition to these two compounds already mentioned, the compound caryophyllene oxide (23.83%) is significantly present in the species V. cymbalaria. Together with phytol and hexadecanoic acid, which are also significantly present in the EO composition of V. officinalis, heptacosane is the most abundant compound (17.21%) (Table 2).
2.1.2. Composition of Hydrosols
The compounds terpinen-4-ol, linalool, (E)-caryophyllene, allo-aromadendrene, caryophyllene oxide, benzaldehyde, benzene acetaldehyde, and β-ionone were detected in the six studied Veronica HYs (Table 3).
In the composition of HY of V. agrestis, four components were identified with a proportion greater than 10%: caryophyllene oxide (14.01%), (E)-β-damascenone (12.42%), benzene acetaldehyde (11.56%), and β-ionone (10.32%). In V. anagalloides HY, β-ionone (16.53%), benzaldehyde (13.56%), methyl eugenol (13.57%), and (E)-β-damascenone (11.55%) dominate. In addition, methyl eugenol accounts for 37.01%, more than one-third of all identified components in the HY composition of V. austriaca ssp. jacquinii. The HY composition of V. beccabunga differs significantly from the other Veronica species studied, as the HY composition is dominated by α-pinene and piperitone with 17.11% and 19.54%, respectively. The HY of V. cymbalaria is rich in the phenolic constituents methyl eugenol (38.61%) and (Z)-methyl isoeugenol (31.32%). Methyl eugenol also predominates in the HY of V. officinalis with 22.01% and is the most abundant compound, followed by the compounds (E)-β-damascenone and β-ionone with an identification percentage of more than 14% (Table 3).
2.2. Cell Viability and Proliferation Using MTT Assay
Cell viability and proliferation after treatment with essential oils (EOs) and hydrosols (HYs) of six Veronica species were determined and presented on two cancer cell lines, breast cancer cell line MDA-MB-231 and bladder cancer cell line T24, using MTT cell proliferation assay. The mentioned cancer cell lines were treated at concentrations of 50, 100, 250, 500, and 1000 µg/mL for EOs, while HYs were tested at different dilutions (10%, 20%, 30%, 40%, and 50%) after MAE for 4, 24, 48, and 72 h. After MTT cell proliferation assay, obtained results were expressed as % of metabolically active cells (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 and Figures S1–S24) and IC50 values (50% cell growth inhibitory concentrations). In this work, only results for the times at which the samples showed antiproliferative activity in tested concentrations and dilutions are presented. In Supplementary Materials all the obtained results on MDA-MB-231 and T24 cancer cell lines are presented (Figures S1–S24).
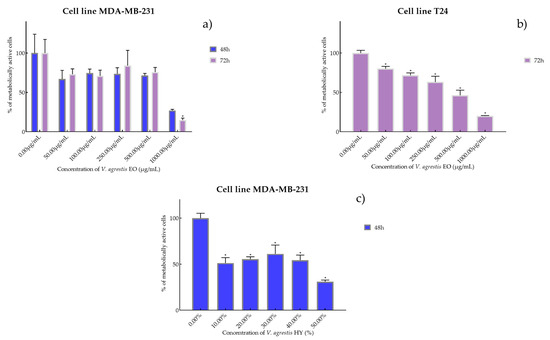
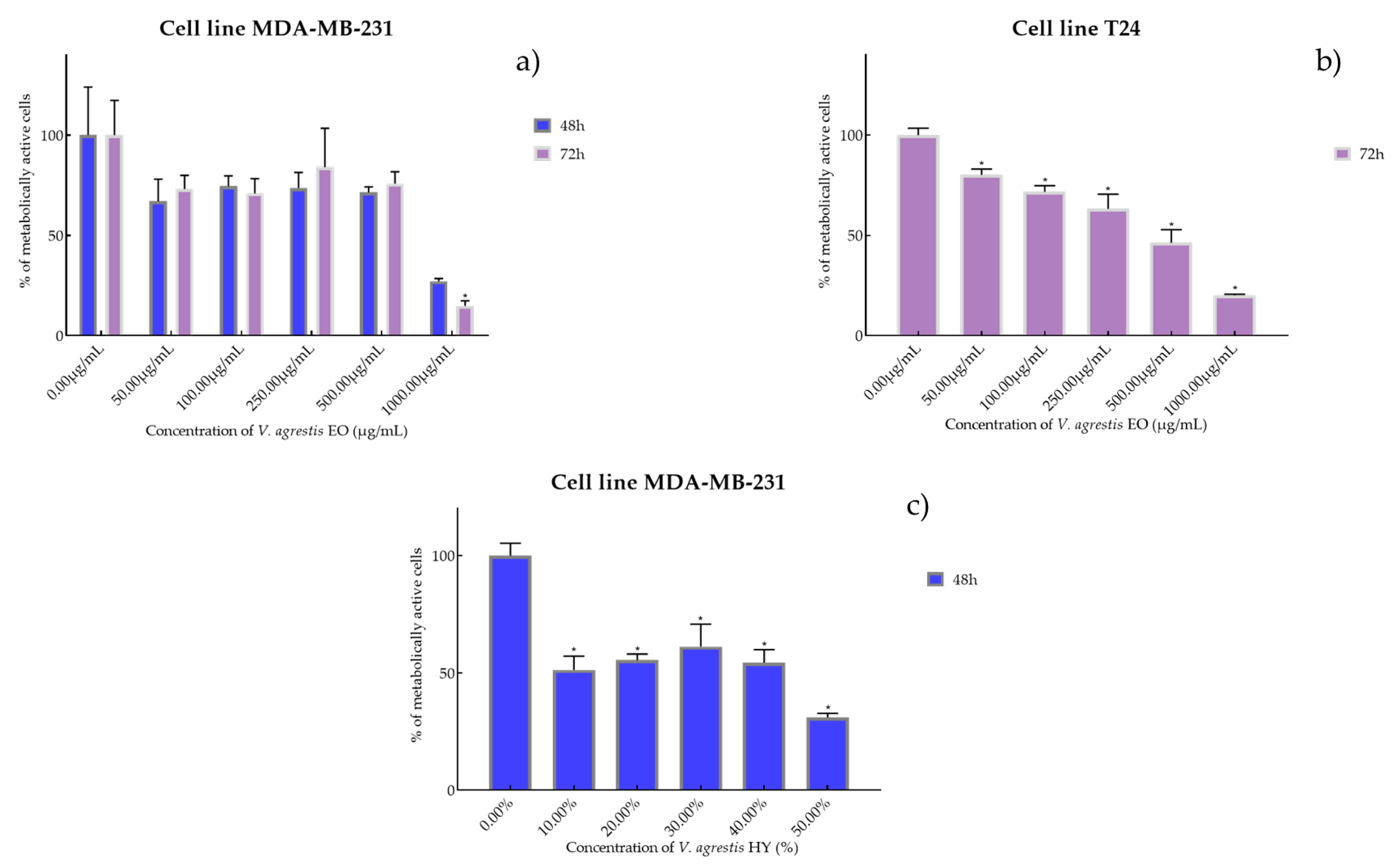
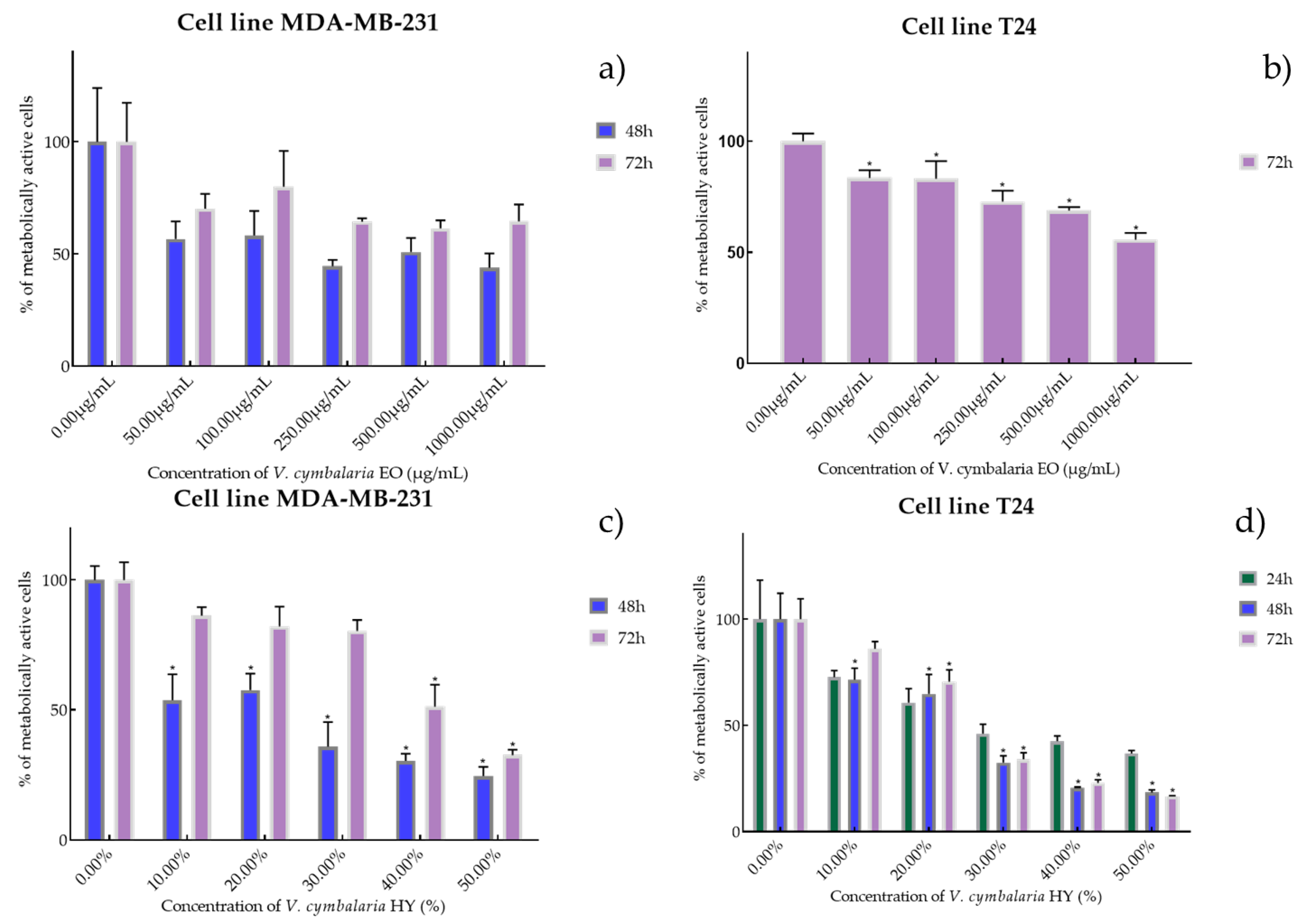
Figure 1.
Metabolic activity of the cells (%) after treatment with V. agrestis EO (a,b) and V. agrestis HY (c) on MDA-MB-231 and T24 cancer cell lines. The results are expressed as means of three independent experiments with SD values (presented as error bars). For statistical analyses, a t-test with unequal variances was performed with the significance set at * p < 0.05.
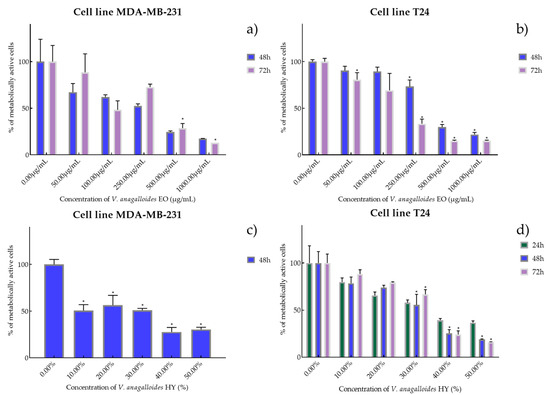
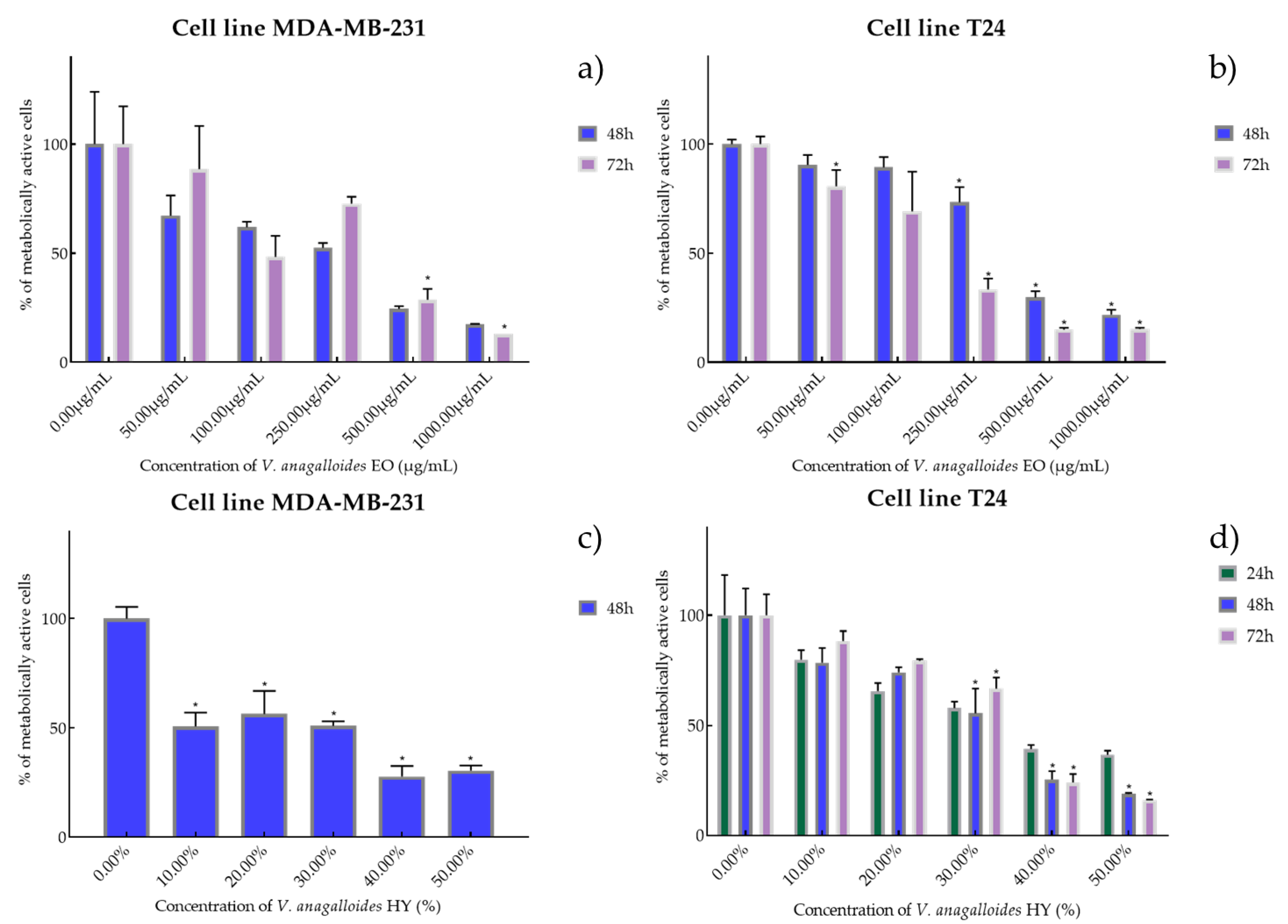
Figure 2.
Metabolic activity of the cells (%) after treatment: with V. anagalloides EO (a,b) and V. anagalloides HY(c,d) on MDA-MB-231 and T24 cancer cell lines. The results are expressed as means of three independent experiments with SD values (presented as error bars). For statistical analyses, a t-test with unequal variances was performed with the significance set at * p < 0.05.
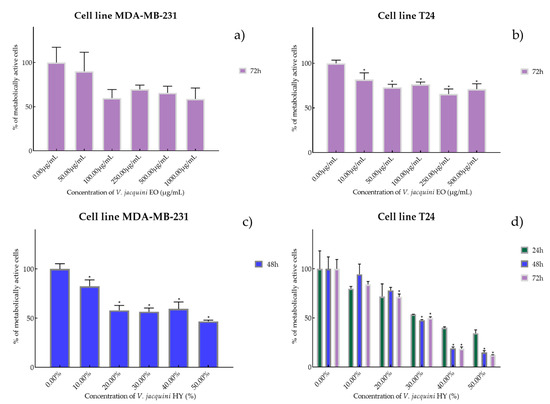
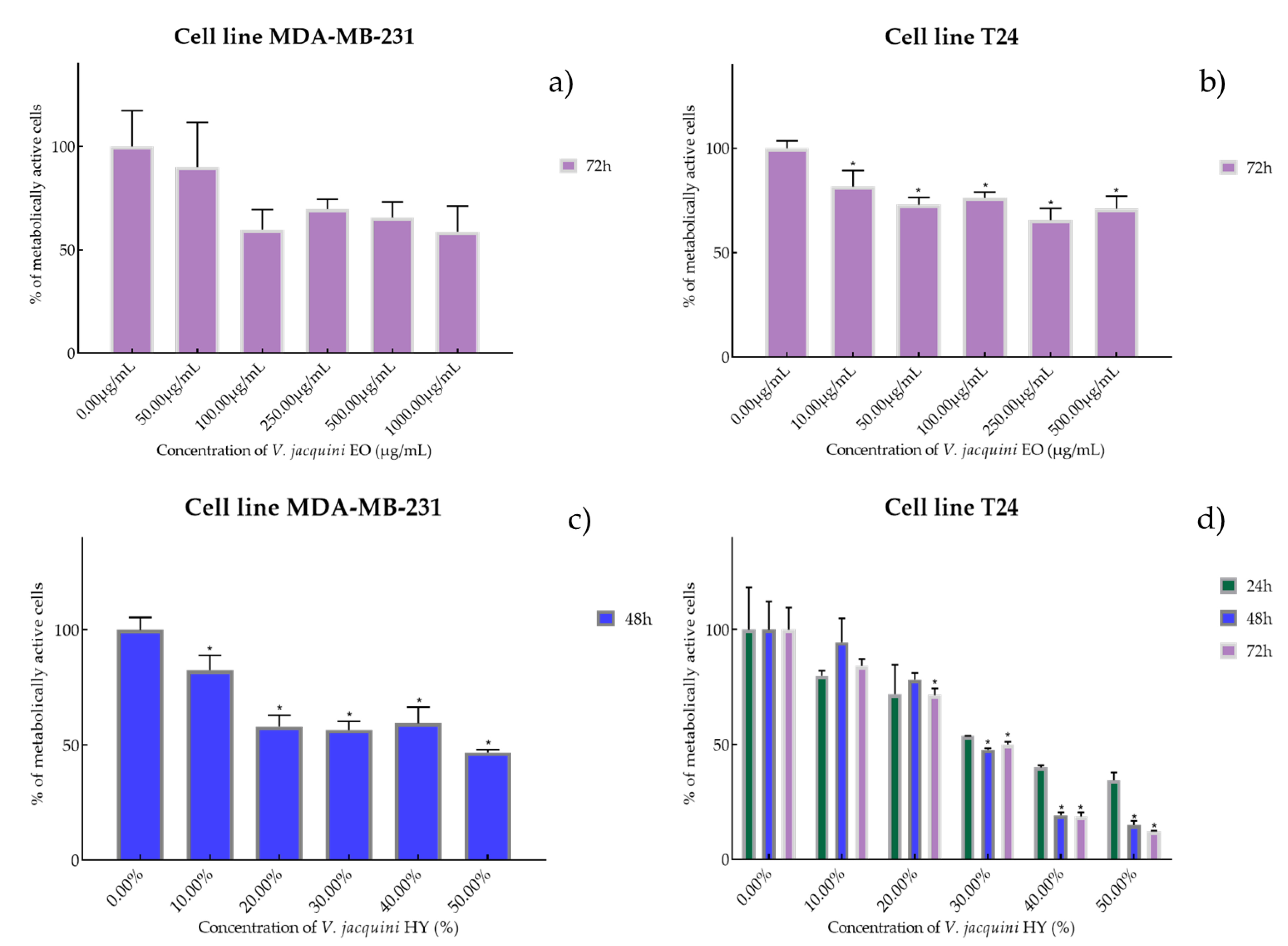
Figure 3.
Metabolic activity of the cells (%) after treatment with V. austriaca ssp. jacquini EO (a,b) and V. jacquini HY (c,d) on MDA-MB-231 and T24 cancer cell lines. The results are expressed as means of three independent experiments with SD values (presented as error bars). For statistical analyses, a t-test with unequal variances was performed with the significance set at * p < 0.05.
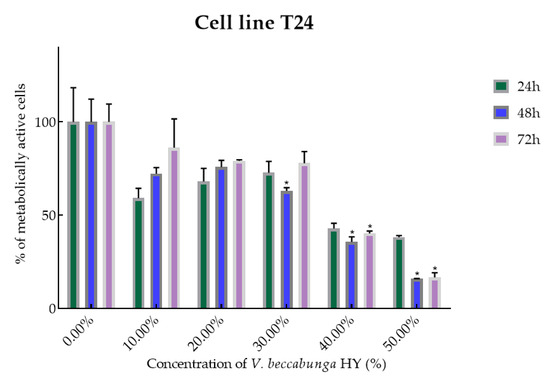
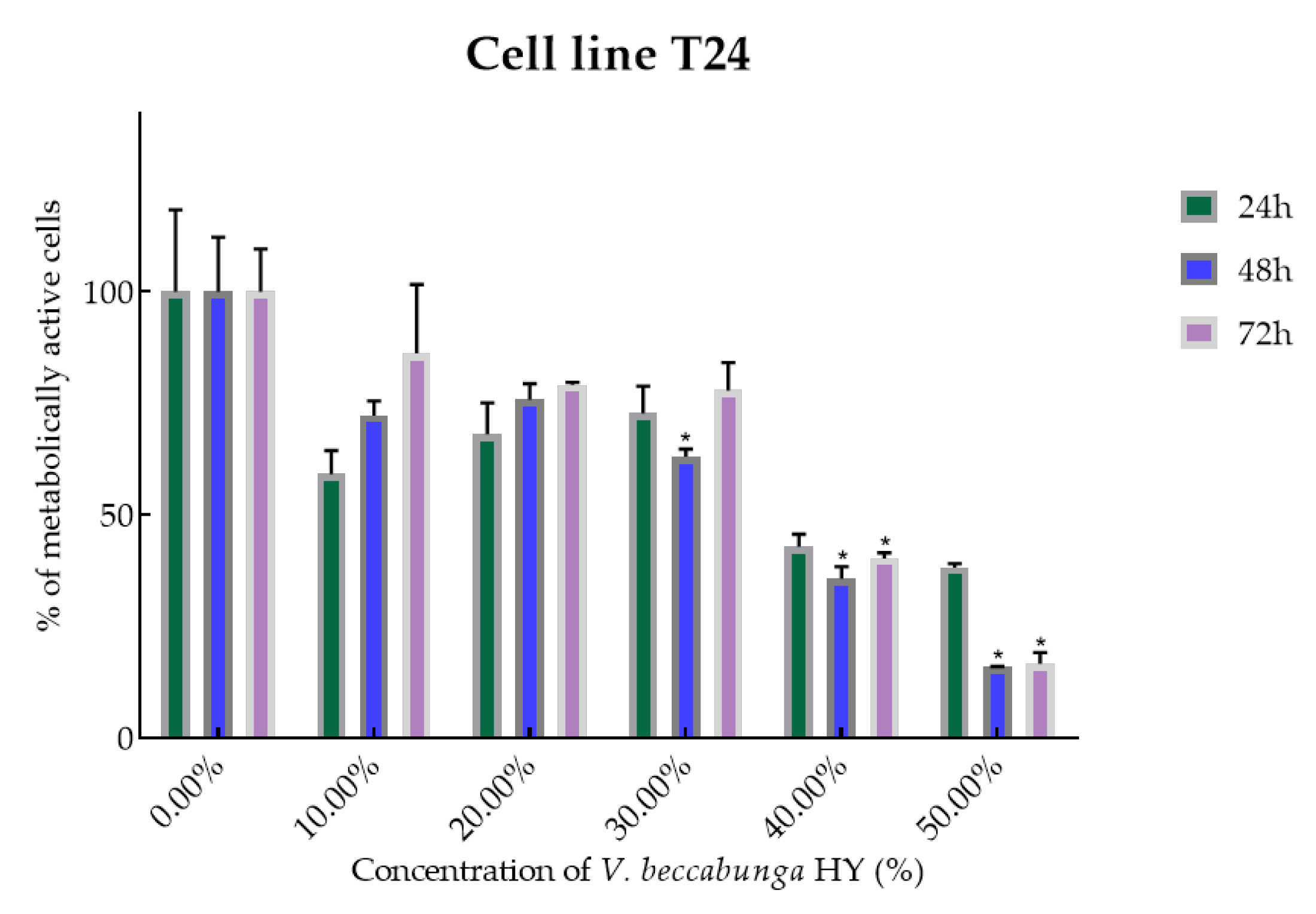
Figure 4.
Metabolic activity of the cells (%) after treatment with V. beccabunga HY on the T24 cancer cell line. The results are expressed as means of three independent experiments with SD values (presented as error bars). For statistical analyses, a t-test with unequal variances was performed with the significance set at * p < 0.05.
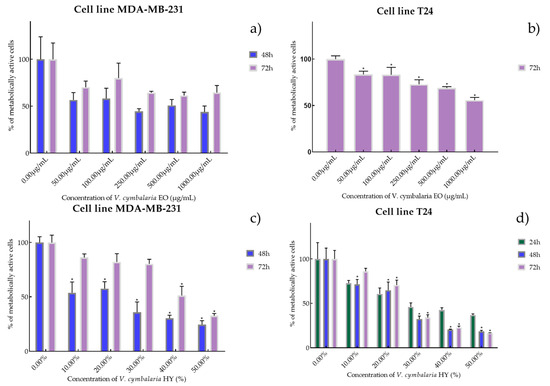
Figure 5.
Metabolic activity of the cells (%) after treatment with V. cymbalaria EO (a,b) and V. cymbalaria HY (c,d) on MDA-MB-231 and T24 cancer cell lines. The results are expressed as means of three independent experiments with SD values (presented as error bars). For statistical analyses, a t-test with unequal variances was performed with the significance set at * p < 0.05.
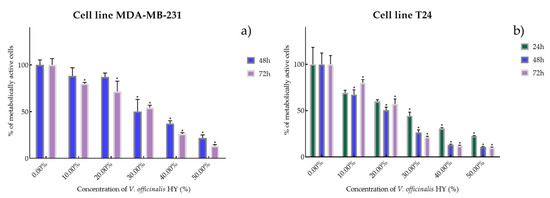
Figure 6.
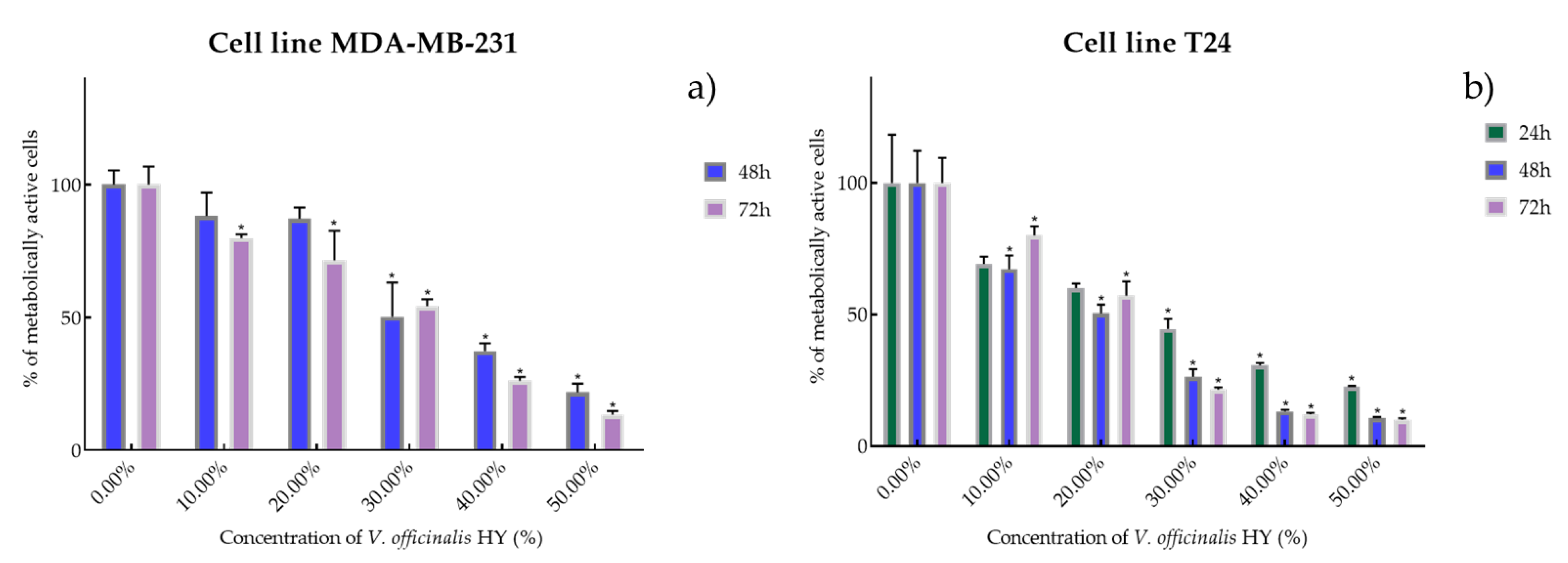
Metabolic activity of the cells (%) after V. officinalis HY on MDA-MB-231 (a) and T24 cancer cell lines (b). The results are expressed as means of three independent experiments with SD values (presented as error bars). For statistical analyses, a t-test with unequal variances was performed with the significance set at * p < 0.05.
Antiproliferative activity of V. agrestis EO on MDA-MB-231 after 48 and 72 h was IC50 572.1 µg/mL and 586.9 µg/mL (percentage of metabolically active cells was 66.98%, 74.43%, 73.57% 71.43%, and 27.15% after 48 h, and 73.11%, 70.98%, 84.05%, 75.79%, and 14.86% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) and IC50 340.6 µg/mL on T24 after 72 h (percentage of metabolically active cells was 80.18%, 71.73%, 63.31%, 46.32%, and 20.06% at concentrations of 50, 100, 250, 500, and 1000 µg/mL) (Figure 1a,b). On the other hand, V. agrestis HY showed only antiproliferative activity on the MDA-MB-231 cancer cell line after 48 h as IC50 28.43%, and the percentage of metabolically active cells was 51.40%, 55.49%, 61.35%, 54.50%, and 31.11% at dilutions of 10%, 20%, 30%, 40%, and 50% (Figure 1c).
V. anagalloides EO showed antiproliferative activity after 48 and 72 h on the MDA-MB-231 cancer cell line with IC50 180.1 µg/mL and 243.4 µg/mL (percentage of metabolically active cells was 67.19%, 61.90%, 52.41%, 24.43%, and 17.35% after 48 h, and 88.51%, 48.23%, 72.66%, 28.69%, 12.91% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) and T24 cancer cell line was IC50 389.9 µg/mL and 158.1 µg/mL (percentage of metabolically active cells was 90.46%, 89.34%, 73.31%, 29.75%, and 21.83% after 48 h, and 80.67%, 69.20%, 33.35%, 15.01%, and 15.20% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) (Figure 2a,b). HY of V. anagalloides showed antiproliferative activity of 19.82% on MDA-MB-231 only after 48 h (percentage of metabolically active cells was 50.69%, 56.39%, 50.87% 27.69%, and 30.43% at dilutions of 10%, 20%, 30%, 40%, and 50%) and 33.70%, 26.96%, and 31.76% after 24, 48, and 72 h on T24 cancer cell lines (percentage of metabolically active cells was 79.91%, 65.66%, 57.93%, 39.38%, and 36.73% after 24 h, and 78.61%, 74.16%, 55.49%, 28.44%, and 18.85% after 48 h, and 88.45%, 79.50%, 66.72%, 24.10%, and 16.10% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50%) (Figure 2c,d).
Species V. austriaca ssp. jacquinii EO showed antiproliferative activity only after 72 h on both mentioned cancer cell lines as IC50 816.0 µg/mL on MDA-MB-231 (percentage of metabolically active cells was 90.08%, 59.77%, 69.73%, 65.67%, and 58.83% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) and 617.1 µg/mL on T24 (percentage of metabolically active cells was 81.67%, 72.96%, 76.37%, 65.60%, and 71.11% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) (Figure 3a,b). In general, V. austriaca ssp. jacquinii HY showed better antiproliferative effect, especially on the T24 cancer cell line, than EO (percentage of metabolically active cells was 82.43%, 58.03%, 56.56%, 59.46%, and 46.73% after 48 h at dilutions of 10%, 20%, 30%, 40%, and 50%). After 48 h on MDA-MB-231, it showed an antiproliferative effect of 42.05% but pointed to the T24 cell line antiproliferative activity of 33.65%, 26.72%, and 23.58% (percentage of metabolically active cells was 79.82%, 71.84%, 53.61%, 40.01%, and 34.44% after 24 h, and 94.35%, 78.07%, 47.72%, 19.14%, and 14.91% after 48 h, and 84.10%, 71.34%, 50.04%, 18.61%, and 12.06% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50%) (Figure 3c,d).
V. beccabunga HY showed only antiproliferative effect on T24 cancer cell line, IC50 36.05%, 29.64%, and 39.29% after 24, 48, and 72 h, and metabolically active cells are presented in Figure 4 (percentage of metabolically active cells was 59.06%, 67.99%, 72.69%, 42.76%, and 38.17% after 24 h, and 72.24%, 75.76%, 62.95%, 35.65%, and 15.78% after 48 h, and 86.11%, 79.07%, 77.84%, 40.19%, and 16.62% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50%). The EO isolated from V. beccabunga did not show any antiproliferative effect.
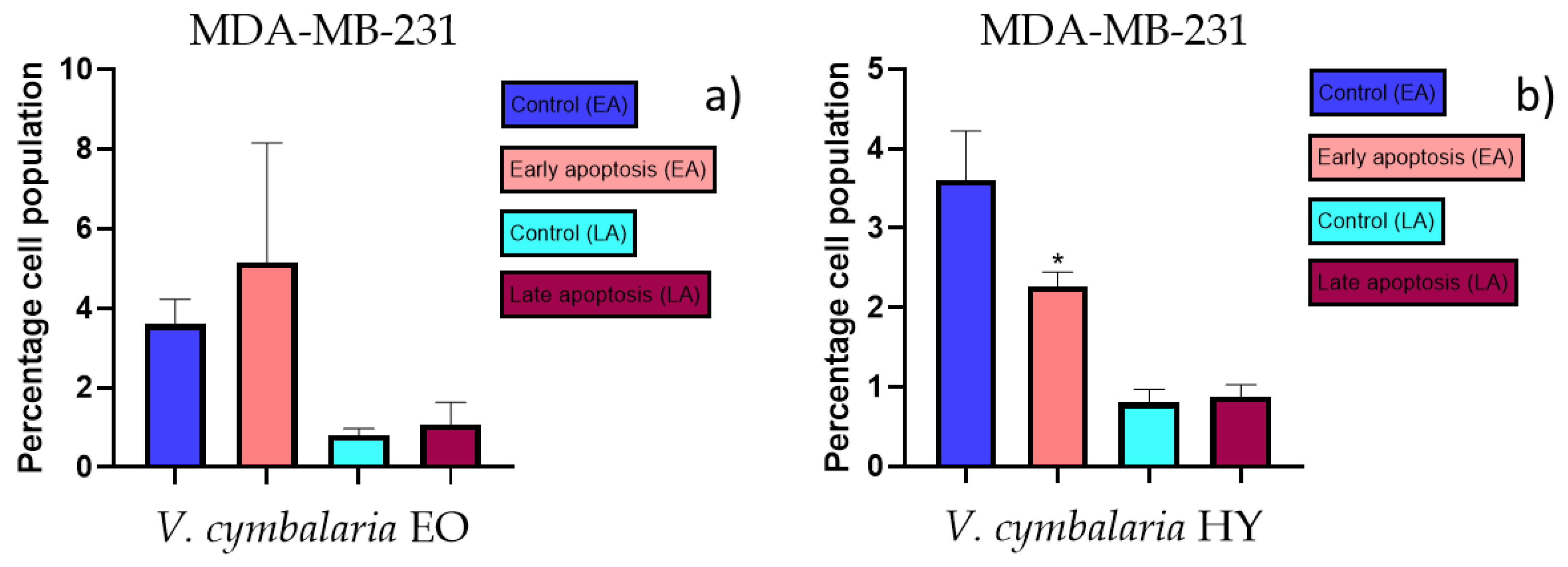
EO of V. cymbalaria showed antiproliferative effect on the MDA-MB-231 cancer cell line (IC50 249.9 µg/mL and 841.3 µg/mL after 48 and 72 h) (percentage of metabolically active cells was 56.72%, 58.29%, 44.57%, 50.92%, and 43.89% after 48 h, and 70.26%, 80.02%, 64.44%, 61.44%, and 64.63% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) compared to V. cymbalaria EO on the T24 cancer cell line (IC50 970.4 µg/mL after 72 h) (percentage of metabolically active cells was 83.52%, 83.19%, 72.82%, 68.86%, and 55.73% after 72 h at concentrations of 50, 100, 250, 500, and 1000 µg/mL) (Figure 5a,b). HY of V. cymbalaria showed better antiproliferative effect than EO on MDA-MB-231 and T24 cancer cell lines as IC50 17.41% and 49.63% on MDA-MB-231 after 48 and 72 h (percentage of metabolically active cells was 53.70%, 57.56%, 35.80%, 30.35%, and 24.51% after 48 h and 85.27%, 81.08%, 80.30%, 51.20%, and 31.54% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50% and IC50 28.30%, 18.64%, and 22.28% on T24 after 24, 48 and 72 h, respectively) (percentage of metabolically active cells was 72.07%, 60.68%, 45.98%, 42.44%, and 36.68% after 24 h, and 71.44%, 64.76%, 32.34%, 20.62%, and 18.42% after 48 h, and 86.04%, 70.59%, 33.96%, 22.82%, and 16.76% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50%) (Figure 5c,d).
It is interesting that the V. officinalis EO did not show antiproliferative activity at all four tested times (4, 24, 48, and 72 h), while V. officinalis HY showed excellent antiproliferative activity on MDA-MB-231 and T24 cancer cell lines. V. officinalis HY showed on MDA-MB-231 an antiproliferative effect of the cells after 48 and 72 h (IC50 34.28% and 25.44%, respectively) (percentage of metabolically active cells was 88.22%, 87.17%, 50.25%, 37.40%, and 21.88% after 48 h, and 79.86%, 71.54%, 54.28%, 26.19%, and 13.43% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50%) and after 24, 48, and 72 h on the T24 cancer cell line as IC50 21.83%, 13.41%, and 15.22% (percentage of metabolically active cells was 69.27%, 60.08%, 44.52%, 30.75%, and 22.55% after 24 h, and 67.25%, 50.52%, 26.40%, 13.23%, and 10.74% after 48 h, and 80.09%, 57.25%, 21.55%, 12.12%, and 10.04% after 72 h at dilutions of 10%, 20%, 30%, 40%, and 50%) (Figure 6a,b).
2.3. Apoptotic Activity
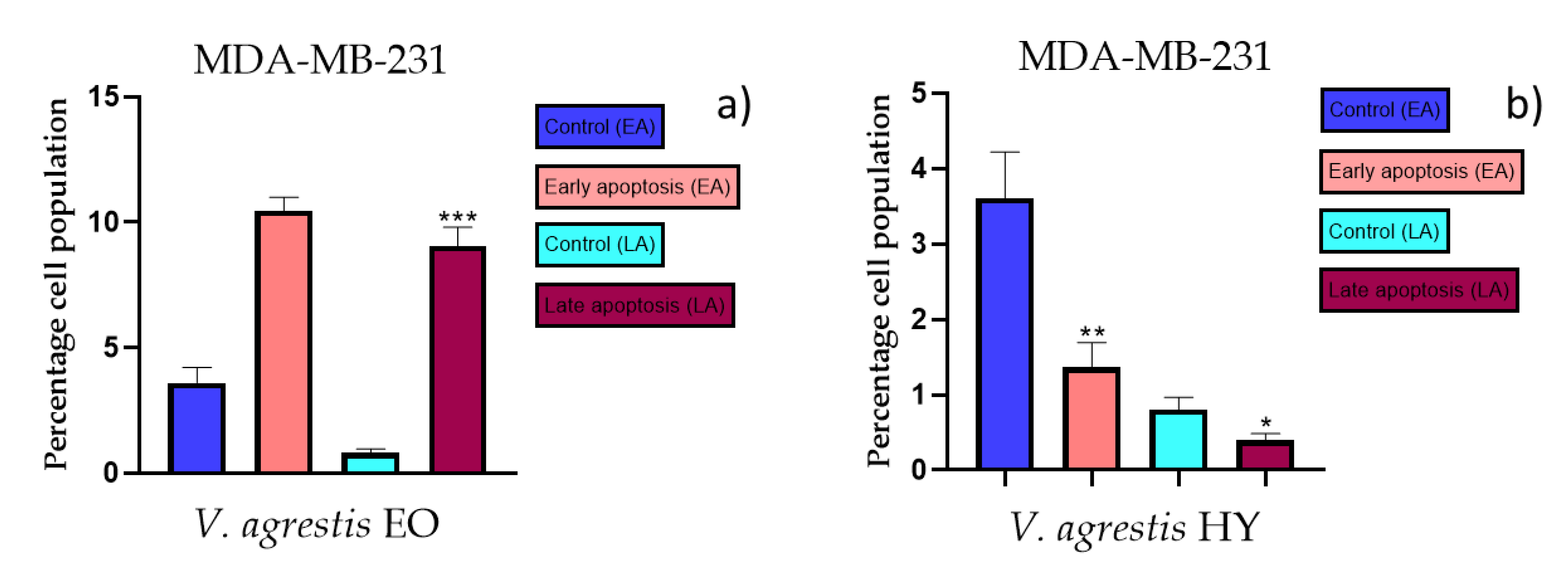
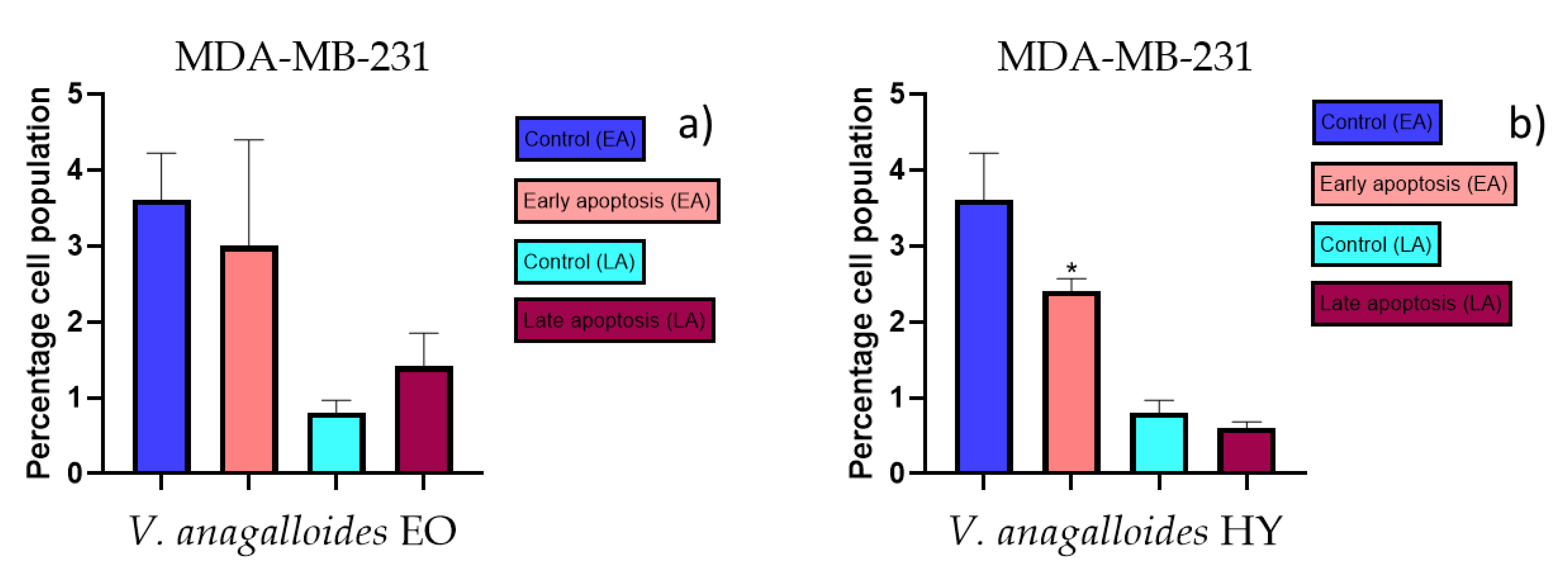
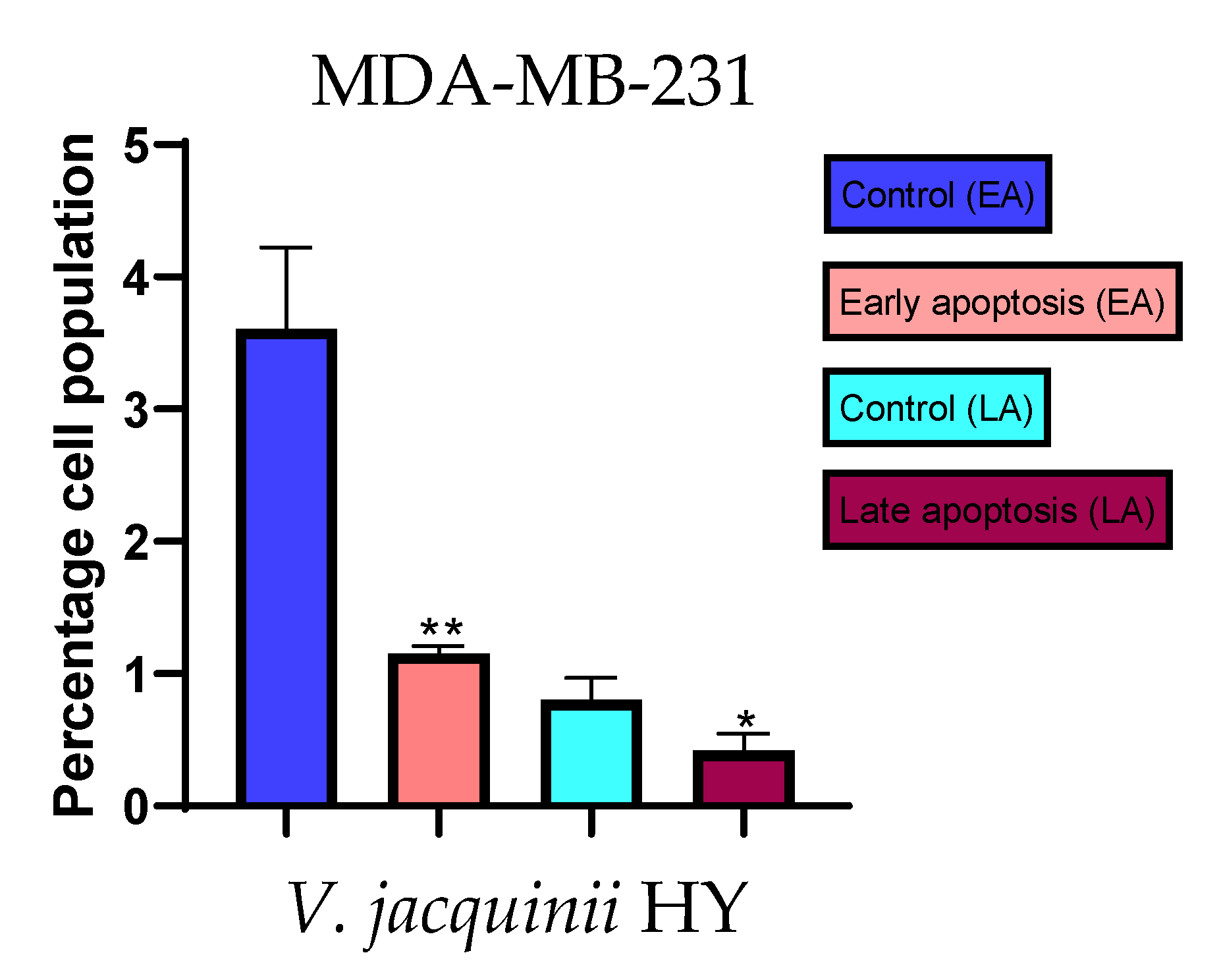
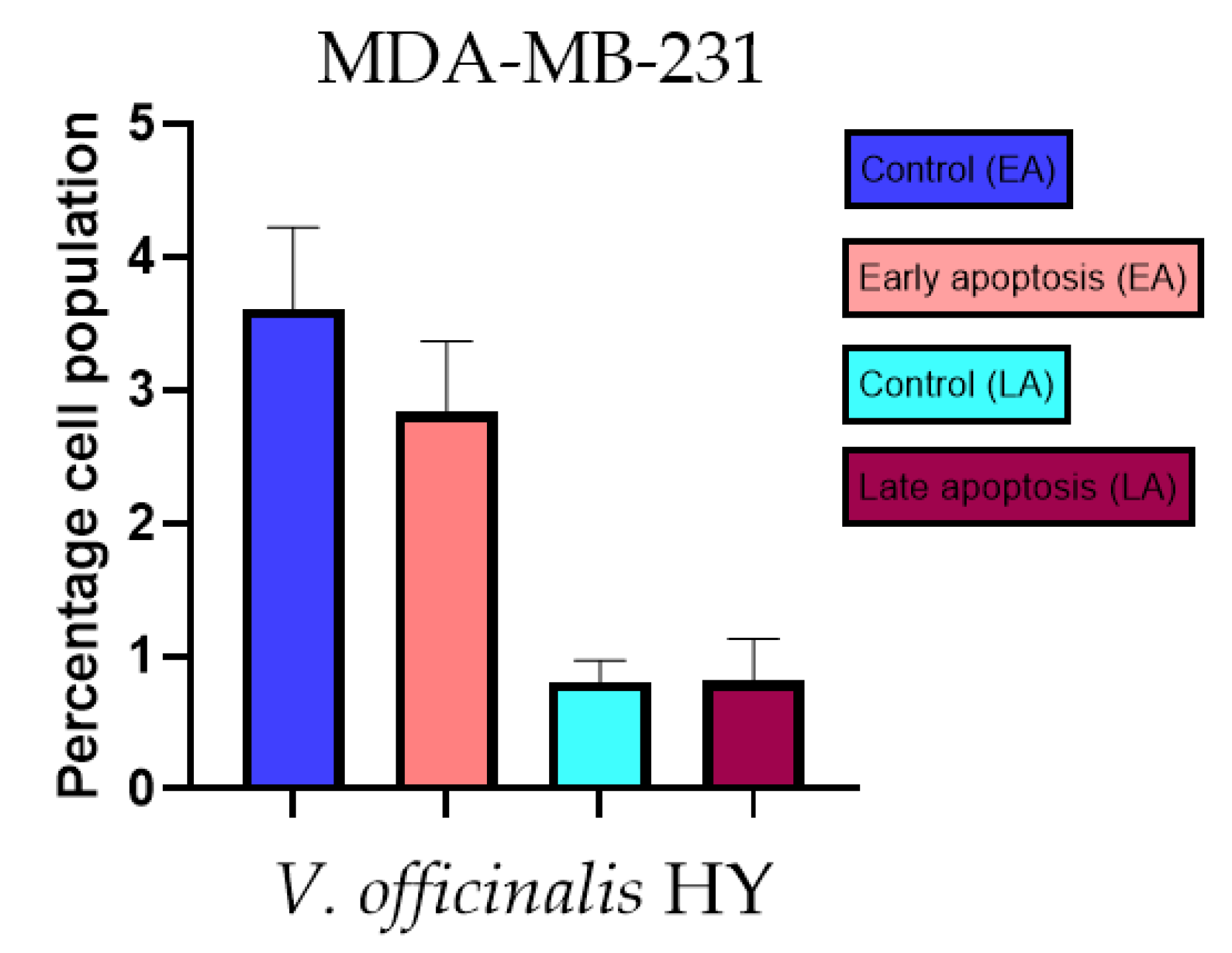
The apoptotic activity of essential oils (EOs) and hydrosols (HYs) of some Veronica species that showed antiproliferative activity after 48 h was determined on two cancer cell lines: breast cancer cell line MDA-MB-231 and bladder cancer cell line T24. Results are expressed as the distinction between early (Annexin-V+/PI−) and late (Annexin-V+/PI+) apoptotic cells. All results are expressed as mean ± SD (n = 3). V. agrestis EO showed apoptotic activity on MDA-MB-231 of 10.47 ± 0.53% and 9.06 ± 0.74%, while V. agrestis HY showed 1.37 ± 0.33% and 0.40 ± 0.09% in early apoptosis and late apoptosis (Figure 7a,b), respectively. On the MDA-MB-231 cancer cell line, V. anagalloides EO showed apoptotic activity of 5.15 ± 2.99% and 1.07 ± 0.56%, while V. anagalloides HY showed on MDA-MB-231 2.41 ± 0.16 and 0.6 ± 0.08% in early apoptosis and late apoptosis (Figure 8a,b), respectively. V. austriaca ssp. jacquinii HY showed 1.15 ± 0.06% and 0.42 ± 0.13% in early apoptosis and late apoptosis, respectively, on the MDA-MB-231 cancer cell line (Figure 9). V. cymbalaria EO showed apoptotic activity on MDA-MB-231 of 5.15 ± 2.99% and 1.07 ± 0.56% in early apoptosis and late apoptosis, respectively (Figure 10a). HY of V. cymbalaria showed apoptotic activity of 2.26 ± 0.19% and 0.88 ± 0.15%, respectively (Figure 10b). V. officinalis HY showed 2.83 ± 0.53% and 0.81 ± 0.32% in early apoptosis and late apoptosis (Figure 11), respectively. Control on MDA-MB-231 showed 3.61 ± 0.62% and 0.80 ± 0.17% in early/late apoptosis, respectively.
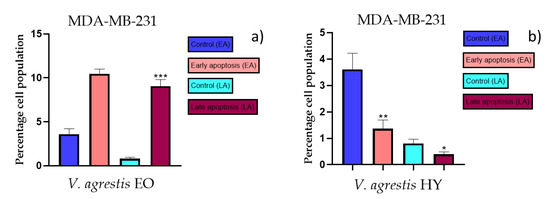
Figure 7.
Apoptotic activity of V. agrestis EO and V. agrestis HY on the MDA-MB-231 cancer cell line (a,b). The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD) with the significance set at * p < 0.05, ** p < 0.01, ***p < 0.001.
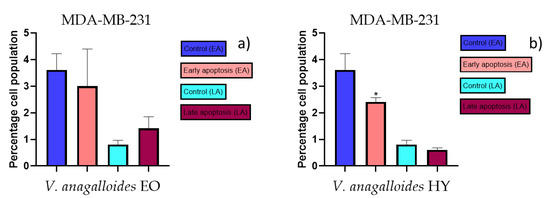
Figure 8.
Apoptotic activity of V. anagalloides EO and V. anagalloides HY on the MDA-MB-231 cancer cell line (a,b). The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD) with the significance set at * p < 0.05.
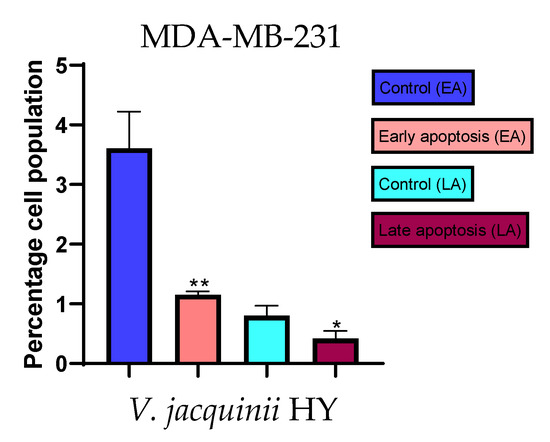
Figure 9.
Apoptotic activity of V. austriaca ssp. jacquinii HY on the MDA-MB-231 cancer cell line. The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD) with the significance set at * p < 0.05, ** p < 0.01.
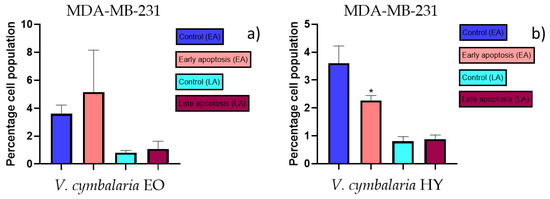
Figure 10.
Apoptotic activity of V. cymbalaria EO and V. cymbalaria HY on the MDA-MB-231 cancer cell line (a,b). The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD) with the significance set at * p < 0.05.
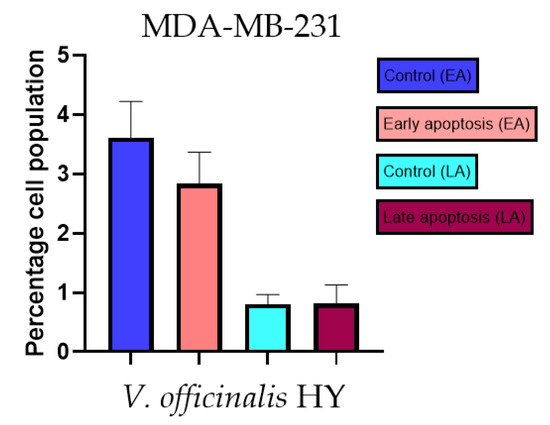
Figure 11.
Apoptotic activity of V. officinalis HY on the MDA-MB-231 cancer cell line. The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD).
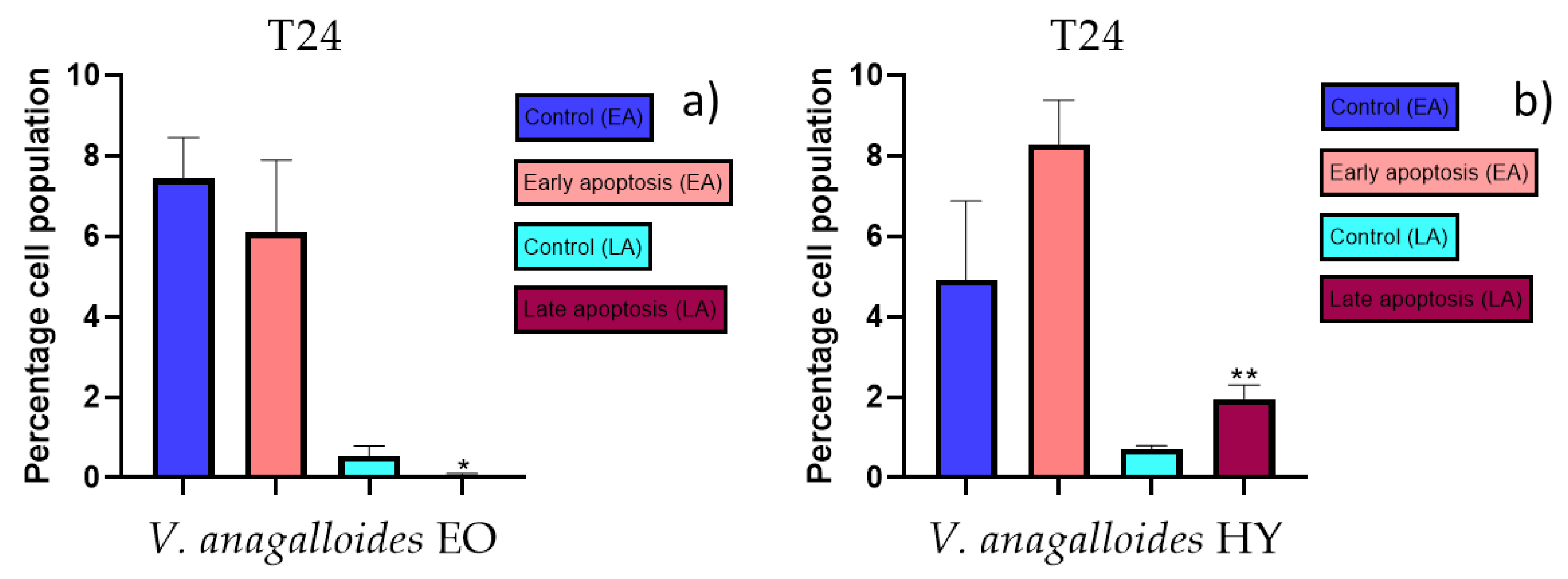
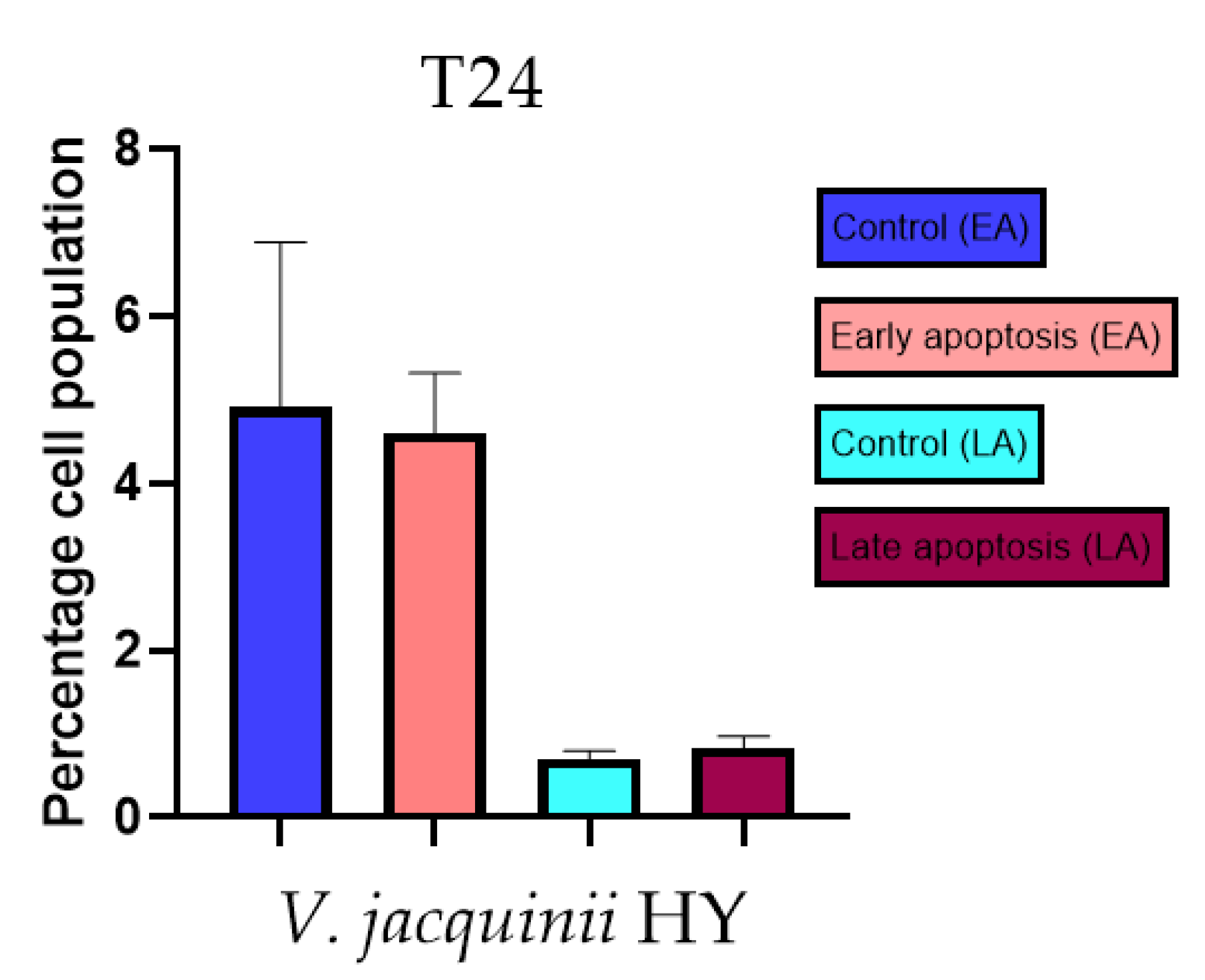
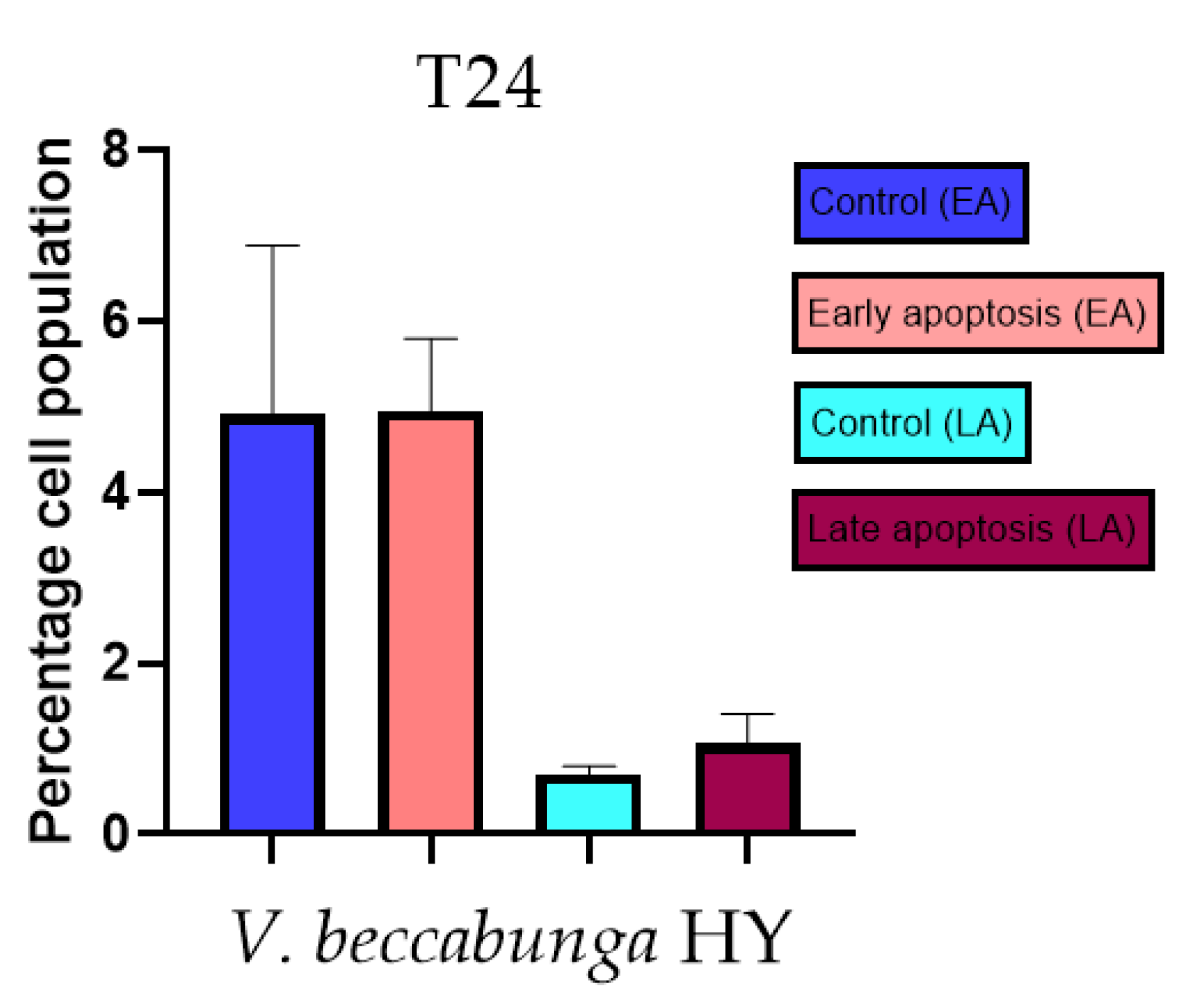
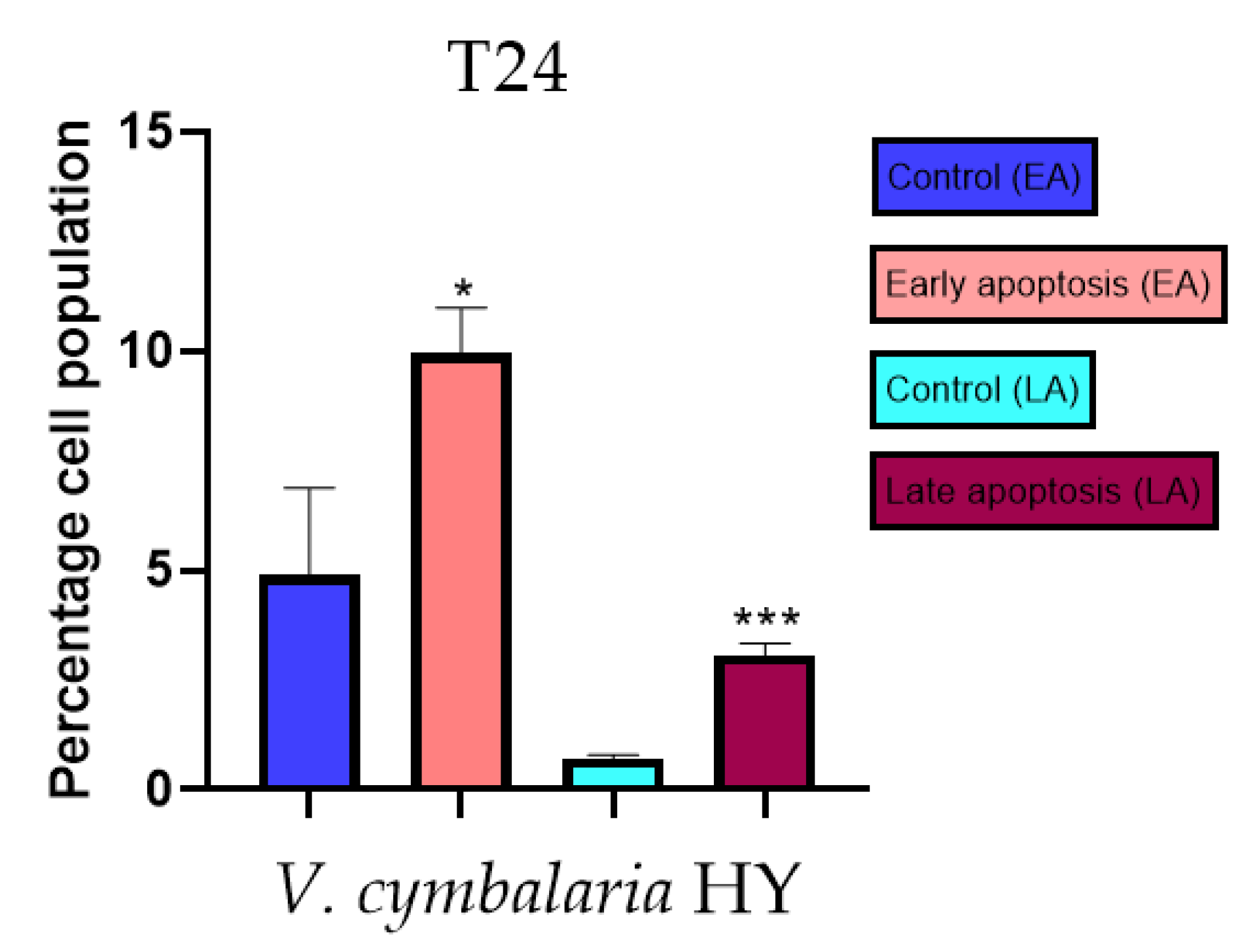
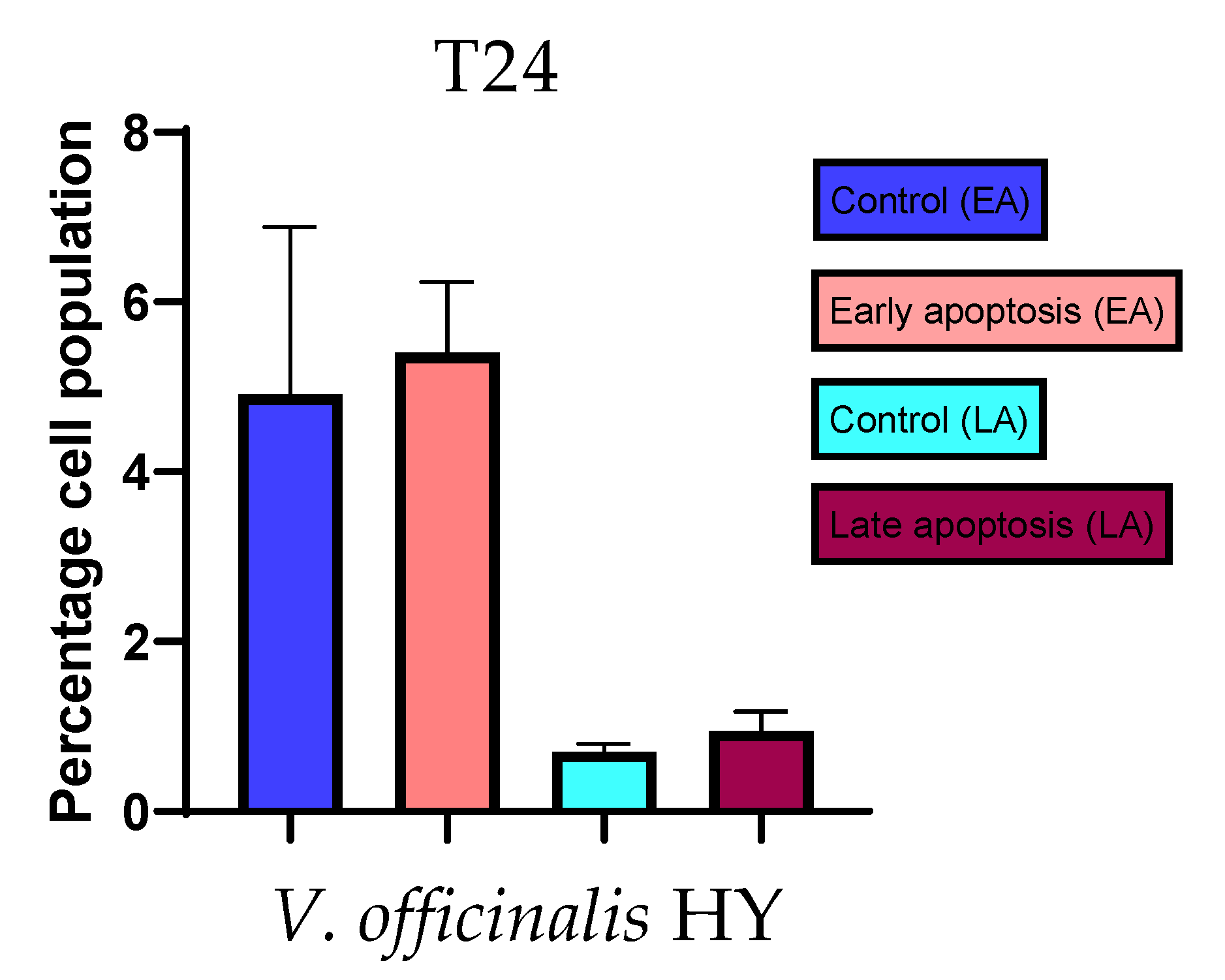
Apoptotic activity of V. anagalloides EO was 6.1 ± 1.79% and 0.05 ± 0.0.5% (Figure 12a), while for V. anagalloides, HY was 8.29 ± 1.09% and 1.95 ± 0.36% (Figure 12b for early and late apoptosis on T24 bladder cancer cell line, respectively. Apoptotic activity of V. austriaca ssp. jacquinii HY on the T24 cancer cell line was 4.60 ± 0.72% for early apoptosis and 0.84 ± 0.13% for late apoptosis (Figure 13). Apoptotic activity of V. beccabunga HY on the T24 cancer cell line was 4.95 ± 0.85% for early apoptosis and 1.06 ± 0.35% for late apoptosis (Figure 14). HY of V. cymbalaria showed apoptotic activity on the T24 cancer cell line of 9.95 ± 1.05% and 3.06 ± 0.28%, respectively (Figure 15). V. officinalis HY showed apoptotic activity on the T24 cancer cell line of 5.41 ± 0.83% and 0.95 ± 0.22% for early and late apoptosis, respectively (Figure 16). Control for EO was 7.45 ± 1.01% and 0.54 ± 0.25%, and for HYs on the T24 cancer cell line was 4.91 ± 1.97% and 0.70 ± 0.09% in early apoptosis/late apoptosis, respectively.
Figure 12.
Apoptotic activity of V. anagalloides EO and V. anagalloides HY on the T24 cancer cell (a,b). The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD) with the significance set at * p < 0.05, ** p < 0.01.
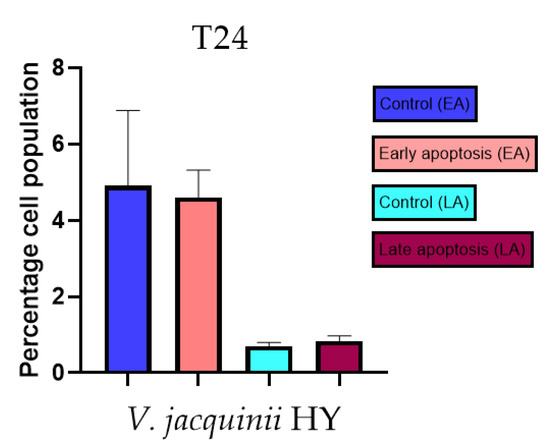
Figure 13.
Apoptotic activity of V. austriaca ssp. jacquinii HY on the T24 cancer cell line. The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD).
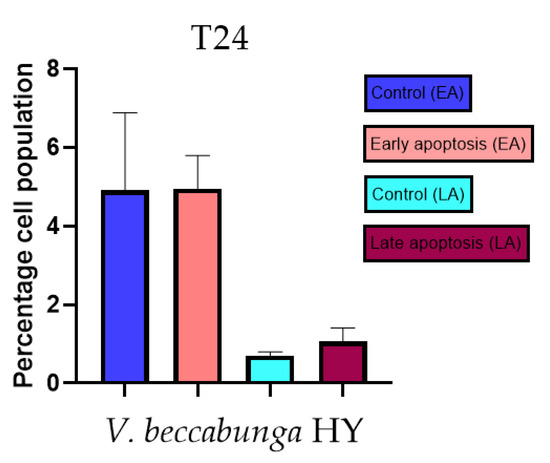
Figure 14.
Apoptotic activity of V. beccabunga HY on the T24 cancer cell line. The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD).
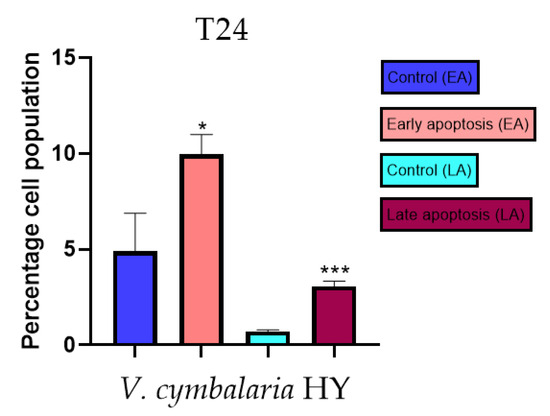
Figure 15.
Apoptotic activity of V. cymbalaria HY on the T24 cancer cell line. The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD) with the significance set at * p < 0.05, ***p < 0.001.
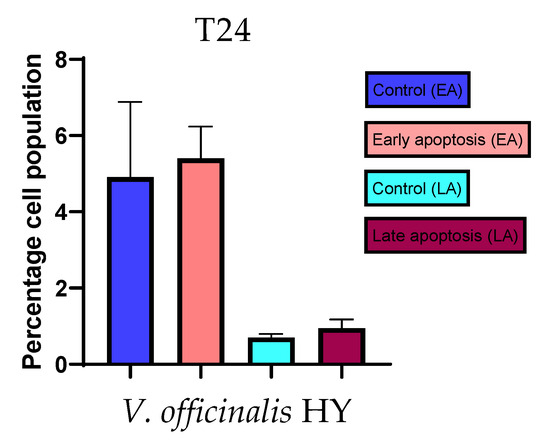
Figure 16.
Apoptotic activity of V. officinalis HY on the T24 cancer cell line. The percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD).
3. Discussion
In this paper, the antiproliferative and apoptotic activities of free volatile compounds (FVCs) of six Croatian Veronica species, V. agrestis, V. anagalloides, V. austriaca ssp. jacquinii, V. beccabunga, V. cymbalaria, and V. officinalis, were investigated. The extraction of FVCs from all plant samples was performed by microwave-assisted extraction (MAE). Twelve samples were obtained—two samples for each species (essential oil (EO) and hydrosol (HY)). All samples were analyzed by gas chromatography–mass spectrometry (GC-MS), and the obtained data are presented in Table 2 and Table 3. The plant material of the mentioned Veronica species was collected in 2022 (Table 1).
In this study, FVCs isolated from the species V. agrestis were analyzed for the first time, and phytol was the dominant component in the fraction EO with 56.57% (Table 2). The fraction HY was dominated by caryophyllene oxide (14.01%), (E)-β-damascenone (12.42%), benzene acetaldehyde (11.56%), and β-ionone (10.32%) (Table 3).
The FVCs of the other five Veronica species studied in this paper were compared with previously published data [2,7,28]. In the composition of the EO extract of V. anagalloides, the dominant compounds are hexahydrofarnesyl acetone (16.17%) and β-ionone (13.13%). Comparing the composition of the oil components of this species with the data previously published in the article by Dunkić et al. [2], we note a similarity in the concentration value of hexahydrofarnesyl acetone. In that research, the composition of oil components obtained by classical (Clevenger apparatus, HD) and modern hydrodistillation (MAE) was compared, so the value of hexahydrofarnesyl acetone in the EO of V. anagalloides was 14.33% for HD and 19.12% for MAE [2]. Moreover, in Nazlić et al. article [7], a much lower β-ionone relative percentage was found in this sample obtained by MAE compared to this study: only 4.22%.
In the composition of V. austriaca ssp. jacquinii EO, hexadecanoic acid (27.66%) and phytol (13.02%) were the predominant compounds (Table 2). Hexadecanoic acid was also the most abundant compound in a previously published study of V. austriaca ssp. jacquinii in MAE extract (22.17%) [2]. Phytol and hexadecanoic acid are also the predominant EO constituents in the species V. beccabunga, with 28.08% and 17.06%, respectively. The dominant compound in V. beccabunga collected in 2021 is oxygenated diterpene phytol with 34.54% for MAE [2], and the biggest difference in the composition of EO compounds in this line comparing 2021 and 2022 is the identification of piperitone: 29.28% in 2021 [2] and only 2.46% in this research.
Phytol was identified in the EO of V. cymbalaria with 3.71% in 2021 and 16.66% in 2022 (Table 2), while caryophyllene oxide with 23.83% was identified as less compared to in 2021 when 32.72% was identified [2]. Specifically, in the EO composition of V. officinalis, heptacosane is the most abundant compound (17.21%) (Table 2), while in the previous year, it was only identified as 5.52% [2].
The most significant differences in the proportions of the following compounds in the HY of V. anagalloides species are the following: in the extracts from 2022, the compound β-ionone was 16.53% and (E)-β-damascenone was 11.55% (Table 3), while in the material from 2021, these compounds were identified in significantly lower proportions, β-ionone with 6.07% and (E)-β-damascenone with 1.52% [7]. The reported differences in the EOs’ relative percentages of some compounds for the species, V. anagalloides, V. austriaca ssp. jacquinii, V. cymbalaria, and V. officinalis, could also be related to different locations of material collection, not just the difference in the year of collection.
Comparing the compositions of HYs from two years, the biggest discrepancy is the identification of methyl eugenol, which is 37.01% in the HY of V. austriaca ssp. jacquinii from 2022, while it was not identified at all in the year before; moreover, not a single phenolic component characteristic of species of the genus Veronica was identified [28]. The composition of V. beccabunga HY is dominated by α-pinene and piperitone at 17.11% and 19.54% (Table 3), respectively, and the differences compared with published compositions are as follows: piperitone was identified at 79.86% and α-pinene was not identified [28].
The HY of V. cymbalaria in this study is rich in the phenolic constituents methyl eugenol (38.61%) and (Z)-methyl isoeugenol (31.32%), while oxygenated sesquiterpene caryophyllene oxide is 6.26% (Table 3). Caryophyllene oxide is the most abundant compound in the previously published manuscript for the species V. cymbalaria HY with 37.12% [7]. The total phenolic components in V. cymbalaria in the research conducted on the material from 2021 are represented by only 5.51%, and the most abundant is thymol with 3.83%; methyl eugenol was not identified, and (Z)-methyl isoeugenol was identified with less than 1% [7]. Methyl eugenol is the most represented phenolic component in HY of V. officinalis with 22.01%, while in the previously published composition, the hydrosols of phenolic compounds was the only one represented by 2-methoxy-4-vinylphenol with 11.12%. The compounds (E)-β-damascenone and β-ionone for V. officinalis were identified in a similar percentage in both years compared (Table 3) [7]. From the comparison of the phytochemical profile of the Veronica species studied, it is evident that the composition of FVCs may vary within the same plant species, which is influenced by many factors such as abiotic and biotic factors, postharvest treatment, extraction methods, and storage conditions of the extract. Among the abiotic factors, microclimatic influence on plant growth is particularly important [29]. This is precisely why it is important to determine the chemical composition of any extract before beginning research into its biological activity. So far, almost 300 natural compounds from species of the genus Veronica have been identified and their biological activity studied [12,14], confirming the importance of this genus as medicinal plants.
Only a few studies have shown that extracts that contain terpenoids can behave synergistically with conventional chemotherapy. In spite of the encouraging results obtained over more than 35 years on the beneficial effects of these components, only a few clinical studies on humans have been conducted in the field. The only existing studies were conducted on limonene and its derivatives with some promising results [25].
So far, only a few Veronica species have been studied for their cytotoxic activity in vitro and in vivo, mostly methanolic and aqueous extracts of various Veronica species. Also, in our review, just two studies regarding antiproliferative activity of EOs and HYs were conducted [19,30]. In the present study, the antiproliferative activity of the EOs and HYs of the above-mentioned six Veronica species was tested on two cancer cell lines (MDA-MB-231 and T24). V. agrestis EO showed significant antiproliferative activity on MDA-MB-231 after 48 and 72 h (572.1 µg/mL and 586.9 µg/mL), and 340.6 µg/mL on T24 after 72 h. According to Nazlić et al. [30], similar activity was shown for V. saturejoides (Kamešnica Sample) on the HeLa, HCT116, and U2OS cell lines. EO of V. anagalloides showed the highest antiproliferative activity among all tested EOs (on the MDA-MB-231 cancer cell line, it was 180.1 µg/mL and 243.4 µg/mL after 48 and 72 h, respectively, and on the T24 cancer cell line, it was 389.9 µg/mL and 158.1 µg/mL after 48 and 72 h, respectively). Nazlić et al. [19] reported that V. austriaca ssp. jacquinii EO showed antiproliferative activity above 1000 µg/mL after 48 h on the tested cell lines, while in our present study, the EO of this species showed slightly better results, but after 72 h on both mentioned cancer cell lines (816.0 µg/mL on MDA-MB-231 and 617.1 µg/mL on T24). EO of V. cymbalaria showed an antiproliferative effect on the MDA-MB-231 cancer cell line (IC50 249.9 µg/mL and 841.3 µg/mL after 48 and 72 h, respectively), while V. cymbalaria EO showed lower activity on the T24 cancer cell line (IC50 970.4 µg/mL after 72 h). According to Nazlić et al. [30], V. officinalis EO showed significant antiproliferative activity on the HCT116 (IC5O > 500 µg/mL), HeLa, and U2OS cancer cell lines (IC5O > 1000 µg/mL). That could be a reason why the EOs of V. officinalis and V. beccabunga did not show antiproliferative activity in this study, as the intervals of tested concentrations were 50–1000 µg/mL. Generally, V. agrestis EO, V. anagalloides EO, and V. cymbalaria EO showed the highest antiproliferative activity of all tested Veronica species’ EOs. This could be due to the higher relative percentage of phytol, (E)-caryophyllene, and caryophyllene oxide in these species. For these compounds, significant antiproliferative and/or apoptotic activity was reported in recent studies [31,32,33].
HYs of V. anagalloides, V. austriaca ssp. jacquinii, and V. cymbalaria showed antiproliferative activity on MDA-MB-231 only after 48 h (17–43%). V. beccabunga HY did not show an antiproliferative effect at the tested dilutions and times on the MDA-MB-231 cancer cell line. HYs of V. anagalloides, V. austriaca ssp. jacquinii, V. cymbalaria, and V. beccabunga showed high antiproliferative activity (18–40%), especially on the T24 cancer cell line after 24, 48, and 72 h. In this study, HY of V. officinalis was the most effective in antiproliferative activity on the MDA-MB-231 (after 48 and 72 h, 34.28% and 25.44%, respectively) and T24 cell lines (after 24, 48, and 72 h on the T24 cancer cell line, 21.83% 13.41%, and 15. 22%, respectively). These results are in accordance with previous results on HYs of V. officinalis on HeLa, HCT116, and U2OS [30]. HY of V. agrestis did not show antiproliferative activity on the T24 cancer cell line. The reason for its biological inactivity potentially lies in the smaller amount of plant material from which the HY was obtained. The reason for the excellent antiproliferative activity of V. officinalis HY could be the presence of methyl eugenol, terpinen-4-ol, linalool, and β-ionone in higher relative percentages than in other tested HYs. For these compounds, significant antiproliferative and/or apoptotic activity was reported in recent studies [34,35,36,37,38,39]. Also, β-ionone was present in a higher percentage in V. anagalloides and V. beccabunga HYs, which could be the explanation for the great antiproliferative activity shown for these extracts, especially on the T24 cancer cell line. V. beccabunga HY also contained α-pinene in a high percentage, which could be explanation for the great antiproliferative activity on the T24 cancer cell line. There are many more studies of antiproliferative activity on various phenolic extracts of the Veronica species than on EOs and HYs. Harput et al. [22] reported that V. hederifolia, V. cymbalaria, V. persica, V. pectinata var. glandulosa, and V. polita methanolic extracts showed cytotoxic, anti-inflammatory, and radical-scavenging activities. V. americana methanolic extract showed antiproliferative activity on two cancer cell lines, HF-6 (colon cancer) and PC-3 (prostate cancer) [40]. According to Saracoglu and Harput [41], some iridoid glucosides of V. persica Poir, V. anagallis-aquatica, and V. thymoides subsp. pseudocinerea showed cytostatic and cytotoxic activities on human and murine cancer cell lines, with verminoside being the most cytotoxic component. Another study on V. cuneifolia subsp. cuneifolia and V. cymbalaria aqueous extracts showed moderate antiproliferative activity on Hep-2 (human epidermoid carcinoma), RD (human rhabdomyosarcoma), and L-20B (transgenic murine L-cells) [42].
Induction of apoptosis is extremely desirable for cancer control [43]. As far as the authors know, there are no previous studies of EOs and HYs from the Veronica species that have been tested for apoptotic activity. In general, EOs have been shown to induce intrinsic (mitochondria-dependent) and extrinsic (or cell death receptor-dependent) pathways of apoptosis. A study on the antitumor properties of caffeine isolated from the EO of Piper cernuum in melanoma cells showed that this component could induce apoptosis by activating the caspase-3 pathway, as well as by activating endoplasmic reticulum (ER) stress signaling. Another study focused on evaluating the mechanism of the action of carvacrol found in oregano and thyme EO. Carvacrol induced apoptosis in the MDA-MB-231 breast cancer cell line via mitochondrial membrane permeabilization resulted in the release of cytochrome C, and the induction of caspases was indicated by DNA cleavage and fragmentation [24]. According to Feng et al. [44], flavonoids extracted from Veronica sibirica (Vtfs) induced dose-dependent apoptosis in MCF-7 breast cancer cells with IC50 of 42 µg/mL. In our present study, apoptotic activity was tested on the previously mentioned Veronica species on MDA-MB-231 and the T24 cancer cell line. Mastelić et al. [45] reported paclitaxel in a concentration of 40 nM (36.24 µg/mL) for apoptotic activity on the MDA-MB-231 cancer cell line. According to Mastelić et al. [45], apoptotic activity of paclitaxel was 7.80% and for control, 1.58%, in apoptosis for the MDA-MB-231 cancer cell line. According to Bilušić et al. [46], cisplatin was used as a positive control against the T24 and A549 cancer cell lines for apoptotic activity in a concentration of 50 µg/mL. Cisplatin showed 1.36 ± 0.82% and 0.86 ± 0.14% in early/late apoptosis, respectively, on the T24 cancer cell line. In the present study, the best apoptotic activity of all tested EOs showed V. agrestis EO on the MDA-MB-231 cancer cell line (10.47 ± 0.53% of early apoptotic and 9.06 ± 0.74% of late apoptotic cells, comparing to control 3.61 ± 0.62% and 0.80 ± 0.17% of early/late apoptosis, respectively), and among the HYs, V. cymbalaria showed 9.95 ± 1.05% of early apoptotic and 3.06 ± 0.28% of late apoptotic cells, and V. anagalloides 8.29 ± 1.09% of early apoptotic and 1.95 ± 0.36% of late apoptotic cells, comparing to control (for EO was 7.45 ± 1.01% and 0.54 ± 0.25%, and for HYs was 4.91 ± 1.97% and 0.70 ± 0.09% of early/late apoptosis, respectively) on the T24 cancer cell line. To our knowledge, no other study involved testing apoptotic activity of the Veronica species’ EOs or HYs.
The results obtained in this research showed comparison of EO and HY composition from the above-mentioned Veronica species, and significant antiproliferative and apoptotic activities of tested extracts and their possible chemotherapeutic properties, which is why they deserve further investigation. Also, one of the most extensive and interesting fields of application of nanobiotechnology is medicine using plant extracts. Ahmadov et al. [47] reported the process of green synthesis by Scutellaria baicalensis extract, and the components present in plant extract were the most important in the formation and stabilization of silver nanoparticles. These bioactive components can be connected to the surface of the silver nanoparticles and function as nanodrugs. Therefore, future research should be focused on FVCs present in the Veronica species on antiproliferative and apoptotic activities, their possible encapsulation for in vivo studies, and as potential nanodrugs in medicine.
4. Materials and Methods
4.1. Preparation, Extraction, and Identification of Volatile Compounds from Six Veronica Species
4.1.1. Preparation of Plant Material from Six Veronica Species
All six Veronica species were collected during the flowering period in May and June 2022 at various locations in Croatia (Table 1). The voucher specimens were deposited in the herbarium of the Laboratory of Botany (HPMF-HR) of the Faculty of Science, University of Split, Croatia. All specimens were air dried in a single layer for 10 days and protected from direct sunlight.
4.1.2. Extraction of Volatile Compounds
Plant material (30–50 g) of each Veronica species studied (Table 1) was hydrodistilled by microwave-assisted extraction (MAE) using an ETHOS X device (Milestone, Italy). MAE was performed at atmospheric pressure for 30 min (extraction process started after 10 min) at 800 W (98 °C). All extracts consisted of two layers: a lipophilic layer (essential oil) and a water layer (hydrosol). The lipophilic layer was collected in a side tube with a pentane/diethyl ether trap (VWR, Radnor, PA, USA), dried over anhydrous sodium sulfate, and stored at −20 °C until analysis. The pentane/diethyl ether trap was used because of the ease of evaporation (in order to exclude their toxicity), determining the yield of essential oil, and the certainty that there will be no loss of thermolabile components. The water extracts were also collected, and 2 g of HY from each sample was placed in a glass bottle and sealed with a stopper. The sample thus prepared was placed in a water bath, and a solid phase micro-extraction (SPME) needle was injected through the septum of the bottle cap. The first part of the process took place at 40 °C for 20 min to allow the compounds to evaporate from the water. The SPME fiber is directly above the liquid sample, which is stirred during the next 20 min of the process. The volatile compounds settled on the resin SPME fiber. The prepared sample was injected into the gas chromatography (GC) inlet and left there for 20 min to ensure that all volatile compounds were reabsorbed by the SPME fiber into the injection liner.
4.1.3. Identification of Volatile Compounds
Chromatographic analyses were performed using a GC (model 3900; Varian Inc., Lake Forest, CA, USA) equipped with a flame ionization detector and a mass spectrometer (model 2100 T; Varian Inc., Lake Forest, CA, USA), a nonpolar capillary column VF-5 ms (30 m × 0.25 mm i.d., coating thickness 0.25 µm, Palo Alto, CA, USA), and a polar CP Wax 52 (30 m × 0.25 mm i.d., coating thickness 0.25 µm, Palo Alto, CA, USA). The chromatographic methods and conditions for hydrosol fraction analysis were the same as described in the article by Dunkić et al. [2]: the condition for the VF-5-ms column was a temperature of 60 °C (isothermal) for 3 min, which was then increased to 246 °C at a rate of 3 °C min−1 and maintained for 25 min (isothermal). The condition for the CP Wax 52 column was a temperature of 70 °C (isothermal) for 5 min, which was then increased to 240 °C at a rate of 3 °C min−1 and maintained for 25 min (isothermal). The injection volume was 2 µL, and the split ratio was 1:20. The MS conditions were as follows: ion source temperature, 200 °C; ionization voltage, 70 eV; mass scan range, 40–350 mass units. The individual peaks of all samples were identified by comparing their retention indices of n-alkanes with those of authentic samples and the studies [27,48] by comparison with our libraries from previous work and by comparison with other previously published material for the Veronica species [49,50,51]. Results are given as the mean of three analyses with standard deviation (n = 3 ± SD).
4.2. Cell Viability and Proliferation Were Determined by Measuring Cellular Metabolism Using MTT Assay
Cell viability and proliferation after treatment with essential oils (EOs) and hydrosols (HYs) of six different Veronica species (V. agrestis, V. anagalloides, V. austriaca ssp. jacquinii, V. beccabunga, V. cymbalaria, and V. officinalis) were performed against two human cancer cell lines, breast cancer cell line MDA-MB-231 and bladder cancer cell line T24, to determine which sample has the best antiproliferative activity. Stock solutions of EOs were prepared in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/mL. Cell lines MDA-MB-231 and T24 were grown in Dulbecco’s modified Eagle’s medium (DMEM, Euroclone, Milano, Italy) in a humidified incubator at 37 °C with 5% CO2. The DMEM medium contained 10% fetal bovine serum (FBS, Euroclone, Milano, Italy) and 1% antibiotics (penicillin and streptomycin, Euroclone, Milano, Italy) and was used as a negative control. An equal number of cells (1 × 104) were transferred into 96 wells and left overnight. The cells were then treated with EOs of the above-mentioned Veronica species at concentrations of 50, 100, 250, 500, and 1000 µg/mL, while HYs were tested at different dilutions (10%, 20%, 30%, 40%, and 50%) after MAE for 4, 24, 48, and 72 h. Cell viability and proliferation were determined by measuring cellular metabolism using an MTT assay. Yellow tetrazoline MTT (3-(4,5-dimethylthiazolid-2)-2,5-diphenyltetrazoline bromide) is reduced in metabolically active cells to the purple formazan. After 2 h, the medium with MTT was removed, and DMSO was added. The plates were incubated for 10 min at 37 °C with shaking. The absorbance was measured at 570 nm by a HiPo MPP-96 microplate photometer (Biosan, Riga, Latvia). All samples were run on three different plates in triplicate per plate. Solvent control was measured: the highest concentration of DMSO was adjusted to 1% (v/v) and did not show antiproliferative activity. According to Mastelić et al. [45], the antiproliferative activity of paclitaxel as positive control on MDA-MB-231 after 4, 24, 48, and 72 h in percentage of metabolically active cells was 82.04%, 83.85%, 49.21%, and 33.11%. According to Bilušić et al. [46], cisplatin was used as a positive control against T24 and A549 cancer cell lines for antiproliferative activity in a concentration of 0.05 mg/mL. Cisplatin showed antiproliferative activity after 4, 24, 48, and 72 h (percentages of metabolically active cells were 91.56%, 86.33%, 56.18%, and 52.32 for the T24 cancer cell line). The experiments and procedures are in accordance with ethical and safety guidelines. For statistical analyses, a t-test with unequal variances was performed using GraphPad Prism 9.0 statistical software (San Diego, CA, USA) with the significance set at * p < 0.05. The calculation of IC50 values was performed with GraphPad Prism software version 9.0 (San Diego, CA, USA), normalizing the data by three independent measurements of untreated controls.
4.3. Apoptotic Activity
An equal number of cells (1 × 104) were seeded in 6-well plates and treated with essential oils (EOs) and hydrosols (HYs) of V. agrestis, V. anagalloides, V. austriaca ssp. jacquinii, V. beccabunga, V. cymbalaria, and V. officinalis for 48 h and then analyzed for apoptosis to determine which sample has the best apoptotic activity. The antiproliferative activities of V. agrestis EO and HY on the MDA-MB-231 cancer cell line after 48 were IC50 572.1 µg/mL and 28.43%. V. anagalloides EO showed antiproliferative activity after 48 on the MDA-MB-231 and T24 cancer cell lines (IC50 180.1 µg/mL and 389.9 µg/mL, respectively). HY of V. anagalloides showed antiproliferative activity of 19.82% on MDA-MB-231 and of 26.96% after 48 h on T24 cancer cell lines. V. austriaca ssp. jacquinii HY showed better antiproliferative effect, especially on the T24 cancer cell line. After 48 h on MDA-MB-231, it showed antiproliferative effect of 42.05%, but it pointed to the T24 cell line antiproliferative activity of 26.72% after 48 h. V. austriaca ssp. jacquinii HY showed on MDA-MB-231 an antiproliferative effect of 42.05% and pointed to the T24 cell line an antiproliferative activity of 26.72% after 48 h. V. beccabunga HY showed an antiproliferative effect on the T24 cancer cell line of 29.64% after 48 h. EO of V. cymbalaria also showed antiproliferative activity on the MDA-MB-231 cancer cell line (IC50 249.9 µg/mL) after 48 h. HY of V. cymbalaria showed antiproliferative effect on the MDA-MB-231 and T24 cancer cell lines (17.41% and 18.64% after 48 h, respectively). V. officinalis HY showed on the MDA-MB-231 and T24 cancer cell lines significant antiproliferative effect after 48 h (34.28% and 13.41%, respectively). A combination of Annexin-V-FITC and propidium iodide staining allows the distinction between early (Annexin-V+/PI−) and late (Annexin-V+/PI+) apoptotic cells, necrotic cells, and live cells. After treatment with EOs and HYs, the cells were trypsinized, washed with PBS, and resuspended in 100 µL of the binding buffer containing 5 µL of Annexin-V-FITC and/or 10 µL of PI (FITC Annexin V Apoptosis Detection Kit with PI, BioLegend, San Diego, CA, USA). The cells were incubated for 15 min at room temperature in the dark and, thereafter, analyzed by flow cytometry (BD Accuri C6, BD Biosciences). Using the FlowLogic Software (Inivai, Mentone, VIC, Australia), the percentages of apoptotic cells (Annexin-V-positive cells) were determined and presented as mean ± standard deviation (SD). For statistical analyses of the apoptosis rate, a t-test with unequal variances, one-way ANOVA followed by a post hoc Tukey test or Kruskal–Wallis test, followed by Dunn’s post hoc test, were performed using GraphPad Prism 7.0 statistical software (San Diego, CA, USA), with the significance set at p < 0.05, p < 0.01 and p < 0.001.
5. Conclusions
Free volatile compounds (FVCs) present in essential oils (EOs) and hydrosols (HYs) of six Veronica species were obtained by microwave-assisted extraction (MAE). The identification of free volatile compounds (FVC) from six Veronica species was analyzed using gas chromatography–mass spectrometry, and the composition of the compounds was compared. The components identified in the EO extracts of all six Veronica species studied are linalool, (E)-caryophyllene, allo-aromadendrene, caryophyllene oxide, phytol, benzene acetaldehyde, β-ionone, hexadecanoic acid, docosane, tricosane, tetracosane, and octocosane. The constituents terpinen-4-ol, (E)-caryophyllene, allo-aromadendrene, caryophyllene oxide, benzaldehyde, benzene acetaldehyde, and β-ionone were identified in all studied HY extracts. These volatile compounds belong to the specialized metabolites, and it is important to know their composition for further research on the pharmaceutical activity of the Veronica species. Also, the antiproliferative and apoptotic effects of essential oils and hydrosols were compared. Generally, hydrosols showed better antiproliferative activity, especially on the T24 cancer cell line. Among the tested EOs, V. anagalloides exerted the best antiproliferative effect on both the MDA-MB-231 and T24 cancer cell lines, while among the tested HYs, V. officinalis HY showed the best antiproliferative activity. The best apoptotic effect of the EOs was shown by V. agrestis EO on the MDA-MB-231 cancer cell line, and among the HYs, V. cymbalaria and V. anagalloides showed the best proapoptotic activities on the T24 cancer cell line. Future research should be focused on the discovery of drugs based on natural plant extracts, which implies testing the combinatorial effect of compounds that show biological activity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants12183244/s1, Figure S1: Antiproliferative activity of V. agrestis EO on MDA-MB-231 cancer cell line; Figure S2. Antiproliferative activity of V. agrestis EO on T24 cancer cell line; Figure S3. Antiproliferative activity of V. agrestis HY on MDA-MB-231 cancer cell line; Figure S4. Antiproliferative activity of V. agrestis HY on T24 cancer cell line; Figure S5. Antiproliferative activity of V. anagalloides EO on MDA-MB-231 cancer cell line; Figure S6. Antiproliferative activity of V. anagalloides EO on T24 cancer cell line; Figure S7. Antiproliferative activity of V. anagalloides HY on MDA-MB-231 cancer cell lines; Figure S8. Antiproliferative activity of V. anagalloides HY on T24 cancer cell line; Figure S9. Antiproliferative activity of V. austriaca ssp. jacquini EO on MDA-MB-231 cancer cell line; Figure S10. Antiproliferative activity of V. austriaca ssp. jacquini EO on T24 cancer cell line; Figure S11. Antiproliferative activity of V. austriaca ssp. jacquini HY on MDA-MB-231 cancer cell line; Figure S12. Antiproliferative activity of V. austriaca ssp. jacquini HY on T24 cancer cell line; Figure S13. Antiproliferative activity of V. beccabunga EO on MDA-MB-231 cancer cell line; Figure S14. Antiproliferative activity of V. beccabunga EO on T24 cancer cell line; Figure S15. Antiproliferative activity of V. beccabunga HY on MDA-MB-231 cancer cell line; Figure S16. Antiproliferative activity of V. beccabunga HY on T24 cancer cell line; Figure S17. Antiproliferative activity of V. cymbalaria EO on MDA-MB-231 cancer cell line; Figure S18. Antiproliferative activity of V. cymbalaria EO on T24 cancer cell line; Figure S19. Antiproliferative activity of V. cymbalaria HY on MDA-MB-231 cancer cell line; Figure S20. Antiproliferative activity of V. cymbalaria HY on T24 cancer cell line; Figure S21. Antiproliferative activity of V. officinalis EO on MDA-MB-231 cancer cell line; Figure S22. Antiproliferative activity of V. officinalis EO on T24 cancer cell line; Figure S23. Antiproliferative activity of V. officinalis HY on MDA-MB-231 cancer cell line; Figure S24. Antiproliferative activity of V. officinalis HY on T24 cancer cell line.
Author Contributions
Conceptualization, I.V., V.Č.Č., M.N. and V.D.; methodology, I.V., M.L., V.Č.Č., M.N. and V.D.; validation, I.V., V.Č.Č., M.N. and V.D.; formal analysis, I.V., V.Č.Č. and V.D.; investigation, I.V., M.L., V.Č.Č., N.D., D.K., M.R., M.N. and V.D.; data curation, I.V., V.Č.Č., M.N. and V.D; writing—original draft preparation, I.V., M.N. and V.D.; writing—review and editing, I.V., M.L., V.Č.Č., N.D., D.K., M.R., M.N. and V.D.; visualization, I.V., V.Č.Č. and V.D.; supervision, V.D.; project administration, V.D.; funding acquisition, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation, grant number: IP-2020-02-8425, Croatian Veronica Species: Phytotaxonomy and Biological Activity (CROVeS-PhyBA) project.
Data Availability Statement
The samples and any additional research data are available from the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2019, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Dunkić, V.; Nazlić, M.; Ruščić, M.; Vuko, E.; Akrap, K.; Topić, S.; Milović, M.; Vuletić, N.; Puizina, J.; Jurišić Grubešić, R.; et al. Hydrodistillation and Microwave Extraction of Volatile Compounds: Comparing Data for Twenty-One Veronica Species from Different Habitats. Plants 2022, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, F.; Liu, J.; Zou, Y.; Chen, X. A Comparison of Volatile Fractions Obtained from Lonicera macranthoides via Different Extraction Processes: Ultrasound, Microwave, Soxhlet Extraction, Hydrodistillation, and Cold Maceration. Integr. Med. Res. 2015, 4, 171–177. [Google Scholar] [CrossRef]
- Vrca, I.; Šćurla, J.; Kević, N.; Burčul, F.; Čulić, V.Č.; Bočina, I.; Blažević, I.; Bratanić, A.; Bilušić, T. Influence of Isolation Techniques on the Composition of Glucosinolate Breakdown Products, Their Antiproliferative Activity and Gastrointestinal Stability of Allyl Isothiocyanate. Eur. Food Res. Technol. 2022, 248, 567–576. [Google Scholar] [CrossRef]
- Vrca, I.; Ramić, D.; Fredotović, Ž.; Možina, S.S.; Blažević, I.; Bilušić, T. Chemical Composition and Biological Activity of Essential Oil and Extract from the Seeds of Tropaeolum majus L. var. altum. Food Technol. Biotechnol. 2022, 60, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L. Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–10. ISBN 978-0-12416-641-7. [Google Scholar]
- Nazlić, M.; Akrap, K.; Kremer, D.; Dunkić, V. Hydrosols of Veronica Species—Natural Source of Free Volatile Compounds with Potential Pharmacological Interest. Pharmaceuticals 2022, 15, 1378. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological Activity and Potential as Antimicrobials for Food Applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Hamedi, A.; Moheimani, S.M.; Sakhteman, A.; Etemadfard, H.; Moein, M. An Overview on Indications and Chemical Composition of Aromatic Waters (Hydrosols) as Functional Beverages in Persian Nutrition Culture and Folk Medicine for Hyperlipidemia and Cardiovascular Conditions. J. Evid.-Based Complement. Altern. Med. 2017, 22, 544–561. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Doğan, Z.; Genç, Y.; Harput, Ü.; Karadeniz Pekgöz, A.; Saraçoğlu, İ. Chemical Profiling and Cytotoxic Activity of Aqueous Extract of Veronica peduncularis M.Bieb.: A Chemotaxonomical Approach. İstanbul J. Pharm. 2021, 51, 372–377. [Google Scholar] [CrossRef]
- Xue, H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Review of the Ethnopharmacology, Phytochemistry, and Pharmacology of the Genus Veronica. Am. J. Chin. Med. 2019, 47, 1193–1221. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, E.P.; Alipieva, K.I.; Kokubun, T.; Taskova, R.M.; Handjieva, N.V. Phenylethanoids, Iridoids and a Spirostanol Saponin from Veronica turrilliana. Phytochemistry 2007, 68, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Shetty, M.S.; Anil Kumar, N.V.; Živković, J.; Calina, D.; Docea, A.O.; Emamzadeh-Yazdi, S.; Kılıç, C.S.; Goloshvili, T.; Nicola, S.; et al. Veronica Plants—Drifting from Farm to Traditional Healing, Food Application, and Phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.M.; Crișan, G. Phytochemical Characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and Their Antioxidant and Antimicrobial Properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Lee, S.M.; Ju, H.K.; Lee, H.J.; Choi, H.K.; Jo, G.S.; Kim, Y.S. Comparison of the Profile and Composition of Volatiles in Coniferous Needles According to Extraction Methods. Molecules 2016, 21, 363. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, A.; Osmachko, A.; Ilina, T.; Goryacha, O.; Omelyanchik, L.; Grytsyk, A.; Koshovyi, O. Chemical Composition of Essential Oils from Flowers of Veronica longifolia L., Veronica incana L. and Veronica spicata L. Sci. Pharm. Sci. 2022, 38, 69–79. [Google Scholar] [CrossRef]
- Küpeli, E.; Harput, U.S.; Varel, M.; Yesilada, E.; Saracoglu, I. Bioassay-Guided Isolation of Iridoid Glucosides with Antinociceptive and Anti-Inflammatory Activities from Veronica anagallis-aquatica L. J. Ethnopharmacol. 2005, 102, 170–176. [Google Scholar] [CrossRef]
- Nazlić, M.; Fredotović, Ž.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Kremer, D.; Jurišić Grubešić, R.; Stabentheiner, E.; Randić, M.; Dunkić, V. Free Volatile Compounds of Veronica austriaca ssp. jacquinii (Baumg.) Eb. Fisch. and Their Biological Activity. Plants 2021, 10, 2529. [Google Scholar] [CrossRef]
- Živković, J.; Barreira, J.C.M.; Stojković, D.; Ćebović, T.; Santos-Buelga, C.; Maksimović, Z.; Ferreira, I.C.F.R. Phenolic Profile, Antibacterial, Antimutagenic and Antitumour Evaluation of Veronica urticifolia Jacq. J. Funct. Foods 2014, 9, 192–201. [Google Scholar] [CrossRef]
- Yin, L.; Lu, Q.; Tan, S.; Ding, L.; Guo, Y.; Chen, F.; Tang, L. Bioactivity-Guided Isolation of Antioxidant and Anti-Hepatocarcinoma Constituents from Veronica ciliata. Chem. Cent. J. 2016, 10, 27. [Google Scholar] [CrossRef]
- Harput, U.S.; Saracoglu, I.; Inoue, M.; Ogihara, Y. Anti-Inflammatory and Cytotoxic Activities of Five Veronica Species. Biol. Pharm. Bull. 2002, 25, 483–486. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. eCAM 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. Biomed. Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Lesgards, J.F.; Baldovini, N.; Vidal, N.; Pietri, S. Anticancer Activities of Essential Oils Constituents and Synergy with Conventional Therapies: A Review. Phytother. Res. 2014, 28, 1423–1446. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2017; ISBN 978-1-932633-21-4. [Google Scholar]
- Nazlić, M.; Kremer, D.; Akrap, K.; Topić, S.; Vuletić, N.; Dunkić, V. Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa. Horticulturae 2023, 9, 16. [Google Scholar] [CrossRef]
- Baptiste, J.; Fokou, H.; Michel, P.; Dongmo, J.; Boyom, F.F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils—Oils of Nature; Intech Open: London, UK, 2020. [Google Scholar]
- Nazlić, M.; Fredotović, Ž.; Vuko, E.; Fabijanić, L.; Kremer, D.; Stabentheiner, E.; Ruščić, M.; Dunkić, V. Wild Species Veronica officinalis L. and Veronica saturejoides Vis. ssp. saturejoides—Biological Potential of Free Volatiles. Horticulturae 2021, 7, 295. [Google Scholar] [CrossRef]
- Sakthivel, R.; Malar, D.S.; Devi, K.P. Phytol Shows Anti-Angiogenic Activity and Induces Apoptosis in A549 Cells by Depolarizing the Mitochondrial Membrane Potential. Biomed. Pharmacother. 2018, 105, 742–752. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide-Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chen, Y.-J.; Chen, J.J.W.; Shieh, J.-J.; Huang, C.-H.; Lin, P.-S.; Chang, G.-C.; Chang, J.-T.; Lin, C.-C. Terpinen-4-Ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer In Vitro and In Vivo. eCAM 2012, 2012, 818261. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Emami, S. β-Ionone and Its Analogs as Promising Anticancer Agents. Eur. J. Med. Chem. 2016, 123, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, Y.S.; Jang, W.J.; Rakib, A.M.; Oh, T.W.; Kim, B.H.; Kim, S.Y.; Kim, J.O.; Ha, Y.L. Anti-Proliferative Effects of β-Ionone on Human Lung Cancer A-549 Cells. J. Life Sci. 2013, 23, 1351–1359. [Google Scholar] [CrossRef]
- Yin, L.; Sun, Z.; Ren, Q.; Su, X.; Zhang, D. Methyl Eugenol Induces Potent Anticancer Effects in RB355 Human Retinoblastoma Cells by Inducing Autophagy, Cell Cycle Arrest and Inhibition of PI3K/MTOR/Akt Signalling Pathway. JBUON 2018, 23, 1174–1178. [Google Scholar] [PubMed]
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. Myricetin and Methyl Eugenol Combination Enhances the Anticancer Activity, Cell Cycle Arrest and Apoptosis Induction of Cis-Platin against HeLa Cervical Cancer Cell Lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127. [Google Scholar] [PubMed]
- Cerchiara, T.; Straface, S.; Brunelli, E.; Tripepi, S.; Gallucci, M.C.; Chidichimo, G. Antiproliferative Effect of Linalool on RPMI 7932 Human Melanoma Cell Line: Ultrastructural Studies. NPC 2015, 10, 547–549. [Google Scholar] [CrossRef]
- Moreno-Escobar, J.A.; Bazalda, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; Rodríguez-López, V. Cytotoxic and Antioxidant Activities of Selected Lamiales Species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Saracoglu, I.; Harput, U.S. In Vitro Cytotoxic Activity and Structure Activity Relationships of Iridoid Glucosides Derived from Veronica Species. Phytother. Res. 2012, 26, 148–152. [Google Scholar] [CrossRef]
- Saracoglu, I.; Oztunca, F.H.; Nagatsu, A.; Harput, U.S. Iridoid Content and Biological Activities of Veronica cuneifolia subsp. cuneifolia and V. cymbalaria. Pharm. Biol. 2011, 49, 1150–1157. [Google Scholar] [CrossRef]
- Okun, I.; Balakin, K.V.; Tkachenko, S.E.; Ivachtchenko, A.V. Caspase Activity Modulators as Anticancer Agents. Med. Chem. 2008, 8, 322–341. [Google Scholar]
- Feng, K.; Jiang, R.; Sun, L.W. Studies on the Anti-Tumor Activity in Vitro of the Flavonoid Extract in Round Leaf Speedwell. In Proceedings of the First International Conference on Cellular, Molecular Biology, Biophysics and Bioengineering, Lushan, China, 17–18 September 2010. [Google Scholar]
- Mastelić, A.; Čulić, V.Č.; Mužinić, N.R.; Vuica-Ross, M.; Barker, D.; Leung, E.Y.; Reynisson, J.; Markotić, A. Glycophenotype of Breast and Prostate Cancer Stem Cells Treated with Thieno[2,3-b]Pyridine Anticancer Compound. Drug Des. Dev. Ther. 2017, 11, 759–769. [Google Scholar] [CrossRef]
- Bilušić, T.; Šola, I.; Rusak, G.; Poljuha, D.; Čikeš Čulić, V. Antiproliferative and Pro-Apoptotic Activities of Wild Asparagus (Asparagus acutifolius L.), Black Bryony (Tamus communis L.) and Butcher’s Broom (Ruscus aculeatus L.) Aqueous Extracts against T24 and A549 Cancer Cell Lines. J. Food Biochem. 2019, 43, e12781. [Google Scholar] [CrossRef]
- Ahmadov, I.S.; Bandaliyeva, A.A.; Nasibova, A.N.; Hasanova, F.V.; Khalilov, R.I. The Synthesis of the Silver Nanodrugs in the Medicinal Plant Baikal Skullcap (Scutellaria baicalensis Georgi) and Their Antioxidant, Antibacterial Activity. Adv. Biol. Earth Sci. 2020, 5, 103–118. [Google Scholar]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/ (accessed on 12 March 2021).
- Ertas, A.; Boga, M.; Kizil, M.; Ceken, B.; Goren, A.C.; Hasimi, N.; Demirci, S.; Topcu, G.; Kolak, U. Chemical Profile and Biological Activities of Veronica thymoides subsp. pseudocinerea. Pharm. Biol. 2015, 53, 334–339. [Google Scholar] [CrossRef]
- Li, F. Analysis of Chemical Constituents of Essential Oil in Veronica linariifolia by Gas Chromatography-Mass Spectrometry. Chin. J. Anal. Chem. 2002, 30, 822–825. [Google Scholar]
- Valyova, M.; Hadjimitova, V.; Stoyanov, S.; Ganeva, Y.; Petkov, I. Free Radical Scavenging Activity of Extracts from Bulgarian Veronica officinalis L. and GC-MS Analysis of Ethanol Extract. Internet J. Aesthetic Antiaging Med. 2008, 2, 2–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).