Abstract
Oxalis latifolia, a perennial herbaceous weed, is a highly invasive species that poses a threat to agricultural lands worldwide. East Asia is under a high risk of invasion of O. latifolia under global climate change. To evaluate this risk, we employed maximum entropy modeling considering two shared socio-economic pathways (SSP2-4.5 and SSP5-8.5). Currently, a small portion (8.02%) of East Asia is within the O. latifolia distribution, with the highest coverages in Chinese Taipei, China, and Japan (95.09%, 9.8%, and 0.24%, respectively). However, our projections indicated that this invasive weed will likely be introduced to South Korea and North Korea between 2041 and 2060 and 2081 and 2100, respectively. The species is expected to cover approximately 9.79% and 23.68% (SSP2-4.5) and 11.60% and 27.41% (SSP5-8.5) of the total land surface in East Asia by these time points, respectively. South Korea and Japan will be particularly susceptible, with O. latifolia potentially invading up to 80.73% of their territory by 2081–2100. Mongolia is projected to remain unaffected. This study underscores the urgent need for effective management strategies and careful planning to prevent the introduction and limit the expansion of O. latifolia in East Asian countries.
1. Introduction
Oxalis latifolia Kunth, commonly called “broadleaf woodsorrel”, is a perennial herbaceous plant native to Mexico and Central and South America. It was introduced to Africa, Asia, and Australasia before 1950 for ornamental purposes [1,2]. It is distributed throughout tropical, Mediterranean, and temperate climates [3]. It is a low-growing plant with three broad leaflets on long petioles and reproduces both vegetatively by means of bulbs and sexually by means of seeds [4,5].
O. latifolia is referred to as a noxious and invasive weed as it has caused losses of 30 different types of crops, including rice, tea, potato, and apple, in 37 countries [3,6,7]. Its uncontrolled growth has resulted in a 56% maize reduction in India [8]. O. latifolia absorbs a relatively high proportion of soil nutrients and moisture and creates an allelopathic effect, inhibiting the growth of surrounding plants [9]. The weed also has a significant impact on nurseries, resulting in a loss of garden plants.
The distribution and invasiveness of plants are strongly influenced by climatic components, including temperature, precipitation, and atmospheric carbon dioxide [10]. Temperature plays important roles in the activation of O. latifolia bulbs [3]. The annual cycle begins when the bulbs are activated, occurring at temperatures above 15 °C in soil, and dormancy can last for more than 1 year [6]. The dormancy of bulbs is broken by a number of circumstances, including chilling at 5 °C for 3 weeks or dry heat at 45 °C for several hours, and continues until autumn in severe droughts [11,12].
East Asian countries, including China, Chinese Taipei, Japan, South Korea, North Korea, and Mongolia, confront a significant threat from invasive plants. These intruders endanger biodiversity, ecosystems, and human well-being. China, Chinese Taipei, Japan, and South Korea, due to increased global trade and tourism, face heightened invasion risks. China’s diverse climates and habitats house 488 invasive species across various environments [13,14]. Chinese Taipei, with tropical landscapes, notes 168 invasive plants, such as O. latifolia. Japan and South Korea record 154 and 320 invasive species, respectively, impacting agriculture and pastures [15,16]. Landlocked Mongolia, boasting diverse ecosystems, grapples with 77 invasive species listed in the Global Register of Introduced and Invasive Species (GRIIS), comprising 4.8% of its flora [17]. These invasions pose serious challenges across the region.
To manage invasive species and preserve native biodiversity, it is important to understand how invasive species are established and spread under current and future climate change [18]. In the past two decades, species distribution models (SDMs) have emerged as one of the most effective methods for investigating the impact of climate change on species habitat characteristics [19]. Among various SDMs, the maximum entropy method (MaxEnt) is the most frequently used machine learning technique for modeling species niches and geographic distributions using presence-only data based on parameters that link environmental variables and habitat suitability [20,21,22]. The MaxEnt algorithm has become an extremely popular tool for predicting the current and future potential habitats of invasive species because absence data are rarely available for these taxa and an equilibrium state has not been reached [23,24,25].
In East Asia, O. latifolia currently occurs in China and Chinese Taipei, with predictions of introduction from South Asia (e.g., India). It is well established in disturbed areas and is rapidly expanding into agriculture and pastures, resulting in productivity loss in such areas [3]. Future climate change is likely to favor the habitat expansion of O. latifolia, extending to other countries in East Asia. While the taxonomy, ecology, and physiology of O. latifolia have been studied [1,3,5,6], its distribution in relation to global climate change and human-induced disturbances has not been explored yet. Furthermore, understanding the potential distribution of O. latifolia is crucial for designing a robust quarantine system and implementing effective control and eradication measures.
Therefore, this study was designed with the main objectives as follows: (1) to identify important bioclimatic factors for the habitat suitability of O. latifolia, (2) to predict the potential distribution of O. latifolia in six East Asian countries using the MaxEnt modeling approach under the current and future climate change scenarios, and (3) to compare and estimate mean habitat suitability in six countries of East Asia under SSP2-4.5 and SSP5-8.5 scenarios. We expect the results to guide the development of a theoretical framework for preventing the introduction and dispersal of the species.
2. Results
2.1. Contribution of Bioclimatic Variables and Evaluation of Model Performance
We evaluated the degree of importance of various bioclimatic variables (Bio1, Bio2, Bio3, Bio12, Bio13, and Bio14) for modeling the distribution of O. latifolia based on the average contribution of each variable in the model for the time periods of 1970–2000, 2041–2060, and 2081–2100 (Table 1). Among six bioclimatic variables, three variables (i.e., Bio1, Bio3, and Bio12) had relatively high contributions to the model, estimated to be 35.23%, 30.08%, and 24.24%, respectively (Table 1). The distribution of O. latifolia was therefore primarily driven by annual mean temperature, isothermality, and annual precipitation, whereas other variables had relatively minor roles in model performance.

Table 1.
Selected bioclimatic variables and their average contribution to the model.
Variable importance was assessed using the jackknife method, which estimates the degree of relevance and distinctness of each variable to a species distribution model. Jackknife tests also revealed that Bio1, Bio3, and Bio12 were relatively important variables for predicting the potential distribution of O. latifolia (Figure S1).
In an evaluation of model performance, AUC, TSS, and Kappa values were 0.91, 0.85, and 0.71, respectively. The AUC value indicates the excellent model performance for predicting the global distribution of O. latifolia. Similarly, the evaluation metrics, TSS and Kappa, showed that there is good agreement in model predictions between observed and predicted data sets. These results confirm that the model exhibited excellent performance in predicting the spatial distribution of O. latifolia using presence-only data.
2.2. Predicting the Distribution of O. latifolia under Global Climate Change
Based on GBIF data, O. latifolia has been reported in three countries in East Asia, China, Japan, and Chinese Taipei, with no records in other countries, such as Mongolia, North Korea, and South Korea, in the wild (Figure 1).
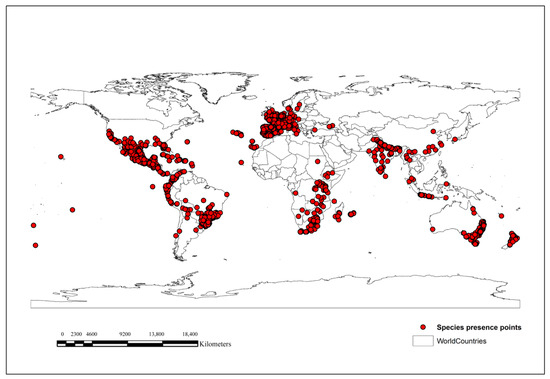
Figure 1.
Worldwide occurrence records of O. latifolia. Red points indicate occurrence records.
MaxEnt modeling was performed to predict the potential distribution of O. latifolia in East Asian countries under current and future climate change scenarios (shared socio-economic pathways SSP2-4.5 and SSP5-8.5) and the results are presented in Figure 2. Currently, Chinese Taipei has highest proportion of suitable habitat relative to the total land surface of the country (95.09%), followed by China (9.78%) and Japan (0.24%) (Table 2). Mongolia, North Korea, and South Korea are climatically unsuitable for the establishment of O. latifolia. Altogether, 8.02% of the area of East Asia (689,716 cells, 2.5 min resolution) was estimated to be invaded by O. latifolia under the current climate.
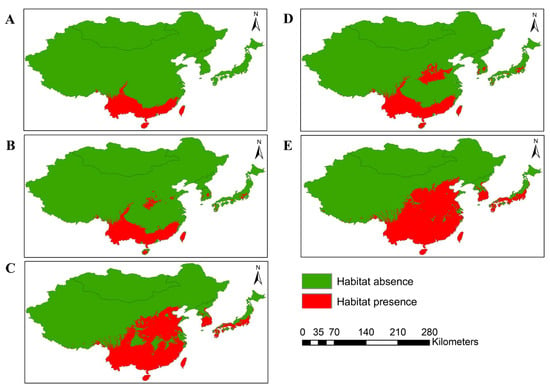
Figure 2.
Distribution of O. latifolia in East Asia under current and future climatic scenarios: (A) habitat distribution of O. latifolia under the current climate (1970–2000); (B,C) potential distribution by 2041–2060 and 2081–2100 under the climate change scenario SSP2-4.5; (D,E) potential distribution by 2041–2060 and 2081–2100 under the climate change scenario SSP5-8.5. Green and red in the legend indicate the absence and presence of habitats of O. latifolia.

Table 2.
Percentage of area covered by O. latifolia in different countries in East Asia under the climate change scenarios SSP2-4.5 and SSP5-8.5.
Under future climate change scenarios, it was predicted that O. latifolia will be introduced to South Korea between 2041 and 2060. It was estimated that the species will cover approximately 9.82% and 15.89% of the total land surface under the SSP2-4.5 and SSP5-8.5 scenarios, respectively. Similarly, by 2081–2100, the suitable habitat for O. latifolia was predicted to expand by 77.29% (SSP2-4.5) and 80.73% (SSP5-8.5) across the country. In addition, O. latifolia was predicted to be introduced to North Korea by 2081–2100, while Mongolia was expected to remain safe from O. latifolia invasion at least until the end of this century. Other countries, such as China, Chinese Taipei, and Japan, had predicted invasion rates ranging from 27.24% to 99.45% (2041–2060) and 31.62% to 100% (2081–2100), as shown in Table 2. In total, by 2041–2060 and 2081–2100, approximately 9.79% and 23.68% (SSP2-4.5) and 11.60% and 27.41% (SSP5-8.5) of the total land surface of East Asia, respectively, could be invaded by O. latifolia. These results revealed that the habitat expansion of O. latifolia would be extensive in East Asia under global climate change.
2.3. Habitat Suitability Index and Future Potential Habitats in East Asia
Mean habitat suitability for O. latifolia was estimated under future climate change scenarios (SSP2-4.5 and SSP5-8.5) in six countries of East Asia, as summarized in Figure 3. The habitat suitability index was highest in North Korea (0.3) in 2081–2100, followed by China, Japan and South Korea, and Chinese Taipei (0.16, 0.14, 0.04, and 0.01, respectively). However, Mongolia was identified as an unsuitable habitat for O. latifolia by the end of this century. Countries with higher habitat suitability indexes in the future will have higher rates of greenhouse gas emissions and greater anthropogenic disturbances affecting natural resources.
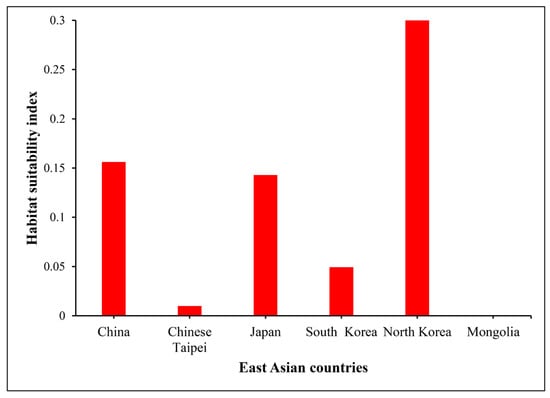
Figure 3.
Graphical representation showing differences in the ratios of the mean habitat suitability index of O. latifolia between climate change scenarios SSP2-4.5 and SSP5-8.5 in various countries of East Asia by the period of year 2081–2100.
3. Discussion
We assessed the risk of O. latifolia invasion under future climate change scenarios. We obtained several remarkable results as follows. First, the annual mean temperature was the most significant determinant of the global distribution of O. latifolia (Table 1). Second, the current potential distribution of O. latifolia was largely in China and Chinese Taipei; however, by 2081–2100, O. latifolia was predicted to expand to all countries in East Asia, except Mongolia (Figure 2). Third, based on the mean habitat suitability index, the rate of habitat expansion is predicted to be relatively high in North Korea, China, and Japan; these countries are at a high risk of invasion under the climate change scenario SSP5-8.5 (Figure 3).
Global climate change refers to the persistent alteration of worldwide climate patterns, including temperature, precipitation, and wind flows. These changes primarily result from the accumulation of greenhouse gases, such as carbon dioxide, methane, and nitrous oxide. These gases trap heat in the atmosphere, contributing to the Earth’s warming. Human activities, particularly the burning of fossil fuels, deforestation, industrial processes, and agriculture, have significantly elevated the levels of these greenhouse gases in the atmosphere. This has led to higher temperatures, rising sea levels, loss of biodiversity, and an increased risk of invasive alien plant species. Over the past century, the global temperature has risen by 0.78 °C. Predictions indicate that by 2100, relative to the average temperature of 1850–1880, global mean temperatures could rise by 1.5 °C under the SSP2-4.5 scenario and 5.2 °C under the SSP5-8.5 scenario [26]. Global climate change might exacerbate the risk of invasive alien plant species by causing damage to ecosystems and intensifying competition within native ecological systems due to higher levels of greenhouse gases [27]. To manage the invasion risk posed by these alien and invasive species, it is essential to anticipate their potential introduction, establishment, and spread, which can be achieved through standardized modeling systems.
SDM is widely used in ecological research, including the prediction of habitat suitability, assessment of biodiversity, and management of nature reserves [28,29,30]. More recently, SDM is used in the prediction of the biological invasion of alien plant species [31,32,33]. Several factors contribute to the predictive performance of SDM, including the size and resolution of the study area and the threshold used for modeling [34]. Other important factors include the type of species, number of species occurrence records, and environmental variables used in the models [35] as well as the modeling algorithms and global circulation models utilized [36] and the methods for model evaluation and validation [36,37].
In this study, we applied the MaxEnt modeling approach because the algorithm has high predictive performance using presence-only data and background data as pseudoabsence data [38]. Moreover, the algorithm can be run easily with many options in default settings [38]. Although MaxEnt is a powerful and widely used modeling technique, it is important to be aware of its limitations, such as the possibility of over-predicting species distributions due to sampling bias in geographic and environmental space [39]. To address this issue, we spatially filtered the species presence points in the model [40]. The scores for three indicators of predictive performance, AUC, TSS, and Kappa, were 0.91, 0.85, and 0.71, respectively, indicating that the model was robust and highly accurate in predicting the global distribution of the species.
Bioclimatic variables, such as temperature and precipitation, have a significant impact on the invasion of plant species [3,18,41]. Among the six bioclimatic varibles used to model the global, O. latifolia distribution, the temperature-related variable Bio1, and precipitation-related variable Bio12, were significant factors. These results indicate that O. latifolia requires a warm and humid environment for its optimal germination, growth, and spread. Therefore, it can survive in equatorial regions, including central America, equatorial South America, and introduced regions of Africa and Australia, with a temperature range of 20–25 °C and annual precipitation of 800–1500 mm [42]. However, in temperate and Mediterranean climates, aboveground vegetative parts disappear during the cold and wet autumn and reappear in late spring when temperatures increase [3,43]. In temperate and Mediterranean climates, the spring season is characterized by high levels of rainfall, resulting in activation of the bulbs. Thus, temperature may be a more significant factor than moisture for activation [43].
Globally, the native range of O. latifolia spans 19 countries in North, Central, and South America; however, it has been introduced to more than 41 countries of Asia, Europe, Australia, and Oceania [44]. O. latifolia has invaded some countries in South Asia (e.g., India, Nepal, and Bhutan) and Southeast Asia (e.g., China and Indonesia). However, it has not been recorded in South Korea, North Korea, or Mongolia. Our model predicted that these countries are climatically unsuitable for O. latifolia growth under the current climate. During the winter season, the average minimum temperature ranges between −2 °C and −20 °C in the southern coastal and northeastern regions of the Korean peninsula, respectively (KMA 2020). Similarly, the average minimum temperature in Mongolia ranges between −15 °C and −35 °C [45]. O. latifolia bulbs can survive short periods of freezing but are killed during exposure to sub-zero temperatures for prolonged periods [42]. Therefore, the climates of the northern part of China, North Korea, South Korea, and Mongolia are unsuitable under the current climate.
The annual mean temperature is expected to rise in many developed countries of East Asia, e.g., China, Japan, and South Korea, with estimated increases of 6.0 °C, 5.85 °C, and 5.38 °C, respectively, under SSP5-8.5 by 2100 [46,47,48], providing suitable climatic conditions for the introduction and habitat expansion of alien and invasive plants (e.g., O. latifolia) with tropical and subtropical origins [10]. Our model showed that O. latifolia would spread to South Korea, Japan, and China, covering approximately 9.82%, 5.4%, and 11.73% of the total area, respectively, by 2041–2060. Similarly, by 2081–2100, the habitat of O. latifolia was predicted to expand in northern regions of East Asia (up to 40° latitude), including North Korea, and spread to about 31.62% of China, 41.73% of Japan, and 77.29% of South Korea under SSP5-8.5. Mongolia is located above the 40° latitude and exhibits extreme cold in the winter, which does not allow the survival of warm-adapted plants, such as O. latifolia. Chinese Taipei is located in the southernmost part of East Asia, with a tropical and subtropical climate, favoring plant survival throughout the year under the current and future climates. In this study, we evaluated the habitat suitability index of O. latifolia in six East Asian countries to predict the rate of invasion under SSP2-4.5 and SSP5-8.5 considering anthropogenic activities, land cover changes, and population growth (and encroachment on natural resources) in 2081–2100. North Korea, China, and Japan had relatively higher values for the habitat suitability index and therefore will have higher rates of invasion under SSP5-8.5 than under SSP2-4.5; in these countries, the O. latifolia is expected to have weaker responses to greenhouse gas management efforts by 2081–2100 [49].
By 2081–2100, under the SSP2-4.5 and SSP5-8.5 climate change scenarios, agricultural land in the western and eastern regions of East Asia is at a high potential risk of O. latifolia invasion. This could result in significant economic losses and have a severe impact on food security, native biodiversity, and ecosystem services. Moreover, the invasion of O. latifolia degrades pastureland, severely affecting wild herbivores, including roe deer, sika deer, and ghorals [50]. Therefore, the control and management of O. latifolia are needed. Three major techniques, mechanical, chemical, and biological control, are widely used for invasive weeds [12]. Among these techniques, mechanical and chemical control are not effective for O. latifolia because they cannot destroy bulbs and roots in the soil [3,12]. Similarly, biological control could be an effective method for controlling O. latifolia, but it has not been studied or employed yet.
In addition to climate change, various other biotic and abiotic factors can accelerate the invasion of O. latifolia in East Asia (e.g., human activities, movement of goods, etc.) [51,52]. To improve the accuracy of predictions, it is crucial to incorporate various environmental variables into the model, including changes in land use and land cover, soil moisture, and soil pH, alongside bioclimatic variables. These are the limitations of our study, and we aim to use them in our next study. Looking ahead, we plan to evaluate the potential invasion risk of O. latifolia on a global scale using other environmental variables incluing bioclimatic variables. Additionally, we are focused on conducting invasion risk assessments tailored to specific countries in the near future.
4. Materials and Methods
4.1. Global Occurrence Points
Global occurrence data for O. latifolia (6406) were downloaded from the open access data portal, Global Biodiversity Information Facility (GBIF, www.gbif.org, accessed on 3 September 2022). To prevent the overfitting and incorrect inflation of model performance owing to spatial correlation [39,53], multiple species presence points within the same grid with a spatial resolution of 2.5 min were eliminated, leaving only a single unique point per grid by using the spatially rarefy occurrence tool in the ArcGIS SDM toolbox v. 2.4 [40]. The total number of O. latifolia occurrence points was reduced to 3595, as shown in Figure 1, and these points were used in the MaxEnt modeling (Table S1) to predict the potential distribution of O. latifolia in East Asia, including China, Chinese Taipei, Japan, Mongolia, North Korea, and South Korea, under the current and future climate change scenarios SSP2-4.5 and SSP5-8.5.
4.2. Environmental Variables
Climatic variables are considered the most significant factors determining the potential distribution of a species [54]. Nineteen bioclimatic variables (Table S2) considered important for predicting the O. latifolia distribution were downloaded from the World Climate (https://www.worldclim.org, accessed on 1 September 2022) [55] and were used to build models for predicting the species distribution at a global scale. Historical data for 1970–2000 were considered current climatic data and are referred to as across throughout the manuscript. Two shared socio-economic pathway (SSP) scenarios, SSP2-4.5 and SSP5-8.5, were employed to depict future climate data for the time periods 2041–2060 and 2081–2100. SSP scenarios describe future changes in socio-economic factors, such as human and economic factors, technology, land use, climate impact, emission of gases, and air pollutants [56,57]. It is predicted that the average global temperature will increase by 2.1–3.5 °C under SSP2-4.5 and 3.3–5.7 °C under SSP5-8.5 [58]. The bioclimatic variables were formed by the global climate model ‘Max Planck Institute for Meteorology Earth System Model (MPI-ESM1-2HR)’ [59] under the Coupled Model Intercomparison Project (CMIP6) [60] at a spatial resolution of 2.5 min (4.5 km at the equator).
The WorldClim portal is widely utilized for obtaining bioclimatic variables for predicting and investigating the potential distribution of species in response to changes in temperature and precipitation [61,62]. These variables helped to illustrate and predict the future distributions of species under various climate scenarios based on their ecologies [63]. Pearson correlation coefficients were evaluated to select bioclimatic variables that were not highly correlated with each other (r > 0.75, p = 0.05); highly correlated variables were removed to improve the predictive performance of the model [33,64]. Then, six bioclimatic variables, including temperature-related variables, e.g., annual mean temperature (Bio01), mean diurnal temperature range (Bio2), and isothermality (Bio03), and precipitation-related variables, e.g., annual precipitation (Bio12), precipitation in the wettest month (Bio13), and precipitation in the driest month (Bio14), were selected among the 19 bioclimatic variables based on the Pearson’s correlation coefficients (Table S3) for modeling the O. latifolia distribution, as described previously [65]. The Pearson’s correlation analysis was performed using the PROC CORR function of SAS 9.4 (SAS Institute, Inc., Cary, NC, USA).
4.3. Model Development
The global distributions of O. latifolia under the current and future climates were predicted using MaxEnt Version 3.4.1 [66]. MaxEnt is an open-source, widely used machine learning technique that exhibits high predictive performance based on limited presence-only data sets [67,68]. It is the most appropriate tool for modeling invasive species because absence data are rarely available for these species and may not be accurate as their ranges may be expanding [69]. Therefore, we determined global background points using ArcGIS 10.8 (ESRI, Redlands, CA, USA) based on previously described methods [32,70]. To evaluate the predictive performance of the model, the species occurrence data were randomly split at a 3:1 ratio for model calibration and validation [28]. The model was replicated 100 times, and all other MaxEnt options were run with default parameters [31].
4.4. Model Evaluation and Validation
Model performance was evaluated using the area under the curve (AUC) of the receiver operator characteristic (ROC) curve (Pearson 2010), True Skill Statistic (TSS), and Kappa statistics [71]. The AUC serves as a threshold-independent method to evaluate model performance by separating presence from absence [72]. AUC values range from 0 to 1, where values of 0.5–0.6, 0.6–0.7, 0.7–0.8, 0.8–0.9, and 0.9–1 indicate fail, poor, fair, good, and excellent model performance, respectively [73]. The ROC curve analysis evaluates the prediction accuracy of the MaxEnt model based on AUC values. TSS is considered an alternative to AUC values for assessing model accuracy; it takes values ranging from −1 to +1 [71], with values closer to +1 indicating excellent sensitivity and values of zero or below denoting commission errors (i.e., expecting presence in the absence of the species) and omission (expecting absence in the presence of the species) [71,74]. Similarly, Kappa values range between −1 and +1, indicating poor agreement and perfect agreement, respectively. Furthermore, jackknife tests were performed to identify the important variables for predicting the potential spread of the target species [66].
4.5. Prediction of the Potential Habitat and Habitat Expansion of O. latifolia in East Asia
Global binary distribution maps of O. latifolia were determined using probability distribution maps produced from the MaxEnt model based on the maximum training sensitivity plus specificity Cloglog threshold. The binary distribution maps represent the presence and absence of O. latifolia habitats under the current and future climate change scenarios (SSP2-4.5 and SSP5-8.5) for the designated time periods, 1970–2000, 2041–2060, and 2081–2100. The binary distribution maps of East Asia for each time period were extracted using global binary distribution maps of O. latifolia by using the extract by mask option of the spatial analyst tool in ArcGIS Desktop 10.8. The numbers of suitable habitat cells under the climate change scenarios, SSP2-4.5 and SSP5-8.5, in each country of east Asia were estimated using zonal statistics of the spatial analyst tool in ArcGIS. Then mean habitat suitability of O. latifolia in each country of East Asia was estimated using number of suitable habitat cells to the total number of cells in such countries during the time period of 2081–2100. Habitat suitability was estimated using the ratio of mean habitat suitability under SSP2-4.5 to SSP5-8.5 subtracted from 1. This provides a basis for evaluating the rate of invasion of O. latifolia with respect to different SSP scenarios and thereby different greenhouse gas emissions, anthropogenic land cover changes, and human population growth rates.
5. Conclusions
O. latifolia is a noxious weed that threatens plant nurseries, gardens, and crop fields, with important economic consequences. Utilizing presence-only data for O. latifolia in East Asia and environmental variables, we employed MaxEnt modeling to determine the current and future distributions of the species. Our results indicate that, at present, the suitable habitat for O. latifolia is limited to a small portion of East Asia (approximately 8.02%), particularly in China, Chinese Taipei, and Japan. However, under global climate change, the coverage is projected to expand to approximately 27.41% of the total land surface of East Asia between 2081 and 2100. Additionally, mean habitat suitability was estimated for each country, revealing that North Korea, China, Japan, and South Korea would become future invasion hotspots for O. latifolia. Our study highlights the potential invasion risks posed by O. latifolia in East Asia. These results could be instrumental in planning preventive measures, such as strong quarantine measures to prevent the introduction and spread of this noxious weed. They can also aid in the development of long-term management strategies at both regional and local scales in East Asian countries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12183254/s1. Table S1: Global occurrence points of O. latifolia; Table S2: List of bioclimatic variables used in modeling of O. latifolia; Table S3: Estimation of Pearson correlation coefficient for selecting most suitable bioclimatic variables; Figure S1: Jackknife test analysis for evaluating test performance of O. latifolia under the current climate (1970–2000) and future climate scenarios (SSP2-4.5 and SSP5-8.5) for the year 2041–2060 and 2081–2100.
Author Contributions
Conceptualization, A.P., P.A. and S.H.H.; methodology, A.P. and P.A.; software, A.P. and P.A.; validation, A.P. and P.A.; formal analysis, A.P. and P.A.; investigation, A.P., P.A., C.S.N., J.W., D.-H.L. and Y.H.L.; resources, S.H.H.; data curation, A.P., P.A., C.S.N., J.W., D.-H.L. and Y.H.L.; writing—original draft preparation, A.P.; writing—review and editing, A.P., P.A. and Y.H.L.; supervision, S.H.H.; project administration, S.H.H.; funding acquisition, S.H.H. All the authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Korea Environment Industry & Technology Institute (KEITI) funded by Korea Ministry of Environment (MOE) (No. 2018002270001 and No. 2022002450002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marshall, G. A review of the biology and control of selected weed species in the genus Oxalis: O. stricta L., O. latifolia HBK and O. pes-caprae L. Crop Prot. 1987, 6, 355–364. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Royo-Esnal, A.; López, M.L. Control of Oxalis latifolia: A review and proposals for its improvement. Cien. Inv. Agr. 2008, 35, 121–136. [Google Scholar] [CrossRef]
- Everard, M.; Gupta, N.; Chapagain, P.S.; Shrestha, B.B.; Preston, G.; Tiwari, P. Can control of invasive vegetation improve water and rural livelihood security in Nepal? Ecosyst. Serv. 2018, 32, 125–133. [Google Scholar] [CrossRef]
- Esler, A. Some aspects of the autecology of Oxalis latifolia HBK. In Proceedings of the New Zealand Weed Control Conference, Christchurch, New Zealand, 3–5 July 1962; pp. 87–90. [Google Scholar]
- Holm, L.; Doll, J.; Holm, E.; Pancho, J.V.; Herberger, J.P. World Weeds: Natural Histories and Distribution; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Shrestha, B.B.; Shrestha, U.B.; Sharma, K.P.; Thapa-Parajuli, R.B.; Devkota, A.; Siwakoti, M. Community perception and prioritization of invasive alien plants in Chitwan-Annapurna Landscape, Nepal. J. Environ. Manag. 2019, 229, 38–47. [Google Scholar] [CrossRef]
- Atwal, B.; Gopal, R. Oxalis latifolia and its control by chemical and mechanical methods in hills. Indian J. Weed Sci. 1972, 4, 74–80. [Google Scholar]
- Thomas, P. The effect of Oxalis latifolia competition in maize. S. Afr. J. Plant Soil 1991, 8, 132–135. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef]
- Jackson, D. A growth study of Oxalis latifolia HBK. N. Z. J. Sci. 1960, 3, 600–609. [Google Scholar]
- Chawdhry, M.; Sagar, G. Control of Oxalis latifolia HBK and O, pes-caprae L. by defoliation. Weed Res. 1974, 14, 293–299. [Google Scholar] [CrossRef]
- Yang, Y.; Bian, Z.; Ren, W.; Wu, J.; Liu, J.; Shrestha, N. Spatial patterns and hotspots of plant invasion in China. Glob. Ecol. Conserv. 2023, 43, e02424. [Google Scholar] [CrossRef]
- Xu, H.; Qiang, S.; Genovesi, P.; Ding, H.; Wu, J.; Meng, L.; Han, Z.; Miao, J.; Hu, B.; Guo, J. An inventory of invasive alien species in China. NeoBiota 2012, 15, 1–26. [Google Scholar] [CrossRef]
- Ministry of the Environment; Ministry of Agriculture, Forestry and Fisheries. The List of Alien Species That May Have adverse Effects on Ecosystems in Japan. 2015. Available online: https://www.env.go.jp/naure/intro/2outline/iaslist.html (accessed on 23 March 2023). (In Japanese).
- Jeong, S.; Lee, J.; Kwon, Y.; Shin, H.; Kim, S.; Ahn, J.; Huh, T. Invasive Alien Plants in South Korea; Korea National Arboretum: Pocheon, Republic of Korea, 2016. [Google Scholar]
- Munkhnast, D.; Chuluunjav, C.; Urgamal, M.; Wong, L.J.; Pagad, S. GRIIS Checklist of Introduced and Invasive Species—Mongolia. GRIIS 2020. Available online: https://www.gbif.org/dataset/ca55b876-88ef-44a1-b752-c38977af7d2f (accessed on 23 March 2023).
- Ahmad, R.; Khuroo, A.A.; Hamid, M.; Charles, B.; Rashid, I. Predicting invasion potential and niche dynamics of Parthenium hysterophorus (Congress grass) in India under projected climate change. Biodivers. Conserv. 2019, 28, 2319–2344. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Rahimian Boogar, A.; Salehi, H.; Pourghasemi, H.R.; Blaschke, T. Predicting habitat suitability and conserving Juniperus spp. habitat using SVM and maximum entropy machine learning techniques. Water 2019, 11, 2049. [Google Scholar] [CrossRef]
- Bosso, L.; De Conno, C.; Russo, D. Modelling the risk posed by the zebra mussel Dreissena polymorpha: Italy as a case study. Environ. Manag. 2017, 60, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, S.; Mortazavi, M.S.; Nozar, S.L.M. Predicting present spatial distribution and habitat preferences of commercial fishes using a maximum entropy approach. Environ. Sci. Pollut. Res. 2023, 30, 75300–75313. [Google Scholar] [CrossRef]
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Shin, M.-S.; Adhikari, P.; Seo, C. Potential impact of climate change on plant invasion in the Republic of Korea. J. Ecol. Environ. 2019, 43, 36. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.-H.; Park, Y.-S.; Hong, S.-H. Assessment of the spatial invasion risk of intentionally introduced alien plant species (IIAPS) under environmental change in South Korea. Biology 2021, 10, 1169. [Google Scholar] [CrossRef]
- López-Tirado, J.; Gonzalez-Andújar, J.L. Spatial weed distribution models under climate change: A short review. PeerJ 2023, 11, e15220. [Google Scholar] [CrossRef]
- Nazarenko, L.S.; Tausnev, N.; Russell, G.L.; Rind, D.; Miller, R.L.; Schmidt, G.A.; Bauer, S.E.; Kelley, M.; Ruedy, R.; Ackerman, A.S. Future climate change under SSP emission scenarios with GISS-E2. 1. J. Adv. Model. Earth Syst. 2022, 14, e2021MS002871. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Midgley, G.F. 12 Will Climate Change Promote Alien Plant Invasions? Ecol. Stud. 2007, 193, 197. [Google Scholar]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Oh, H.-S.; Adhikari, P.; Seo, C. Northward range expansion of southern butterflies according to climate change in South Korea. J. Clim. Change 2020, 11, 643–656. [Google Scholar] [CrossRef]
- Kim, H.W.; Adhikari, P.; Chang, M.H.; Seo, C. Potential distribution of amphibians with different habitat characteristics in response to climate change in South Korea. Animals 2021, 11, 2185. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, Y.H.; Lee, G.; Lee, D.-H.; Adhikari, P. Predicting impacts of climate change on northward range expansion of invasive weeds in South Korea. Plants 2021, 10, 1604. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.-H.; Poudel, A.; Lee, G.; Hong, S.-H.; Park, Y.-S. Predicting the Impact of Climate Change on the Habitat Distribution of Parthenium hysterophorus around the World and in South Korea. Biology 2023, 12, 84. [Google Scholar] [CrossRef]
- Adhikari, P.; Kim, B.-J.; Hong, S.-H.; Lee, D.-H. Climate change induced habitat expansion of nutria (Myocastor coypus) in South Korea. Sci. Rep. 2022, 12, 3300. [Google Scholar] [CrossRef]
- Vale, C.G.; Tarroso, P.; Brito, J.C. Predicting species distribution at range margins: Testing the effects of study area extent, resolution and threshold selection in the Sahara–Sahel transition zone. Divers. Distrib. 2014, 20, 20–33. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Hong, S.H.; Park, Y.-S. Global spatial distribution of Chromolaena odorata habitat under climate change: Random forest modeling of one of the 100 worst invasive alien species. Sci. Rep. 2023, 13, 9745. [Google Scholar] [CrossRef]
- Steen, V.; Sofaer, H.R.; Skagen, S.K.; Ray, A.J.; Noon, B.R. Projecting species’ vulnerability to climate change: Which uncertainty sources matter most and extrapolate best? Ecol. Evol. 2017, 7, 8841–8851. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, H.; Feng, Q.; Jin, C.; Cao, A.; He, L. A new strategy for the prevention and control of Eupatorium adenophorum under climate change in China. Sustainability 2017, 9, 2037. [Google Scholar] [CrossRef]
- CABI. Oxalis latifolia (Sorrel); CABI International: Wallingford, UK, 2022. [Google Scholar]
- Royo, A. Study of the Biology and Ecology of Oxalis latifolia Kunth: Effect of Environmental and Cultural Factors on Its Etiology. Ph. D. Thesis, University of Navarra, Pamplona, Spain, 2004. [Google Scholar]
- Telenius, A.; Jonsson, C. Molluscs of the Gothenburg Natural History Museum (GNM); GBIF: Stockholm, Sweden, 2017. [Google Scholar]
- Batima, P.; Natsagdorj, L.; Gombluudev, P.; Erdenetsetseg, B. Observed climate change in Mongolia. Assess. Imp. Adapt. Clim. Change Work. Pap. 2005, 12, 1–26. [Google Scholar]
- Qin, J.; Su, B.; Tao, H.; Wang, Y.; Huang, J.; Jiang, T. Projection of temperature and precipitation under SSPs-RCPs Scenarios over northwest China. Front. Earth Sci. 2021, 15, 23–37. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Li, Z.; Mihara, K.; Kuramochi, K.; Toma, Y.; Hatano, R. Climate change multi-model projections in CMIP6 scenarios in Central Hokkaido, Japan. Sci. Rep. 2023, 13, 230. [Google Scholar] [CrossRef]
- Yu, I.; Jung, H.; Lee, D.-K.; Lee, S.-H.; Hong, S.-I. Multi-risk assessment due to global warming under the SSP climate scenario in the Republic of Korea. No. EGU23–5301. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M. IPCC Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Iintergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Adhikari, P.; Park, S.-M.; Kim, T.-W.; Lee, J.-W.; Kim, G.-R.; Han, S.-H.; Oh, H.-S. Seasonal and altitudinal variation in roe deer (Capreolus pygargus tianschanicus) diet on Jeju Island, South Korea. J. Asia Pac. Biodivers 2016, 9, 422–428. [Google Scholar] [CrossRef][Green Version]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.-B.; Pe’er, G.; Singer, A.; Bridle, J.; Crozier, L.; De Meester, L.; Godsoe, W. Improving the forecast for biodiversity under climate change. Science 2016, 353, aad8466. [Google Scholar] [CrossRef] [PubMed]
- McDougall, K.L.; Lembrechts, J.; Rew, L.J.; Haider, S.; Cavieres, L.A.; Kueffer, C.; Milbau, A.; Naylor, B.J.; Nuñez, M.A.; Pauchard, A. Running off the road: Roadside non-native plants invading mountain vegetation. Biol. Invasions 2018, 20, 3461–3473. [Google Scholar] [CrossRef]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Yi, Y.-j.; Zhou, Y.; Cai, Y.-p.; Yang, W.; Li, Z.-w.; Zhao, X. The influence of climate change on an endangered riparian plant species: The root of riparian Homonoia. Ecol. Indic. 2018, 92, 40–50. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hejazi, M.; Santos Da Silva, S.R.; Miralles-Wilhelm, F.; Kim, S.; Kyle, P.; Liu, Y.; Vernon, C.; Delgado, A.; Edmonds, J.; Clarke, L. Impacts of water scarcity on agricultural production and electricity generation in the Middle East and North Africa. Front. Environ. Sci. 2023, 11, 157. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.; Park, C.; Kim, S. Comparison of the results of climate change impact assessment between RCP8. 5 and SSP2 scenarios. In Proceedings of the American Geophysical Union (AGU) Fall Meeting Abstracts, New Orleans, LA, USA, 11–15 December 2017. [Google Scholar]
- Lee, J.-Y.; Marotzke, J.; Bala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fischer, E.; Fyfe, J.C.; Jones, C. Future Global Climate: Scenario-Based Projections and Near-Term Information; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Gutjahr, O.; Putrasahan, D.; Lohmann, K.; Jungclaus, J.H.; von Storch, J.-S.; Brüggemann, N.; Haak, H.; Stössel, A. Max planck institute earth system model (MPI-ESM1. 2) for the high-resolution model intercomparison project (HighResMIP). Geosci. Model Dev. 2019, 12, 3241–3281. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Anand, V.; Oinam, B.; Singh, I.H. Predicting the current and future potential spatial distribution of endangered Rucervus eldii eldii (Sangai) using MaxEnt model. Environ. Monit. Assess 2021, 193, 1–17. [Google Scholar] [CrossRef]
- Bosso, L.; Smeraldo, S.; Russo, D.; Chiusano, M.L.; Bertorelle, G.; Johannesson, K.; Butlin, R.K.; Danovaro, R.; Raffini, F. The rise and fall of an alien: Why the successful colonizer Littorina saxatilis failed to invade the Mediterranean Sea. Biol. Invasions 2022, 24, 3169–3187. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef]
- Shin, M.-S.; Seo, C.; Lee, M.; Kim, J.-Y.; Jeon, J.-Y.; Adhikari, P.; Hong, S.-B. Prediction of potential species richness of plants adaptable to climate change in the Korean Peninsula. J. Environ. Impact Assess. 2018, 27, 562–581. [Google Scholar]
- Adhikari, P.; Shin, M.-S.; Jeon, J.-Y.; Kim, H.W.; Hong, S.; Seo, C. Potential impact of climate change on the species richness of subalpine plant species in the mountain national parks of South Korea. J. Ecol. Environ. 2018, 42, 36. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (version 3.4.1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 24 October 2022).
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods. Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).