Diverse Interactions: Root-Nodule Formation and Herb-Layer Composition in Black Locust (Robinia pseudoacacia) Stands
Abstract
:1. Introduction
2. Results
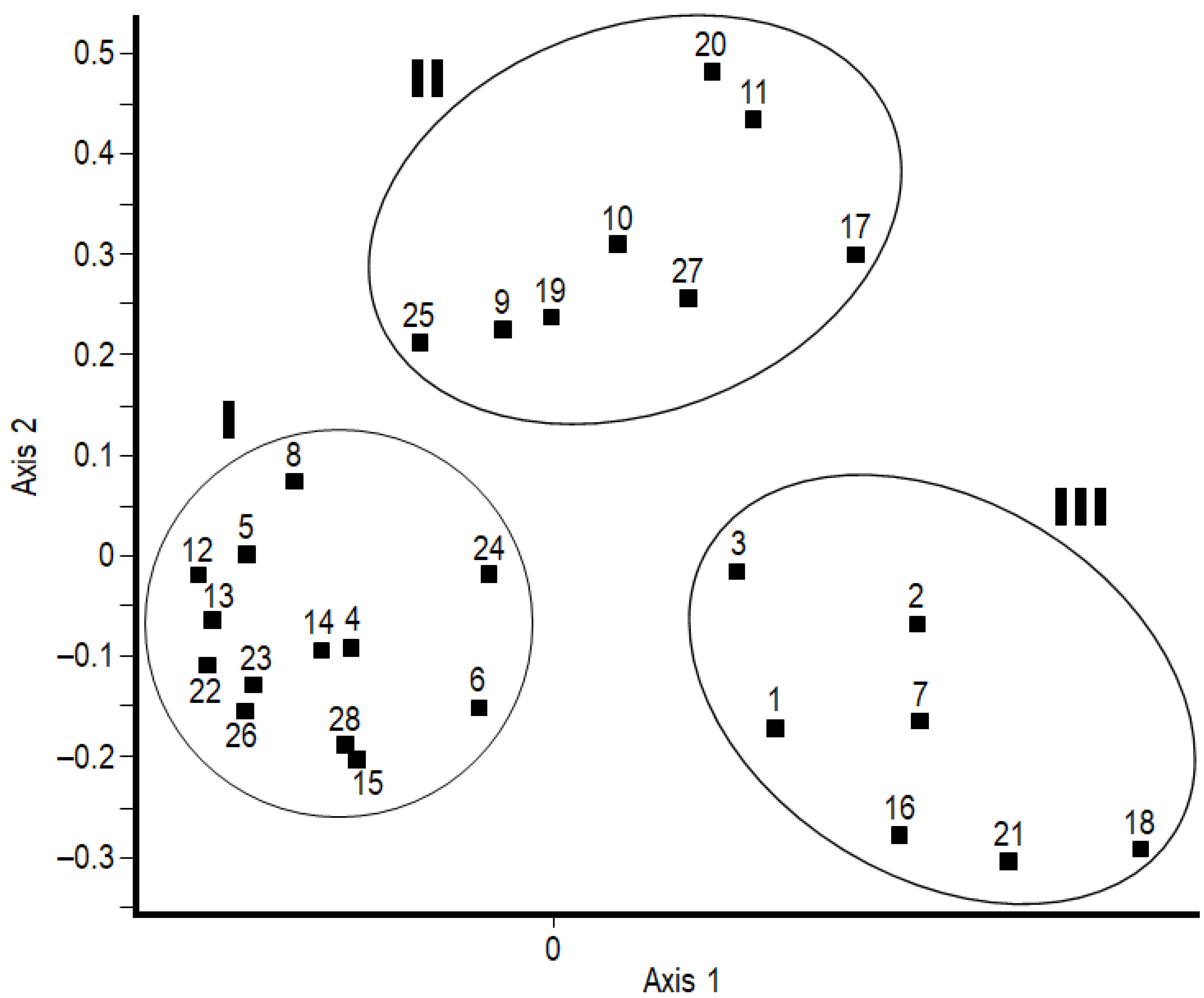
2.1. Herb-Layer Composition
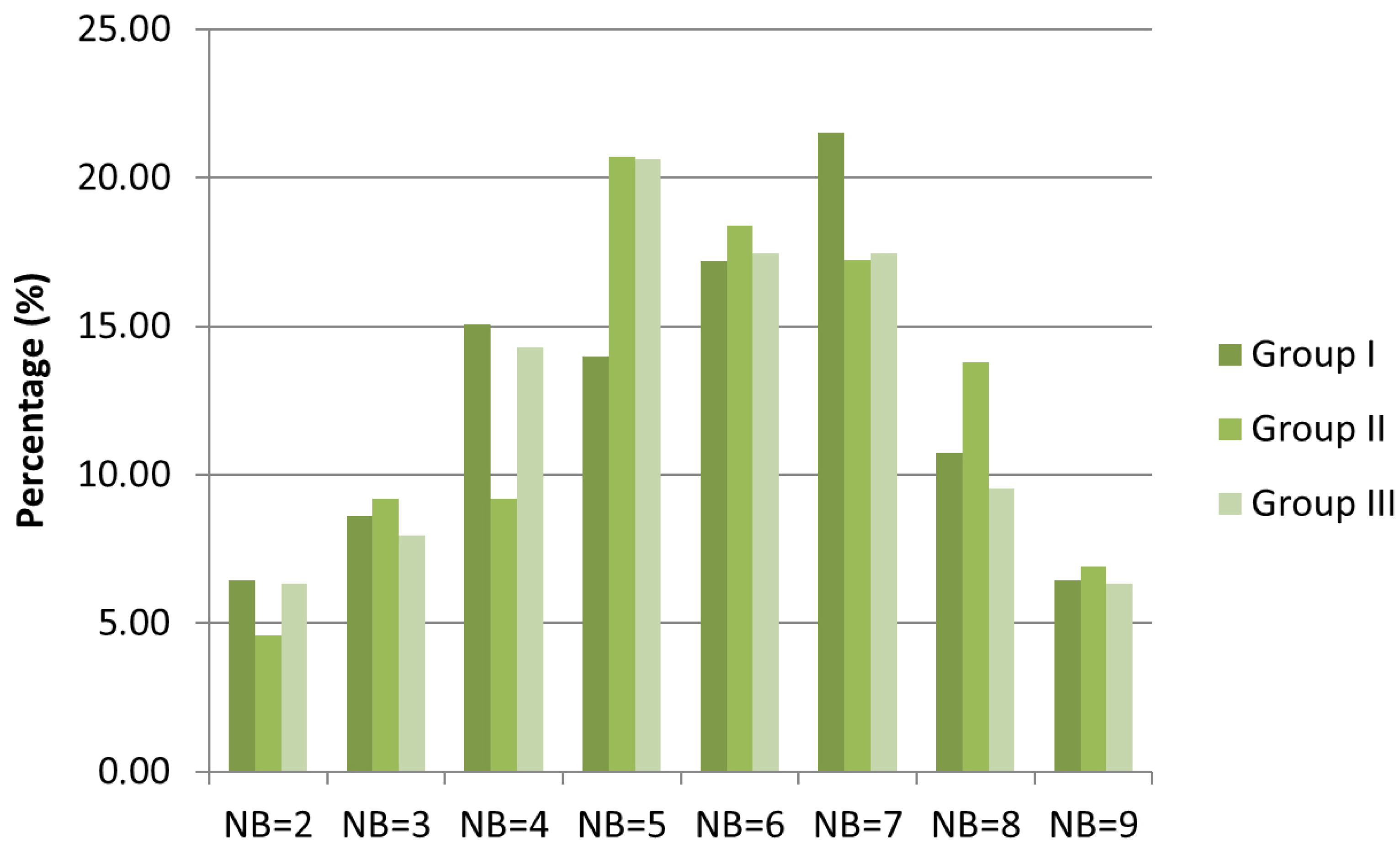
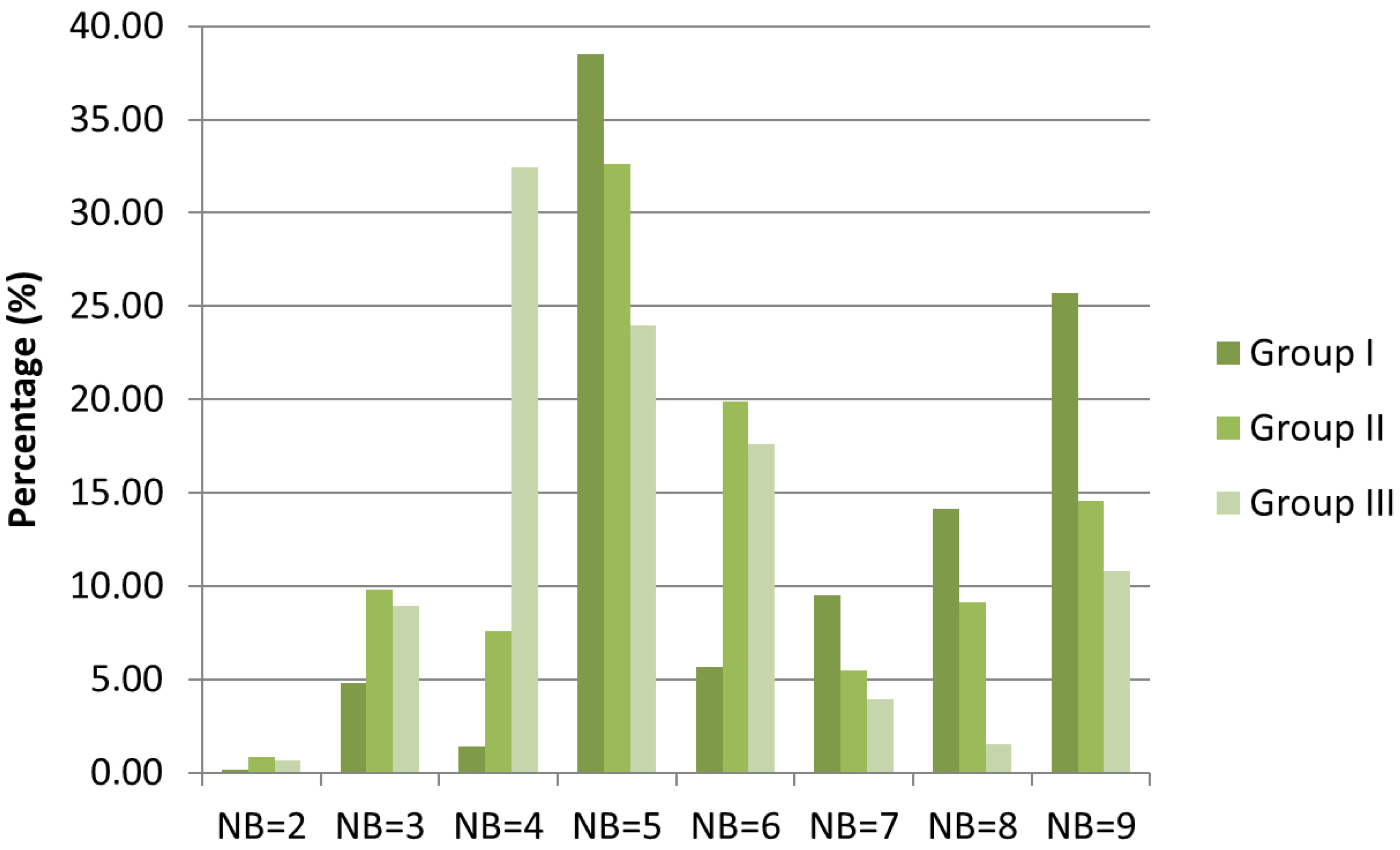
2.2. Root-Nodule Formation
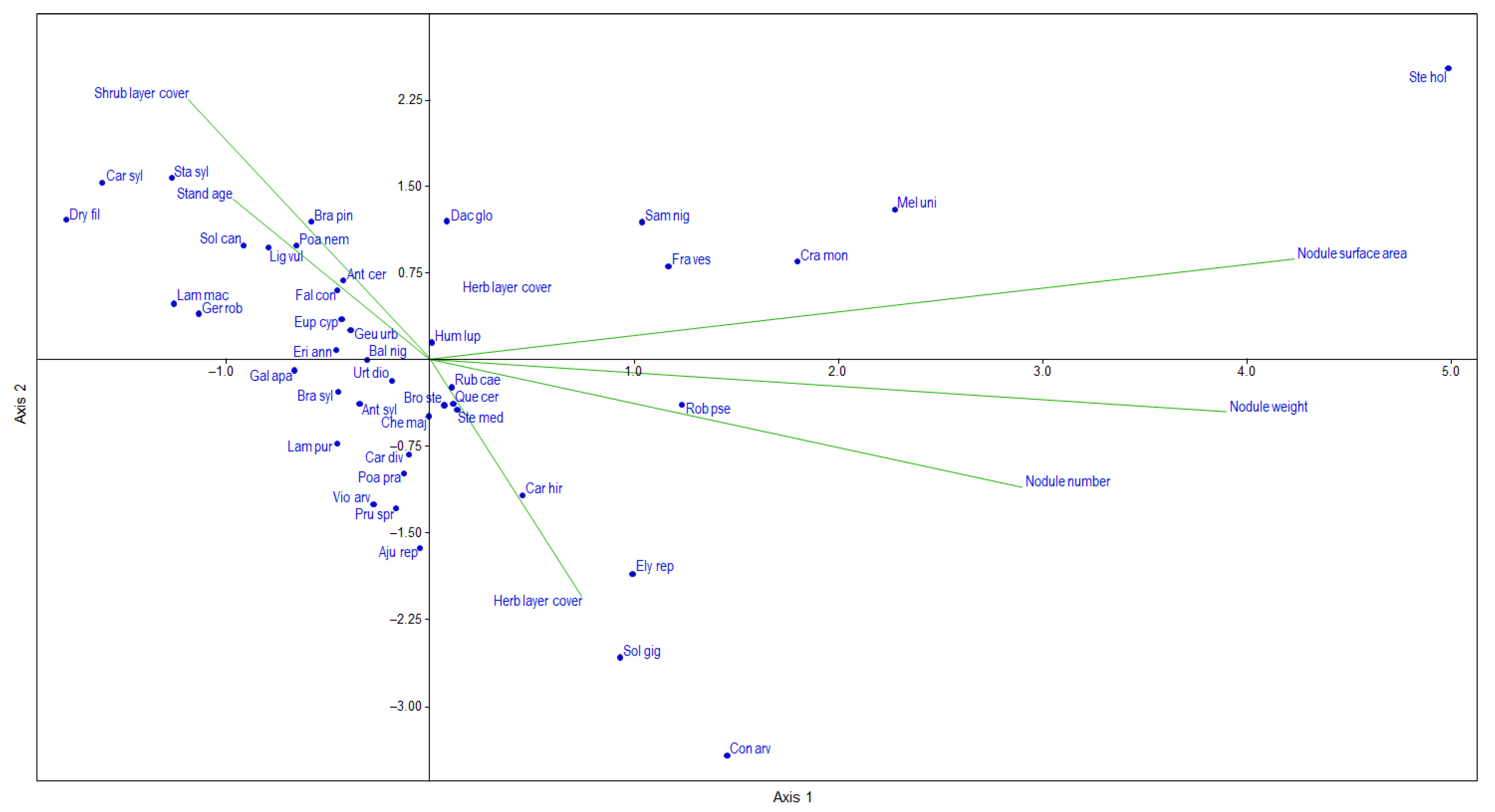
2.3. Root-Nodule and Herb-Layer Interactions
3. Discussion
3.1. Herb-Layer Composition
3.2. Root-Nodule Formation
3.3. Root-Nodule and Herb-Layer Interactions
4. Materials and Methods
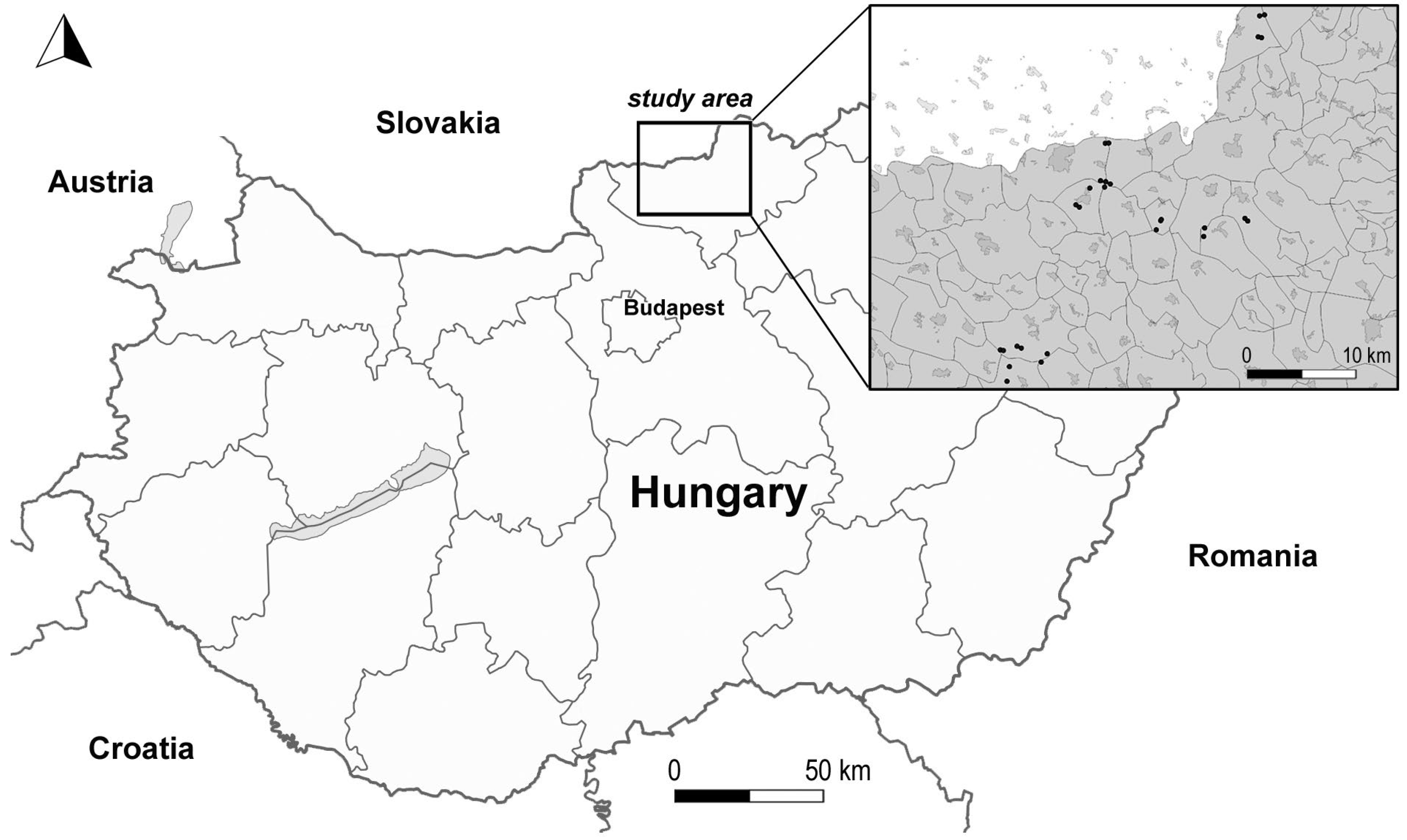
4.1. Study Area
4.2. Field Surveys and Laboratory Work
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Change Biol. 2020, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Bartha, D.; Csiszár, Á.; Zsigmond, V. Black locust (Robinia pseudoacacia L.). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Hungarian Academy of Sciences, Institute of Ecology and Botany: Vácrátót, Hungary, 2008; pp. 63–76. [Google Scholar]
- Kidawa, J.; Chmura, D.; Molenda, T. The Hydrological-Hydrochemical Factors that Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines. Plants 2021, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Środek, D.; Rahmonov, O. The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas. Forests 2022, 13, 7. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Seedling survival of Prunus serotina Ehrh., Quercus rubra L. and Robinia pseudoacacia L. in temperate forests of Western Poland. For. Ecol. Manag. 2019, 450, 117498. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D’Antonio, C.M.; Milton, S.J.; Rejmánek, M. Plant invasions—The role of mutualisms. Biol. Rev. Camb. Philos. Soc. 2000, 75, 65–93. [Google Scholar] [CrossRef]
- Vítková, M.; Tonika, J.; Müllerová, J. Black locust—Successful invader of a wide range of soil conditions. Sci. Total Environ. 2015, 505, 315–328. [Google Scholar] [CrossRef]
- Nasir, H.; Iqbal, Z.; Hiradate, S.; Fujii, Y. Allelopathic potential of Robinia pseudoacacia L. J. Chem. Ecol. 2005, 31, 2179–2192. [Google Scholar] [CrossRef]
- Csiszár, Á.; Korda, M.; Schmidt, D.; Šporčić, D.; Süle, P.; Teleki, B.; Tiborcz, V.; Zagyvai, G.; Bartha, D. Allelopathic potential of some invasive plant species occurring in Hungary. Allelopath. J. 2013, 31, 309–318. [Google Scholar]
- Gillespie, A.R.; Pope, P.E. Rhizosphere acidification increases phosphorus recovery of black locust: I. Induced acidification and soil response. Soil Sci. Sot. Amer. J. 1990, 54, 533–537. [Google Scholar] [CrossRef]
- Olesniewicz, K.S.; Thomas, R.B. Effects of mycorrhizal colonization on biomass production and nitrogen fixation of black locust (Robinia pseudoacacia) seedlings grown under elevated atmospheric carbon dioxide. New Phytol. 1999, 142, 133–140. [Google Scholar] [CrossRef]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing Black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine-oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Von Holle, B.; Joseph, K.A.; Largay, E.F.; Lohnes, R.G. Facilitations between the Introduced Nitrogen-fixing Tree, Robinia pseudoacacia, and Nonnative Plant Species in the Glacial Outwash Upland Ecosystem of Cape Cod, MA. Biodivers. Conserv. 2006, 15, 2197–2215. [Google Scholar] [CrossRef]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.D.; Wang, S.Q.; Hou, Q.C. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau, China. Forest Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Khan, B.; Ablimit, A.; Mahmood, R.; Qasim, M. Robinia pseudoacacia leaves improve soil physical and chemical properties. J. Arid Land 2010, 2, 266–271. [Google Scholar]
- Qiu, L.P.; Zhang, X.C.; Cheng, J.M.; Yin, X.Q. Effects of black locust (Robinia pseudoacacia) on soil properties in the loessial gully region of the Loess Plateau, China. Plant Soil 2010, 332, 207–217. [Google Scholar] [CrossRef]
- Kastler, B.; Samimi, C. The Impact of Robinia pseudoacacia on Ground Vegetation and Plant Nutrient Matter in Donau-Auen National Park. In Proceedings of the 5th Symposium for Research in Protected Areas, Mittersill, Austria, 10–12 June 2013; pp. 345–351. [Google Scholar]
- Rabie, P.A. Overview of Vol. 6, No. 3—Invasive Nitrogen Fixers. Restor. Reclam. Rev. 2000, 6, 1–4. [Google Scholar]
- Carpenter, A.T.; Moore, J.C.; Redente, E.F.; Stark, J.C. Plant community dynamics in a semi-arid ecosystem in relation to nutrient addition following a major disturbance. Plant Soil 1990, 126, 91–99. [Google Scholar] [CrossRef]
- Wilson, S.D.; Tilman, D. Components of plant competition along an experimental gradient of nitrogen availability. Ecology 1991, 72, 1050–1065. [Google Scholar] [CrossRef]
- Nentwig, W. (Ed.) Biological Invasions; Springer: Bern, Switzerland, 2006. [Google Scholar]
- Sherer-Lorenzen, M.; Venterik, H.O.; Buschmann, H. Nitrogen Enrichment and Plant Invasion: The Importance of Nitrogen-Fixing Plants and Anthropogenic Eutrophication. In Biological Invasions; Nentwig, W., Ed.; Springer: Bern, Switzerland, 2006; pp. 163–180. [Google Scholar]
- Burke, I.C.; Grime, J.P. An experimental study of plant community invasibility. Ecology 1996, 77, 776–790. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Dudley, T.L.; Mack, M.C. Disturbance and biological invasions: Direct effects and feedbacks. In Ecosystems of Disturbed Ground; Walker, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 413–452. [Google Scholar]
- Werner, D.; Rohm, M.; Schafers, B.; Scheidemann, P.; Wetzel, A. Signalling in the Robinia-Rhizobium symbiosis. In Trees: Contributions to Modern Tree Physiology, 1st ed.; Rennenberg, H., Eschrich, W., Ziegler, H., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1997; pp. 329–337. [Google Scholar]
- Wei, G.H.; Chen, W.M.; Zhu, W.F.; Chen, C.; Young, J.P.W.; Bontemps, C. Invasive Robinia pseudoacacia in China is nodulated by Mesorhizobium and Sinorhizobium species that share similar nodulation genes with native American symbionts. FEMS Microbiol. Ecol. 2009, 68, 320–328. [Google Scholar] [CrossRef]
- Mierzwa, B.; Lotocka, B.; Wdowiak-Wrobel, S.; Kalita, M.; Gnat, S.; Malek, W. Insight into the evolutionary history of symbiotic genes of Robinia pseudoacacia rhizobia deriving from Poland and Japan. Arch. Microbiol. 2010, 192, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Bedmar, E.J.; Reinhart, K.O.; Silvan, G.G.; Klironomos, J. Effects of soil biota from different ranges on Robinia invasion: Acquiring mutualists and escaping pathogens. Ecology 2011, 92, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tang, M.; Chen, H.; Zhang, H.; Cong, W.; Zhang, H. Community diversity of bacteria and arbuscular mycorrhizal fungi in the rhizosphere of Amorpha fruticosa L., Hippophae rhamnoides L. and Robinia pseudoacacia L. in different ecological regions of Loess Plateau in Shaanxi Province of China. Afr. J. Microbiol. Res. 2011, 5, 4787–4795. [Google Scholar] [CrossRef]
- Bokor, R. Adatok az akácnak nitrogéngyűjtő baktériumokkal való oltásához. [Data to inoculation of black locust by nitrogen fixing bacteria]. Erdészeti Lapok 1938, 77, 623–632. [Google Scholar]
- Boring, L.R.; Swank, W.T. Symbiontic nitrogen fixation in regenerating Black Locust (Robinia pseudoacacia L.) stands. For. Sci. 1984, 30, 528–537. [Google Scholar]
- Danso, S.K.A.; Zapata, F.; Awonaike, K.O. Measurement of biological N2-fixation in field-grown Robinia pseudoacacia L. Soil Biol. Biochem. 1995, 27, 415–419. [Google Scholar] [CrossRef]
- Noh, N.J.; Son, Y.; Koo, J.W.; Seo, K.W.; Kim, R.H.; Lee, Y.Y.; Yoo, K.S. Comparison of nitrogen fixation for north- and south-facing Robinia pseudoacacia stands in Central Korea. J. Plant Biol. 2010, 53, 61–69. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Thomas, R.B. A test of a potential short cut in the nitrogen cycle: The role of exudation of symbiotically fixed nitrogen from the roots of a N-fixing tree and the effects of increased atmospheric CO2 and temperature. Plant Soil 1999, 210, 21–32. [Google Scholar] [CrossRef]
- Kowarik, I. Robinie (Robinia pseudoacacia). Neophyten in mitteleuropäischen Lebensräumen. In Biologische Invasionen: Neophyten und Neozoen in Mitteleuropa, 2nd ed.; Kowarik, I., Ed.; Ulmer: Stuttgart, Germany, 2010; pp. 189–201. [Google Scholar]
- Hanzelka, J.; Reif, J. Responses to the black locust (Robinia pseudoacacia) invasion differ between habitat specialists and generalists in central European forest birds. J. Ornithol. 2015, 156, 1015–1024. [Google Scholar] [CrossRef]
- Tobisch, T.; Csontos, P.; Rédei, K.; Führer, E. Fehér akác (Robinia pseudoacacia L.) faállományok vizsgálata aljnövényzetük összetétele szempontjából. [Survey of black locust (Robinia pseudoacacia L.) stands in aspect of composition of herb-layer]. Tájökológiai Lapok 2003, 1, 193–202. [Google Scholar]
- Cho, K.J.; Kim, J.W. Syntaxonomy and synecology of the Robinia pseudoacacia forests. Kor. J. Ecol. 2005, 28, 15–23. [Google Scholar] [CrossRef]
- Vítková, M.; Kolbek, J. Vegetation classification and synecology of Bohemian Robinia pseudacacia stands in a Central European context. Phytocoenologia 2010, 40, 205–241. [Google Scholar] [CrossRef]
- Sitzia, T.; Campagnaro, T.; Dainese, M.; Cierjacks, A. Plant species diversity in alien black locust stands: A paired comparison with native stands across a north-Mediterranean range expansion. For. Ecol. Manag. 2010, 285, 85–91. [Google Scholar] [CrossRef]
- Rahmonov, O. The chemical composition of plant litter of black locust (Robinia pseudoacacia L.) and its ecological role in sandy ecosystems. Acta Ecol. Sin. 2009, 29, 237–243. [Google Scholar] [CrossRef]
- Klauck, E.J. Die Sambucus nigra-Robinia pseudoacacia-Gesellschaft und ihre geographische Gliederung. Tuexenia 1988, 8, 281–286. [Google Scholar]
- Zagyvai, G. Közösségi jelentőségű erdei élőhelyek spontán regenerációjának esélyei a Cserhátban—Lehetőségek és veszélyek. [Chances and risks of spontaneous regeneration in potential site of forest habitats of community interest in Cserhát Hill]. In Az erdőgazdálkodás Hatása az Erdők Biológiai Sokféleségére; Korda, M., Ed.; Duna-Ipoly National Park Directory: Budapest, Hungary, 2016; pp. 575–602. [Google Scholar]
- Zagyvai, G.; Csiszár, Á.; Korda, M.; Schmidt, D.; Šporčić, D.; Teleki, B.; Tiborcz, V.; Bartha, D. Előzetes eredmények száraz és félszáraz élőhelyek szukcessziós változásainak vizsgálatáról [Preliminary results of dry and semi-dry grassland succession research]. Bot. Kozlem. 2012, 99, 123–141. [Google Scholar]
- Wurzburger, N.; Miniat, C.F. Drought enhances symbiotic dinitrogen fixation and competitive ability of a temperate forest tree. Oecologia 2013, 174, 1117–1126. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Boldt-Burisch, K.; Fritsch, S.; Koning, L.A.; Freese, D. Carbon allocation, nodulation, and biological nitrogen fixation of black locust (Robinia pseudoacacia L.) under soil water limitation. Ann. For. Res. 2015, 58, 259–274. [Google Scholar] [CrossRef]
- Mueller, G.; Broll, G.; Tarnocai, C. Biological activity as influenced by microtopography in a cryosolic soil, Baffin Island, Canada. Perm. Perigl. 1999, 10, 279–288. [Google Scholar] [CrossRef]
- Koponen, P.; Nygren, P.; Domenach, A.M.; Roux, C.L.; Saur, E.; Roggy, J.C. Nodulation and dinitrogen fixation of legume trees in a tropical freshwater swamp forest in French Guiana. J. Trop. Ecol. 2003, 19, 655–666. [Google Scholar] [CrossRef]
- Florinsky, I.V.; McMahon, S.; Burton, D.L. Topographic control of soil microbial activity: A case study of denitrifiers. Geoderma 2004, 119, 33–53. [Google Scholar] [CrossRef]
- Akatov, V.V.; Akatova, T.V.; Shadzhe, A.E. Species richness of tree and shrub layers in riparian forests of the Western Caucasus dominated by alien species. Russ. J. Ecol. 2012, 43, 294–301. [Google Scholar] [CrossRef]
- Benesperi, R.; Giuliani, C.; Zanetti, S.; Gennai, M.; Mariotti Lippi, M.; Guidi, T.; Nascimbene, J.; Foggi, B. Forest plant diversity is threatened by Robinia pseudoacacia (black-locust) invasion. Biodivers. Conserv. 2012, 21, 3555–3568. [Google Scholar] [CrossRef]
- Trentanovi, G.; Lippe, M.; Sitzia, T.; Ziechmann, U.; Kowarik, I.; Cierjacks, A. Biotic homogenization at the community scale: Disentangling the roles of urbanization and plant invasion. Divers. Distrib. 2013, 19, 738–748. [Google Scholar] [CrossRef]
- Yang, H.; Gao, J.; Pan, C.; Qing, S.; Wu, Y.; Jiang, L.; Wang, Z.; Wang, D. Species composition and influencing factors of understory woody species in Robinia pseudoacacia plantations on the Loess Plateau. J. For. Res. 2023. [Google Scholar] [CrossRef]
- Röhm, M.; Werner, D. Nitrate levels affect the development of the black locust-Rhizobium symbiosis. Trees 1991, 5, 227–231. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Thomas, R.B. Effects of increased atmospheric CO2, temperature, and soil N availability on root exudation of dissolved organic carbon by a N-fixing tree (Robinia pseudoacacia L.). Plant Soil 2000, 222, 191–202. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.R.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.B.; Su, H.; Jones, C.H.; Chu, X.T.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell. Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, R.; Yuan, H.; Zhou, M.; Liu, Z.; Hu, B.; Rennenberg, H. Interaction of nitrogen availability in the soil with leaf physiological traits and nodule formation of Robinia pseudoacacia-rhizobia symbiosis depends on provenance. Plant Soil 2023. [Google Scholar] [CrossRef]
- Dövényi, Z.; Ambrózy, P.; Juhász, Á.; Marosi, S.; Mezősi, G.; Michalkó, G.; Somogyi, S.; Szalai, Z.; Tiner, T. (Eds.) Inventory of Microregions in Hungary; HAS Geographical Research Institute: Budapest, Hungary, 2010. (In Hungarian) [Google Scholar]
- Mersich, I.; Práger, T.; Ambrózy, P.; Hunkár, M.; Dunkel, Z. (Eds.) Magyarország Éghajlati Atlasza; Országos Meteorológiai Szolgálat: Budapest, Hungary, 2002. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Veperdi, G. Fatermési fok meghatározása az egészállomány átlagnövedéke alapján. [Determination of the degree of wood productivity based on the average growth of the entire stand]. Erdészettudományi Közlemények 2014, 4, 101–107. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer: Wien, Austria, 1928. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology: The Study of Plant Communities; McGraw-Hill: New York, NY, USA, 1932. [Google Scholar]
- Thomsen, K. Surface Area of an Ellipsoid. 2004. Available online: http://www.numericana.com/answer/ellipsoid.htm (accessed on 14 April 2023).
- Podani, J. SYN-TAX 2000. Computer Programs for Data Analysis in Ecology and Systematics; Scientia: Budapest, Hungary, 2001. [Google Scholar]
- Borhidi, A. Social behaviour types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian flora. Acta Bot. Hung. 1995, 39, 97–181. [Google Scholar]
- IBM Corp. Released. In IBM SPSS Statistics for Windows, Version 20.0; IBM Corp: Armonk, NY, USA, 2011. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Hammer, Ř.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Wang, X.; Guo, X.; Yu, Y.; Cui, H.; Wang, R.; Guo, W. Increased nitrogen supply promoted the growth of non-N-fixing woody legume species but not the growth of N-fixing Robinia pseudoacacia. Sci. Rep. 2018, 8, 17896. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. Forest Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Tamás, J.; Csontos, P.; Farkas, J.Z.; Hoyk, E.; Hardi, T. Különböző mértékben szuburbanizálódott Kecskemét környéki falvak előkertjeiben található fásszárú növények felmérése. [A survey of woody plants chosen for the establishmentof front gardens in villages suburbanized to varying degrees around Kecskemét, Hungary]. J. Landsc. Ecol. 2022, 20, 95–113. [Google Scholar] [CrossRef]
| Characteristics | Min | Max | Mean | SE |
|---|---|---|---|---|
| Species richness | 8 | 40 | 21.25 | 1.48 |
| Shrub layer cover (%) | 5 | 80 | 42.46 | 3.84 |
| Herb-layer cover (%) | 38 | 100 | 78.32 | 3.92 |
| Shannon–Wiener diversity | 1 | 2.27 | 1.71 | 0.07 |
| Proportion of nitrophilous species (%) | 0.15 | 0.58 | 0.30 | 0.02 |
| Cover of nitrophilous species (%) | 1 | 88 | 39.29 | 5.30 |
| Number of root-nodules (Nr.) | 0 | 476 | 116.61 | 26.00 |
| Surface area of root-nodules (mm2) | 0 | 10,487 | 1196.79 | 394.46 |
| Weight of root-nodules (g) | 0 | 4.84 | 0.69 | 0.22 |
| Species | Nodule Number | Nodule Surface Area | Nodule Weight | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Bromus sterilis | −0.099 | 0.615 | 0.009 | 0.963 | −0.063 | 0.749 |
| Chelidonium majus | −0.109 | 0.581 | −0.027 | 0.892 | −0.041 | 0.838 |
| Poa nemoralis | −0.438 | 0.020 | −0.413 | 0.029 | −0.388 | 0.041 |
| Anthriscus sylvestris | −0.239 | 0.220 | −0.247 | 0.205 | −0.242 | 0.214 |
| Melica uniflora | 0.062 | 0.753 | −0.031 | 0.875 | −0.065 | 0.743 |
| Brachypodium sylvaticum | −0.196 | 0.317 | −0.266 | 0.171 | −0.315 | 0.103 |
| Urtica dioica | −0.242 | 0.215 | −0.355 | 0.064 | −0.343 | 0.074 |
| Poa pratensis | −0.332 | 0.085 | −0.244 | 0.212 | −0.312 | 0.106 |
| Stellaria holostea | 0.253 | 0.194 | 0.163 | 0.408 | 0.136 | 0.490 |
| Geum urbanum | −0.324 | 0.092 | −0.407 | 0.031 | −0.381 | 0.045 |
| Elymus repens | 0.053 | 0.790 | 0.167 | 0.396 | 0.139 | 0.482 |
| Anthriscus cerefolium | −0.172 | 0.381 | −0.108 | 0.584 | −0.111 | 0.575 |
| Rubus caesius | 0.096 | 0.628 | 0.092 | 0.643 | 0.080 | 0.687 |
| Humulus lupulus | −0.052 | 0.792 | −0.061 | 0.759 | −0.039 | 0.845 |
| Brachypodium pinnatum | −0.300 | 0.121 | −0.240 | 0.219 | −0.253 | 0.194 |
| Galium aparine | −0.479 | 0.010 | −0.381 | 0.046 | −0.369 | 0.053 |
| Stellaria media | −0.036 | 0.858 | 0.006 | 0.976 | −0.007 | 0.974 |
| Stand Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Species richness | 0.770 | 0.446 | −0.111 |
| Cover of nitrophilous species | 0.926 | 0.198 | 0.064 |
| Proportion of nitrophilous species | 0.708 | −0.306 | 0.364 |
| Shannon–Wiener diversity | 0.812 | 0.146 | −0.185 |
| Herb-layer cover | 0.150 | −0.846 | −0.170 |
| Shrub layer cover | 0.169 | 0.818 | −0.010 |
| Stand age | 0.044 | 0.127 | 0.909 |
| Wood productivity | 0.831 | 0.005 | 0.177 |
| Eigenvalue | 3.410 | 1.740 | 1.028 |
| Explained variance % | 42.63 | 21.75 | 12.85 |
| Cumulated variance % | 42.63 | 64.38 | 77.21 |
| Nodule Characteristics | PC1 | PC2 | PC3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | F | p | r | F | p | r | F | p | |
| Nodule number | −0.556 | 11.66 | 0.002 | 0.042 | 0.046 | 0.833 | −0.403 | 5.056 | 0.033 |
| Nodule surface area | −0.662 | 20.315 | 0.0001 | −0.113 | 0.339 | 0.566 | −0.454 | 3.660 | 0.015 |
| Nodule weight | −0.601 | 14.691 | 0.0007 | −0.091 | 0.329 | 0.644 | −0.514 | 9.320 | 0.005 |
| Site (Forest Subcompartments) | Elevation (m) | Exposure | Slope (°) | Stand Age (year) | Wood Productivity (m3/year/ha) |
|---|---|---|---|---|---|
| Alsópetény 29D | 200 | S | 20 | 19 | 8 |
| Alsópetény 7F | 200 | S | 15 | 23 | 11 |
| Iliny 1A | 200 | N | 15 | 15 | 14 |
| Iliny 1B | 200 | S | 15 | 22 | 13 |
| Nógrádmarcal 1A | 200 | N | 25 | 20 | 13 |
| Nógrádmarcal 1D | 200 | S | 15 | 20 | 10 |
| Nógrádmarcal 9C | 200 | N | 10 | 32 | 10 |
| Nógrádszakál 12G | 300 | S | 15 | 12 | 8 |
| Nógrádszakál 12I | 200 | S | 10 | 8 | 8 |
| Nógrádszakál 5C | 300 | W | 10 | 26 | 10 |
| Nógrádszakál 5D | 300 | E | 10 | 25 | 10 |
| Patvarc 1A | 150 | N | 5 | 21 | 11 |
| Patvarc 1B | 150 | * | 0 | 24 | 10 |
| Rimóc 4 B | 300 | S | 20 | 18 | 9 |
| Rimóc 4G | 300 | S | 15 | 8 | 13 |
| Romhány 12A | 200 | * | 15 | 9 | 8 |
| Romhány 1E | 300 | N | 15 | 25 | 7 |
| Romhány 21D | 300 | N | 10 | 8 | 4 |
| Romhány 22C | 300 | N | 10 | 15 | 8 |
| Romhány 4B | 300 | N | 10 | 9 | 7 |
| Romhány 8C | 200 | * | 10 | 28 | 8 |
| Szügy 25B | 200 | * | 0 | 21 | 12 |
| Szügy 25D | 200 | * | 0 | 3 | 11 |
| Szügy 2D | 200 | W | 10 | 27 | 10 |
| Szügy 3B | 200 | * | 15 | 8 | 15 |
| Szügy 5G | 200 | N | 5 | 14 | 9 |
| Varsány 14C | 200 | * | 10 | 17 | 13 |
| Varsány 17I | 200 | S | 10 | 29 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csiszár, Á.; Winkler, D.; Bartha, D.; Zagyvai, G. Diverse Interactions: Root-Nodule Formation and Herb-Layer Composition in Black Locust (Robinia pseudoacacia) Stands. Plants 2023, 12, 3253. https://doi.org/10.3390/plants12183253
Csiszár Á, Winkler D, Bartha D, Zagyvai G. Diverse Interactions: Root-Nodule Formation and Herb-Layer Composition in Black Locust (Robinia pseudoacacia) Stands. Plants. 2023; 12(18):3253. https://doi.org/10.3390/plants12183253
Chicago/Turabian StyleCsiszár, Ágnes, Dániel Winkler, Dénes Bartha, and Gergely Zagyvai. 2023. "Diverse Interactions: Root-Nodule Formation and Herb-Layer Composition in Black Locust (Robinia pseudoacacia) Stands" Plants 12, no. 18: 3253. https://doi.org/10.3390/plants12183253
APA StyleCsiszár, Á., Winkler, D., Bartha, D., & Zagyvai, G. (2023). Diverse Interactions: Root-Nodule Formation and Herb-Layer Composition in Black Locust (Robinia pseudoacacia) Stands. Plants, 12(18), 3253. https://doi.org/10.3390/plants12183253







