Teline monspessulana Can Harm the Chilean Native Tree Nothofagus obliqua: Effects on Germination and Initial Growth
Abstract
1. Introduction
2. Results
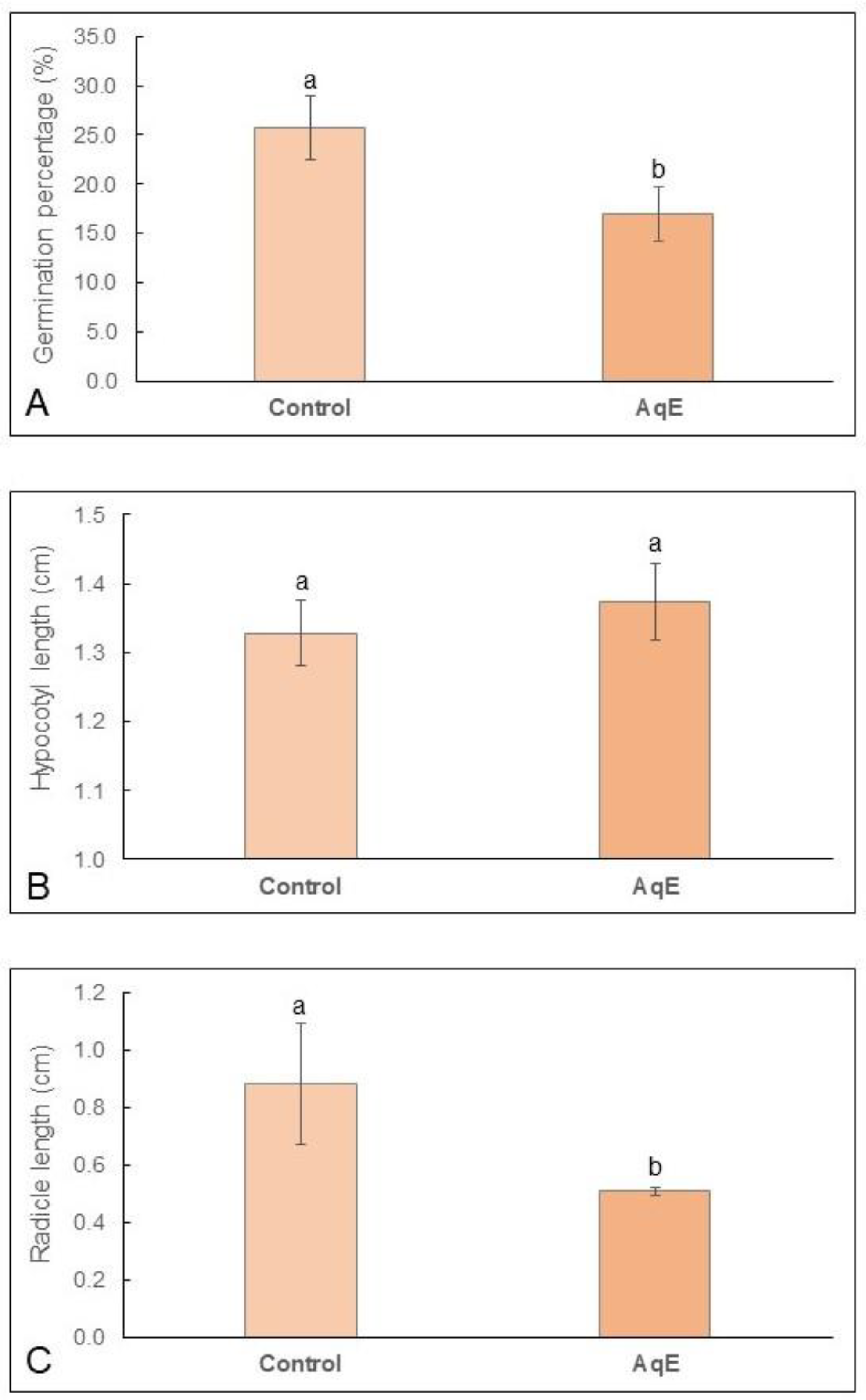
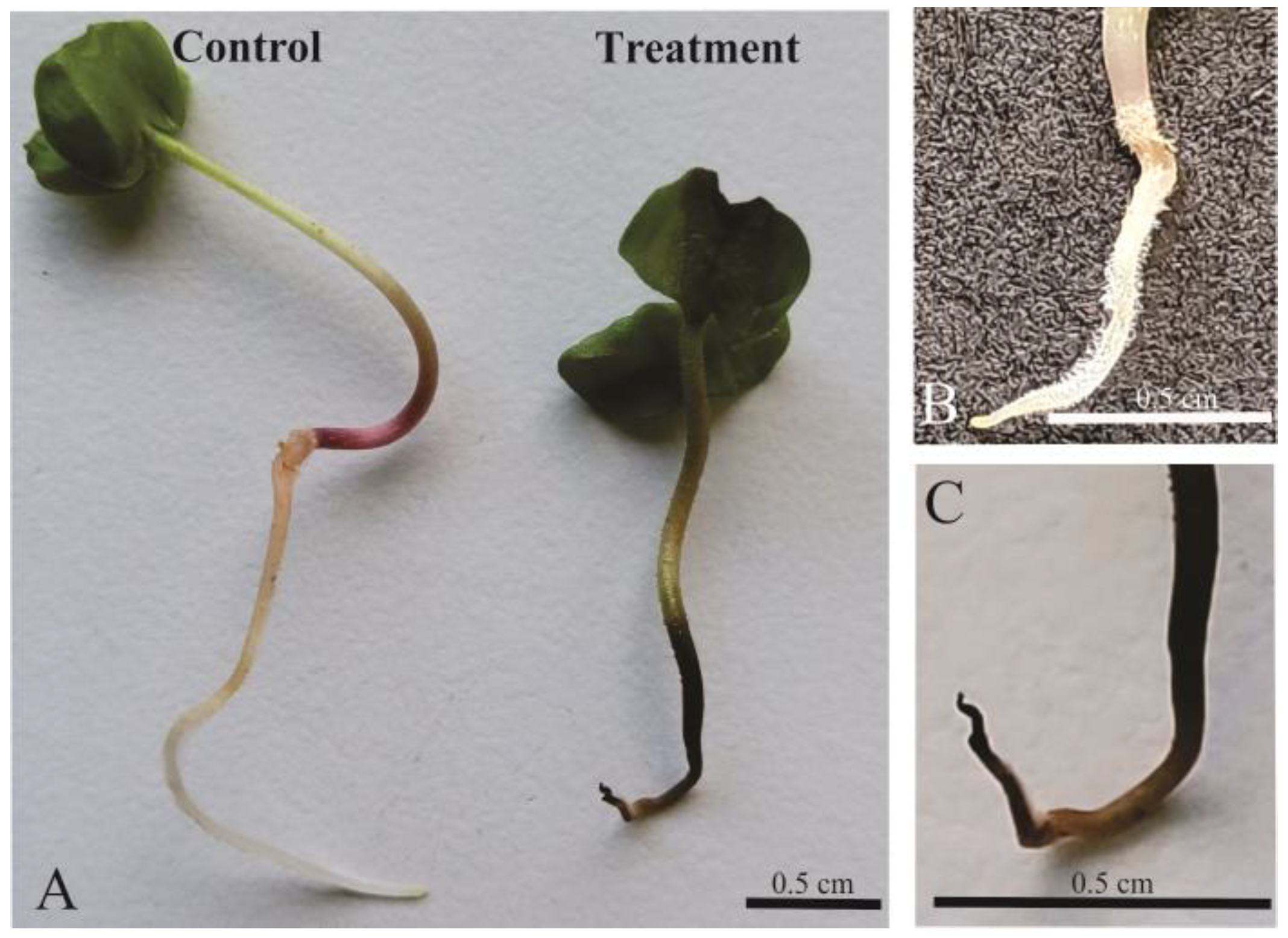
2.1. In Vitro Assay: Morphometric Measurements
2.2. Assay in Substrate
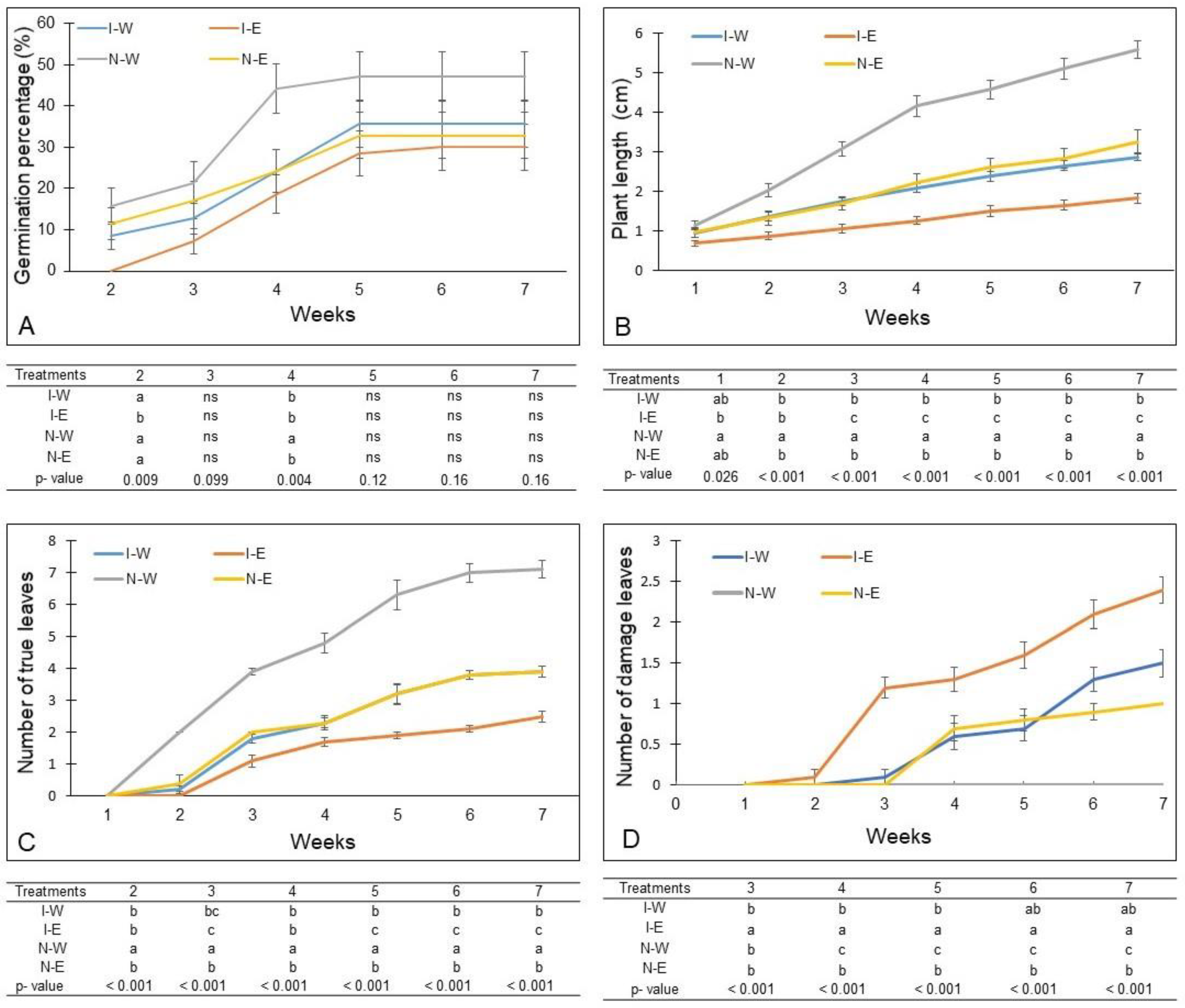
2.2.1. Dynamics of Germination and Morphometric Variables
2.2.2. Anatomical Analysis
2.2.3. Chemical Characteristics of the Substrates
2.2.4. Alkaloid Profile of T. monspessulana
2.2.5. Phenol Profile of T. monspessulana
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling Sites and Plant Material
5.2. Preparation of Aqueous Extract
5.3. In Vitro Bioassays
5.4. Bioassays in Substrate
5.4.1. Substrate Collection and Preparation
5.4.2. Bioassay Establishment
5.4.3. Anatomical Analysis
5.5. Chemical Substrate Analysis
5.6. Characterization of the Alkaloid Profile
5.7. Characterization of Phenol Profiles
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Oduor, A.M.O.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.; van Kleunen, M. Do Invasive Alien Plants Benefit More from Global Environmental Change than Native Plants? Glob. Chang. Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Hierro, J.L. Community Assembly Theory as a Framework for Biological Invasions. Trends Ecol. Evol. 2018, 33, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, E.W.A.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H.S. Controlling Invasive Plant Species in Ecological Restoration: A Global Review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Hussain, W.S. Allelopathy: Allelochemicals a Brief Review. Plant Arch. 2020, 20, 5556–5560. [Google Scholar]
- Šoln, K.; Klemenčič, M.; Koce, J.D. Plant Cell Responses to Allelopathy: From Oxidative Stress to Programmed Cell Death. Protoplasma 2022, 259, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and Physiological Mechanisms Mediated by Allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; González, L. Allelopathic Interference of Invasive Acacia dealbata Link on the Physiological Parameters of Native Understory Species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Mojarad, A.K.; Majad, A.; Islamic, F. Allopathic Effects of Nerium oleander L. on Growth and Anatomy Structure of Hordeum vulgare (Monocotyledon) and Vicia sativa (Dicotyledon) Seedlings 1A. Adv. Environ. Biol. 2013, 7, 766–771. [Google Scholar]
- Inderjit; Wardle, D.A.; Karban, R.; Callaway, R.M. The Ecosystem and Evolutionary Contexts of Allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Kleunen, M. Van Effect of Allelopathy on Plant Performance: A Meta-Analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint Action of Phenolic Acid Mixtures and its Significance in Allelopathy Research. Physiol. Plant. 2002, 114, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Duke, S.O. Ecophysiological Aspects of Allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Blanco, Y. Revisión Bibliografica La Utilización de la Alelopatía y sus Efectos. Cultiv. Trop. 2006, 27, 5–16. [Google Scholar]
- Mushtaq, W.; Siddiqui, M.B.; Hakeem, K.R. Allelopathy Potential for Green Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030408060. [Google Scholar]
- Lamarque, L.J.; Delzon, S.; Lortie, C.J. Tree Invasions: A Comparative Test of the Dominant Hypotheses and Functional Traits. Biol. Invasions 2011, 13, 1969–1989. [Google Scholar] [CrossRef]
- Aguilera, N.; Guedes, L.M.; Becerra, J.; González, L. Is Autotoxicity Responsible for Inhibition Growth of New Conspecific Seedlings under the Canopy of the Invasive Acacia dealbata Link? Gayana. Botánica 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy Is Pervasive in Invasive Plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- LPWG A New Subfamily Classification of the Leguminosae Based on a Taxonomically Comprehensive Phylogeny. Taxon 2017, 66, 44–77. [CrossRef]
- Wink, M. Evolution of Secondary Metabolites from an Ecological and Molecular Phylogenetic Perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Feitoza, R.B.B.; Lima, H.R.P. Chemosystematic and Evolutionary Trends of the Genistoid Clade Sensu Stricto (Papilionoideae, Fabaceae). Phytochemistry 2021, 183, 112616. [Google Scholar] [CrossRef]
- Fuentes, N.; Sánchez, P.; Pauchard, A.; Urrutia, J.; Cavieres, L.; Marticorena, A. Plantas Invasoras Del Centro-Sur de Chile: Una Guía de Campo; Laboratorio de Invasiones Biologicas (LIB): Concepción, Chile, 2014; ISBN 9789563580310. [Google Scholar]
- García, R.; Pauchar, A.; Cavieres, L.; Peña, E.; MF, R. El Fuego Favorece la Invasión de Teline monspessulana (Fabaceae) al Aumentar su Germinación. Rev. Chil. Hist. Nat. 2010, 83, 443–452. [Google Scholar] [CrossRef]
- Fuentealba, J.; Saez-Orellana, F. Neuroactive Alkaloids that Modulate the Neuronal Nicotinic Receptor and Provide Neuroprotection in an Alzheimer’s Disease Model: The Case of Teline monspessulana. Neural Regen. Res. 2014, 9, 1880–1881. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Cely-Veloza, W.; Coy-Barrera, E. Identification of Anti-Proliferative Compounds from Genista monspessulana Seeds through Covariate-Based Integration of Chemical Fingerprints and Bioactivity Datasets. Molecules 2022, 27, 3996. [Google Scholar] [CrossRef] [PubMed]
- Meza, A.; Rojas, P.; Cely-Veloza, W.; Guerrero-Perilla, C.; Coy-Barrera, E. Variation of Isoflavone Content and DPPH• Scavenging Capacity of Phytohormone-Treated Seedlings after in Vitro Germination of Cape Broom (Genista monspessulana). S. Afr. J. Bot. 2020, 130, 64–74. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and Their Various Applications. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 2–15. [Google Scholar]
- Aguilera, N.; Becerra, J.; Guedes, L.; Villaseñor-Paradas, C.; González, L.; Hernández, V. Allelopathic Effect of the Invasive Acacia dealbata Link (Fabaceae) on Two Native Plant Species in South-Central Chile. Gayana Botánica 2015, 72, 231–239. [Google Scholar] [CrossRef]
- Mittermeier, R.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zacos, F., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. ISBN 9783642209918. [Google Scholar]
- Santelices, R.; Drake, F.; Mena, C.; Ordenes, R.; Navarro-Cerrillo, R.M. Current and Potential Distribution Areas for Nothofagus alessandrii, an Endangeredtree Species from Central Chile. Cienc. Investig. Agrar. 2012, 39, 521–531. [Google Scholar] [CrossRef]
- Ortiz, J.; Dube, F.; Neira, P.; Panichini, M.; Stolpe, N.B.; Zagal, E.; Martínez-Hernández, P.A. Soil Quality Changes within a (Nothofagus obliqua) Forest under Silvopastoral Management in the Andes Mountain Range, South Central Chile. Sustainability 2020, 12, 6815. [Google Scholar] [CrossRef]
- Urrutia-Jalabert, R.; Barichivich, J.; Rozas, V.; Lara, A.; Rojas, Y.; Bahamondez, C.; Rojas-Badilla, M.; Gipoulou-Zuñiga, T.; Cuq, E. Climate Response and Drought Resilience of Nothofagus obliqua Secondary Forests across a Latitudinal Gradient in South-Central Chile. For. Ecol. Manag. 2021, 485, 118962. [Google Scholar] [CrossRef]
- Sadzawka, R.; Carrasco, M.; Grez, R.; Mora, M.; Flores, H.; Neaman, A. Métodos de Análisis Recomendados para los Suelos de Chile. Revisión 2006; Instituto de Investigaciones Agropecuarias, Series Actas INIA: Santiago de Chile, Chile, 2006. [Google Scholar]
- Medina, A.; Ipinza, R. Análisis Genético de Rasgos Morfológicos de La Semilla, Germinación y Crecimiento Inicial en Raulí (Nothofagus alpina (Poepp. & Endl.) Oerst.) y Roble (Nothofagus obliqua (Mirb.) Oerst.) En Chile. Cienc. Investig. For. INFOR Chile 2012, 18, 7–23. [Google Scholar]
- Corvalán, P.; Jiménez, J.E. Seed Consumption of Southern Beech (Nothofagus obliqua) by Burrowing Parakeets (Cyanoliseus patagonus) in the Andean Foothills of Curicó. Boletín Chil. Ornitol. 2010, 16, 17–20. [Google Scholar]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant Allelochemicals: Agronomic, Nutritional and Ecological Relevance in the Soil System. Plant Soil 2019, 42, 23–48. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects. Molecules 2019, 24, 4539. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, L.H.P.; de Lima, G.P.; Martins, G.I.; Meneghetti, A.M. Germinação de Sementes e Crescimento de Plântulas de Soja (Glycine max L. Merrill) Sob Cobertura Vegetal. Acta Sci. Agron. 2009, 31, 465. [Google Scholar] [CrossRef][Green Version]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for Weed Control in Agricultural Systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll Conductance to CO2: Current Knowledge and Future Prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll Diffusion Conductance to CO2: An Unappreciated Central Player in Photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the Use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Aguilera, N.; Guedes, L.M.; Becerra, J.; Baeza, C.; Hernández, V. Morphological Effects at Radicle Level by Direct Contact of Invasive Acacia dealbata Link. Flora 2015, 215, 54–59. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, L.Y.; Hu, L.Y.; Cao, W.; Sun, K.; Sun, Q.B.; Siddikee, A.; Shi, R.H.; Dai, C.C. Evidence for the Involvement of Auxin, Ethylene and ROS Signaling During Primary Root Inhibition of Arabidopsis by the Allelochemical Benzoic Acid. Plant Cell Physiol. 2018, 59, 1889–1904. [Google Scholar] [CrossRef]
- Al-Johani, N.S.; Aytah, A.A.; Boutraa, T. Allelopathic Impact of Two Weeds, Chenopodium murale and Malva parviflora on Growth and Photosynthesis of Barley (Hordeum vulgare L.). Pakistan J. Bot. 2012, 44, 1865–1872. [Google Scholar]
- Santos, E.; Macedo, F.; Zanchim, B.; Camacho, M.; Lavres, J. Macronutrients Uptake Rate and Biomass Partitioning during Early Growth of Jatropha Plants. Rev. Ciência Agronômica 2017, 48, 565–575. [Google Scholar] [CrossRef]
- Lima, N.M.; Santos, V.N.C.; La Porta, F.A. Chemodiversity, Bioactivity and Chemosystematics of the Genus Inga (Fabaceae): A Brief Review. Rev. Virtual Quim. 2018, 10, 459–473. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusiox, S.; Weston, L. Allelopathy and the Role of Allelochemicals in Plant Defence. In How Plants Communicate with Their Biotic Environment; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 20–47. [Google Scholar]
- Bermúdez-Torres, K.; Martínez Herrera, J.; Figueroa Brito, R.; Wink, M.; Legal, L. Activity of Quinolizidine Alkaloids from Three Mexican Lupinus against the Lepidopteran Crop Pest Spodoptera frugiperda. BioControl 2009, 54, 459–466. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Allam, A.E.; Farag, S.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. New Quinolizidine Alkaloid and Insecticidal Activity of Sophora secundiflora and Sophora tomentosa against Culex pipiens (Diptera: Culicidae). Nat. Prod. Res. 2022, 36, 2722–2734. [Google Scholar] [CrossRef] [PubMed]
- Mott, I.W.; Cook, D.; Lee, S.T.; Stonecipher, C.A.; Panter, K.E. Phylogenetic Examination of Two Chemotypes of Lupinus leucophyllus. Biochem. Syst. Ecol. 2016, 65, 57–65. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, B.R.; Chen, C.K.; Wang, J.R.; Lee, S.S. Four New Fluorenone Alkaloids and One New Dihydroazafluoranthene Alkaloid from Caulophyllum robustum Maxim. Fitoterapia 2011, 82, 793–797. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z. Insecticidal Activities of Sophora flavescens Alt. towards Red Imported Fire Ants (Solenopsis invicta Buren). Toxins 2023, 15, 105. [Google Scholar] [CrossRef]
- Cely-Veloza, W.; Yamaguchi, L.; Quiroga, D.; Kato, M.J.; Coy-Barrera, E. Antifungal Activity against Fusarium oxysporum of Quinolizidines Isolated from Three Controlled-Growth Genisteae Plants: Structure–Activity Relationship Implications. Nat. Prod. Bioprospect. 2023, 13, 9. [Google Scholar] [CrossRef]
- Lee, S.T.; Cook, D.; Molyneux, R.J.; Marcolongo-Pereira, C.; Stonecipher, C.A.; Gardner, D.R. The Alkaloid Profiles of Sophora nuttalliana and Sophora stenophylla. Biochem. Syst. Ecol. 2013, 48, 58–64. [Google Scholar] [CrossRef]
- Zamora, F.; García, P.; Ruiz, M.; Salcedo, E. Composición de Alcaloides En Semillas de Lupinus mexicanus (Fabaceae) y Evaluación Antifúngica y Alelopática del Extracto Alcaloideo. Agrociencia 2007, 42, 185–192. [Google Scholar]
- O’Sullivan, E.C.; Miller, C.M.; Deane, F.M.; McCarthy, F.O. Emerging Targets in the Bioactivity of Ellipticines and Derivatives. In Studies in Natural Products Chemestry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 39, pp. 190–205. ISBN 9786021018187. [Google Scholar]
- Osorio-Castiblanco, D.F.; Peyre, G.; Saldarriaga, J.F. Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes. Sustainability 2020, 12, 57. [Google Scholar] [CrossRef]
- Wink, M. Evolution of Secondary Metabolites in Legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Stavropoulou, M.I.; Angelis, A.; Aligiannis, N.; Kalpoutzakis, E.; Mitakou, S.; Duke, S.O.; Fokialakis, N. Phytotoxic Triterpene Saponins from Bellis longifolia, an Endemic Plant of Crete. Phytochemistry 2017, 144, 71–77. [Google Scholar] [CrossRef]
- Ho, T.L.; Nguyen, V.L.; Phan, L.K.; Nguyen, C.T.; Nguyen, T.H.D.; Van, V.L.; Reid, S.J. Phytotoxicity in Aqueous Methanolic Extracts of Rice against Junglerice and Total Activities of Identified Phytotoxic Compounds. Ann. Appl. Biol. 2022, 180, 196–210. [Google Scholar] [CrossRef]
- Bruxel, F.; Rodrigues, K.F.; Gastmann, J.; Winhelmann, M.C.; Silva, S.M.; Hoehne, L.; Ethur, E.M.; Sperotto, R.A.; de Freitas, E.M. Phytotoxicity of Aqueous Extract of Ilex paraguariensis A.St.-Hil on Conyza bonariensis (L). Cronquist. S. Afr. J. Bot. 2022, 146, 546–552. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal Activity and Metabolic Profiling of Potent Allelopathic Plant Fractions Against Major Weeds of Wheat—Way Forward to Lower the Risk of Synthetic Herbicides. Front. Plant Sci. 2021, 12, 632390. [Google Scholar] [CrossRef]
- Vitalini, S.; Palmioli, A.; Orlando, F.; Scarì, G.; Airoldi, C.; De Noni, I.; Bocchi, S.; Iriti, M. Phytotoxicity, Nematicidal Activity and Chemical Constituents of Peucedanum ostruthium (L.) WDJ Koch (Apiaceae). Ind. Crops Prod. 2021, 166, 113499. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Nakamura, K.; Ohno, O.; Suenaga, K.; Okuda, N. Asparagus Decline: Autotoxicity and Autotoxic Compounds in Asparagus rhizomes. J. Plant Physiol. 2017, 213, 23–29. [Google Scholar] [CrossRef]
- de las Heras, P.; Medina-Villar, S.; Pérez-Corona, M.E.; Vázquez-de-Aldana, B.R. Leaf Litter Age Regulates the Effect of Native and Exotic Tree Species on Understory Herbaceous Vegetation of Riparian Forests. Basic Appl. Ecol. 2020, 48, 11–25. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. Allelopathy and Exotic Plant Invasion. Plant Soil 2003, 256, 29–39. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Villaseñor-Parada, C.; Lorenzo, P.; González, L.; Hernández, V. Effects and Identification of Chemical Compounds Released from the Invasive Acacia dealbata Link. Chem. Ecol. 2015, 31, 479–493. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company, Inc.: London, UK, 1940; Volume 147. [Google Scholar] [CrossRef]
- Kraus, J.; Arduin, M. Manual Básico de Métodos Em Morfologia Vegetal; EDUR: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Bissing, D.R. Haupt’s Gelatin Adhesive Mixed with Formalin for Affixing Paraffin Sections to Slides. Stain Technol. 1974, 49, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Bukatsch, F. Bermerkungen Zur Doppelf€arbung Astrablau-Safranin. Mikrokosmos 1972, 65, 255. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Ogunsusi, M.; Akinlalu, A.O.; Komolafe, I.J.; Oyedapo, O.O. Allelopathic Effects of Alkaloid Fraction of Crotalaria retusa Linn on Growth and Some Biochemical Parameters of Bean Seedlings (Phaseolus vulgaris). Int. J. Plant Physiol. Biochem. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Guedes, L.M.; Aguilera, N.; Becerra, J.; Hernández, V.; Isaias, R.M.d.S. Leaf and Stem Galls of Schinus polygamus (Cav.) Cabr (Anacardiaceae): Anatomical and Chemical Implications. Biochem. Syst. Ecol. 2016, 69, 266–273. [Google Scholar] [CrossRef]
- Berkov, S.; Bastida, J.; Viladomat, F.; Codina, C. Analysis of Galanthamine-Type Alkaloids by Capillary Gas Chromatography–Mass Spectrometry in Plants. Phytochem. Anal. 2008, 19, 285–293. [Google Scholar] [CrossRef]
- Soto, C.; Caballero, E.; Pérez, E.; Zúñiga, M.E. Effect of Extraction Conditions on Total Phenolic Content and Antioxidant Capacity of Pretreated Wild Peumus boldus Leaves from Chile. Food Bioprod. Process. 2014, 92, 328–333. [Google Scholar] [CrossRef]


| Morphometric Variables | ||||
|---|---|---|---|---|
| PL (cm) | NTL | LMR (cm) | ||
| Model | Mean | 3.59 ± 0.15 | 4.55 ± 0.19 | 3.45 ± 0.16 |
| Statistician | 50.2 | 54.3 | 48.8 | |
| p value | <0.0001 | <0.0001 | <0.0001 | |
| Substrate | Invaded | 2.89 ± 0.18 b | 3.70 ± 0.21 b | 2.79 ± 0.19 b |
| Native | 4.30 ± 0.20 a | 5.40 ± 0.26 a | 4.11 ± 0.22 a | |
| Statistician | 59.88 | 21.24 | 46.67 | |
| p value | <0.0001 | <0.0001 | <0.0001 | |
| Irrigation | Water | 4.45 ± 0.20 a | 5.70 ± 0.26 a | 4.40 ± 0.18 a |
| Extract | 2.73 ± 0.14 b | 3.40 ± 0.13 b | 2.49 ± 0.16 b | |
| Statistician | 88.48 | 35.29 | 98.93 | |
| p value | <0.0001 | <0.0001 | <0.0001 | |
| I-W | 3.61 ± 0.24 | 4.55 ± 0.29 | 3.67 ± 0.18 | |
| I-E | 2.16 ± 0.13 | 2.85 ± 0.17 | 1.91 ± 0.17 | |
| Interaction | N-W | 5.30 ± 0.17 | 6.85 ± 0.22 | 5.14 ± 0.22 |
| N-E | 3.31 ± 0.17 | 3.95 ± 0.11 | 3.08 ± 0.20 | |
| Statistician | 2.18 | 0.23 | 0.70 | |
| p value | 0.1447 | 0.6322 | 0.4049 | |
| Elements | Unit of Measurement | Native Value | Content Level (*) | Invaded Value | Content Level (*) |
|---|---|---|---|---|---|
| pH in water | 6.24 | Medium | 6.03 | Medium | |
| Organic material | % | 8.57 | High | 8.15 | High |
| Nitrates (N-NO3) | mg kg−1 | 65.60 | Medium | 45.49 | Medium |
| Ammonium (N-NH4) | mg kg−1 | 7.40 | Low | 7.40 | Low |
| N available | mg kg−1 | 73.00 | High | 53.30 | High |
| Olsen phosphorus | mg kg−1 | 19.10 | Medium | 3.50 | Low |
| K available | mg kg−1 | 227.60 | High | 135.8 | Medium |
| K interchangeable | cmol kg−1 | 0.58 | High | 0.35 | Medium |
| Ca interchangeable | cmol kg−1 | 9.82 | High | 11.16 | High |
| Mg interchangeable | cmol kg−1 | 3.03 | High | 1.69 | High |
| Compounds Name | Molecular Formula | Molecular Weight (g mol−1) | RA (%) | |||
|---|---|---|---|---|---|---|
| Leaves | Flowers | Stems | Pods | |||
| Caulophylline | C15H20N2O | 204.27 | 2.19 | 16.10 | - | - |
| Lupanine | C15H24N2O | 248.36 | - | 12.20 | - | - |
| Aphylline | C15H24N2O | 248.36 | 3.97 | 20.30 | 27.70 | 20.81 |
| Anagyrine | C15H20N2O | 244.33 | 3.00 | 4.59 | - | 7.53 |
| Sophocarpine | C15H22N2O | 246.35 | 0.73 | 0.14 | - | - |
| Ellipticine | C17H14N2 | 246.313 | 1.36 | - | - | - |
| Cytisine | C11H14N2O | 190.246 | 0.60 | - | - | - |
| Compounds | Phenolic Concentrations (mg mL−1) | p Value | |||
|---|---|---|---|---|---|
| Leaves | Flowers | Stems | Pods | ||
| 14-hydroxy benzoic | 3.9 ± 0.06 c | 6.64 ± 0.07 b | 58.07 ± 0.05 c | - | <0.001 |
| Ellagic acid | - | 4.09 ± 0.01 | - | ||
| Gallic acid | 0.28 ± 4 × 10−3 c | 0.44 ± 0.02 b | 1.48 ± 2 × 10−3 a | 0.47 ± 0.02 b | <0.001 |
| Acid 5-(hydroxy methyl)furfural | - | - | 4.41 ± 0.10 | - | |
| p-tyrosol | - | - | 2.10 ± 0.22 | - | |
| Catechin | 0.15 ± 1.9 × 10−3 | - | - | ||
| Vanillic acid | 5.7 ± 0.01 c | 6.49 ± 0.03 b | 7.74 ± 0.02 a | 6.53 ± 0.02 b | <0.001 |
| Epicatechin | - | 0.37 ± 0.02 | - | - | |
| 3,4-dimetho-ybenzyl alcohol | - | - | 9.28 ± 0.1 | - | |
| Vanillin | 1.56 ± 0.13 c | 3.39 ± 0.10 a | 1.82 ± 0.02 b | - | <0.001 |
| Pinocembrin | - | 1.58 ± 0.03 | - | - | |
| Chlorogenic acid | 0.41 ± 0.02 c | 2.24 ± 0.03 a | 0.47 ± 0.01 b | 2.08 ± 0.01 a | <0.001 |
| Caffeic acid | 0.08 ± 0.03 b | 0.36 ± 0.22 b | 0.88 ± 1 × 10−3 a | - | <0.001 |
| p-coumaric acid | 0.17 ± 0.01 c | 6.97 ± 0.01 a | 0.26 ± 1 × 10−4 b | 6.31 ± 0.01 a | <0.001 |
| Trans-ferulic | 0.35 ± 0.01 b | 2.02 ± 0.01 a | - | 1.98 ± 0.01 a | <0.001 |
| Acid apigenin | 4.64 ± 0.01 | - | - | - | |
| Quercetin 3-rutinoside | 1.9 ± 0.03 | 9.77 ± 5 × 10−2 | - | - | <0.001 |
| Quercetin 3- glucoside | 5.01 ± 0.71 b | 31.11 ± 6 × 10−3 a | 1.64 ± 1 × 10−4 c | - | <0.001 |
| Myricetin | 1.30 ± 0.01 | - | - | ||
| Quercetin | 0.14 ± 4 × 10−3 b | 0.14 ± 2 × 10−3 b | 0.24 ± 1 × 10−3 a | - | <0.001 |
| Kaempferol | 2.82 ± 7 × 10−3 a | 1.05 ± 4 × 10−3 b | 0.34 ± 6 × 10−3 c | - | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera, N.; Guedes, L.M.; Alvarado, U.; Sáez-Carrillo, K. Teline monspessulana Can Harm the Chilean Native Tree Nothofagus obliqua: Effects on Germination and Initial Growth. Plants 2023, 12, 3419. https://doi.org/10.3390/plants12193419
Aguilera N, Guedes LM, Alvarado U, Sáez-Carrillo K. Teline monspessulana Can Harm the Chilean Native Tree Nothofagus obliqua: Effects on Germination and Initial Growth. Plants. 2023; 12(19):3419. https://doi.org/10.3390/plants12193419
Chicago/Turabian StyleAguilera, Narciso, Lubia M. Guedes, Ulises Alvarado, and Katia Sáez-Carrillo. 2023. "Teline monspessulana Can Harm the Chilean Native Tree Nothofagus obliqua: Effects on Germination and Initial Growth" Plants 12, no. 19: 3419. https://doi.org/10.3390/plants12193419
APA StyleAguilera, N., Guedes, L. M., Alvarado, U., & Sáez-Carrillo, K. (2023). Teline monspessulana Can Harm the Chilean Native Tree Nothofagus obliqua: Effects on Germination and Initial Growth. Plants, 12(19), 3419. https://doi.org/10.3390/plants12193419






