The Effect of Different Rhizobial Symbionts on the Composition and Diversity of Rhizosphere Microorganisms of Chickpea in Different Soils
Abstract
1. Introduction
2. Results
2.1. Bacterial Species (OTU) Richness in the Chickpea Rhizosphere with the Different Treatments
2.2. Bacterial Diversity in the Chickpea Rhizosphere with the Different Treatments
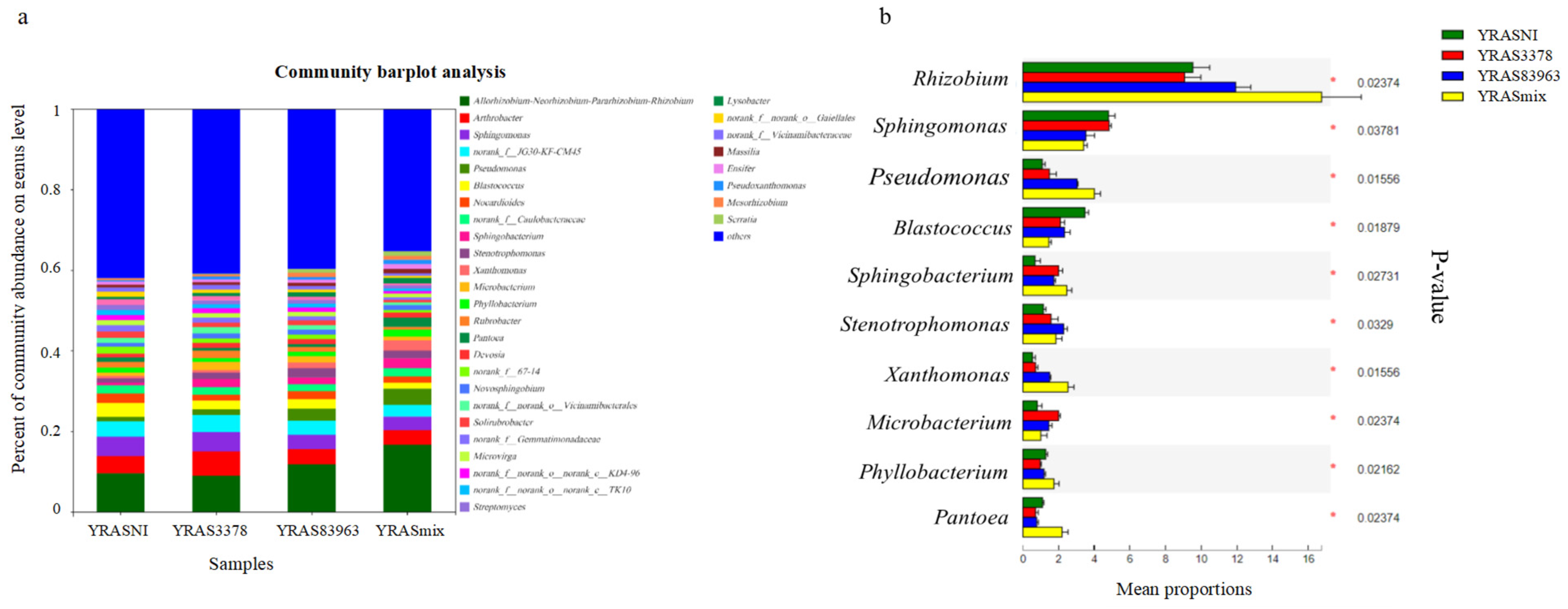
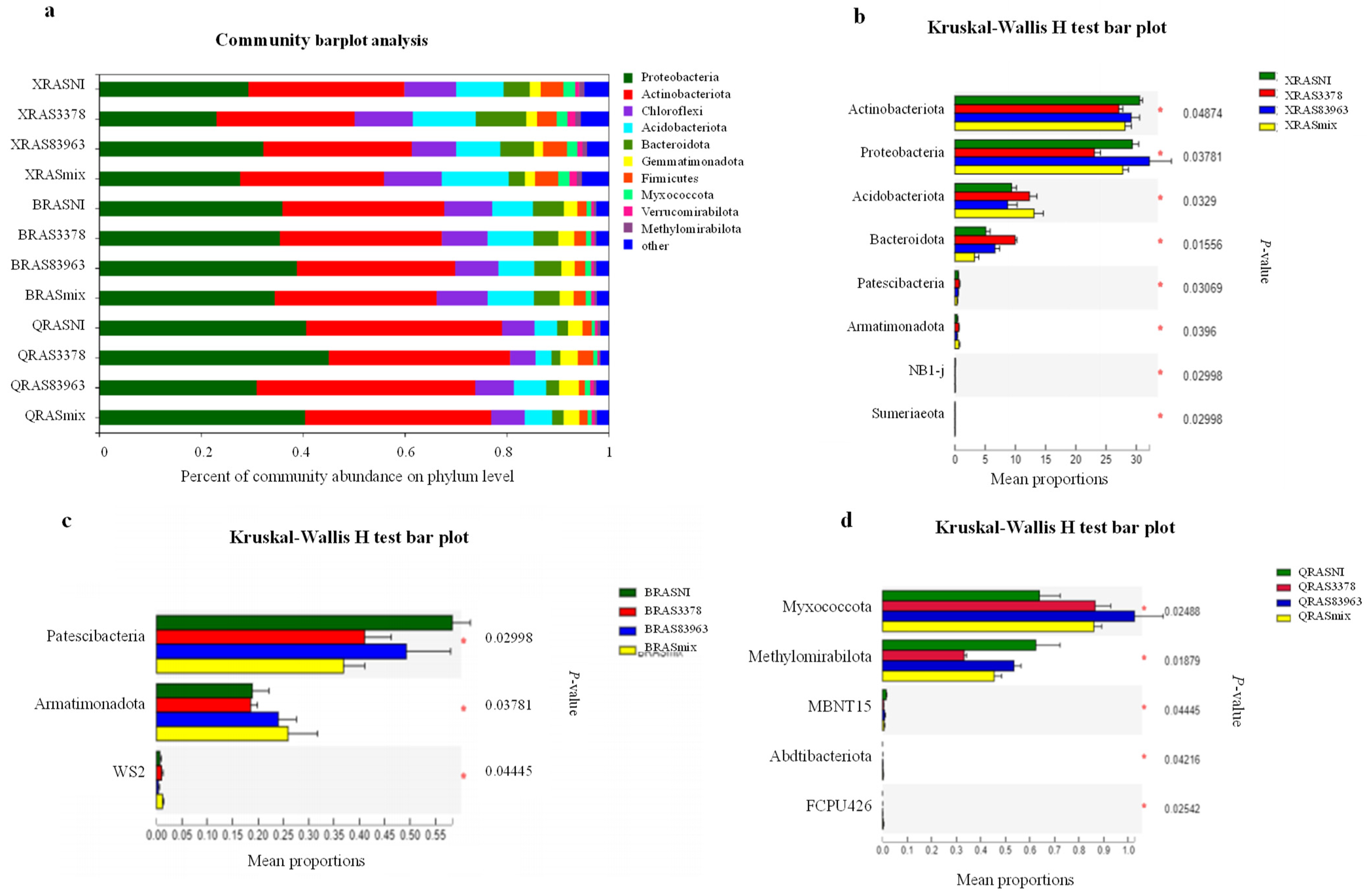
2.3. Bacterial Community in the Rhizosphere of Chickpea with the Different Treatments
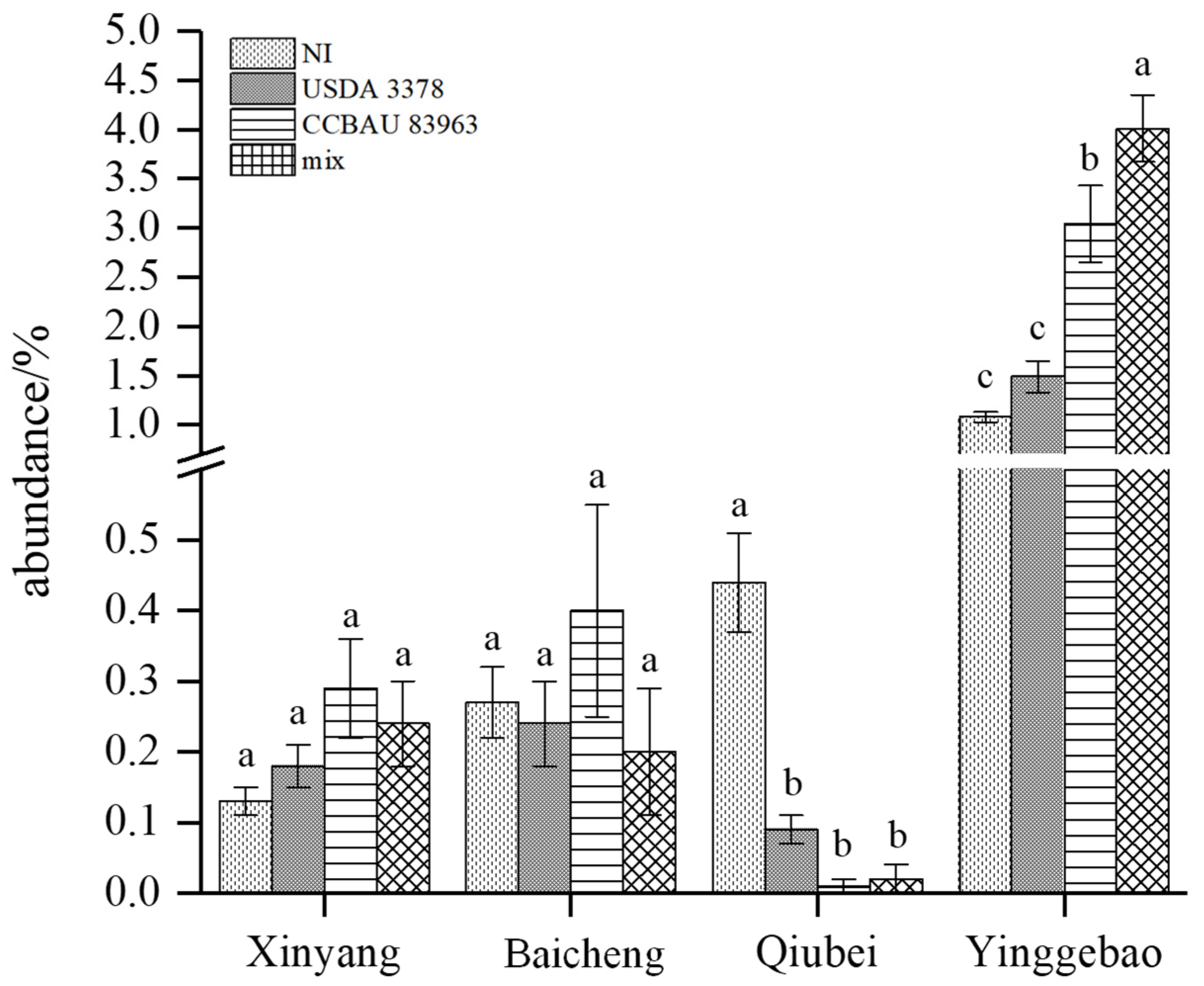
2.4. Inoculation Effects on Rhizosphere Microbiota and Exploration of Key Microorganisms
2.5. Soil Characteristics and Correlation between Soil Characteristics and Bacterial Composition from Rhizosphere Soils
3. Discussion
4. Materials and Methods
4.1. Experimental Soil and Rhizobia
4.2. Experimental Design and Growth Conditions
4.3. Sampling of Rhizosphere Soil
4.4. Bacterial 16S rRNA Sequencing
4.5. Bioinformatics and Statistical Analysis
4.6. Correlation between Soil Characteristics and Bacterial Composition from Rhizosphere Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, F.; Diwakar, B. (Eds.) Chickpea Botany and Production Practices; ICRISAT: Patancheru, India, 1995; p. 1. [Google Scholar]
- Ladizinsky, G. A New Cicer from Turkey; Springer: Edinburgh, UK, 1975; Volume 34, pp. 201–202. [Google Scholar]
- Shcherbakova, E.; Shcherbakov, A.; Andronov, E.; Gonchar, L.; Kalenskaya, S.; Chebotar, V. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis 2017, 73, 57–69. [Google Scholar] [CrossRef]
- Singh, Z.; Singh, G.; Saravaiya, S. Role of Rhizobium in chickpea (Cicer arietinum) production—A review. Agr. Rev. 2018, 38, 31–39. [Google Scholar] [CrossRef]
- Aslam, M.; Shah, J.; Hussain, N.; Ghaffar, A.; Abbas, M.; Hassan, M.; Khan, A.; Nadeem, M.; Irshad, M. Chickpea advanced lines screening for sources of resistance against two major diseases of chickpea “wilt and blight” . Pak. J. Phytopathol. 2021, 33, 369–382. [Google Scholar]
- Chen, X.; Ge, J.; Ma, D.; Ma, L.; Liu, W.; Qiang, S. Characterization and Identification of an epidemic strain of Ascochyta rabieion chickpeas in Northwest China. J. Phytopathol. 2017, 165, 355–360. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Q.; Chen, Y.; Tian, B.; Xu, L.; Bai, Y.; Chen, W.; Li, X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J. 2020, 14, 1915–1928. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Taylor, B.N.; Menge, D.N.L. Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants 2018, 4, 655–661. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.M.; Kronzucker, H.J. How plant root exudates shape the nitrogen cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Schumpp, O.; Deakin, W.J. How inefficient rhizobia prolong their existence within nodules. Trends Plant Sci. 2010, 15, 189–195. [Google Scholar] [CrossRef]
- Wang, D.; Yang, S.M.; Tang, F.; Zhu, H.Y. Symbiosis specificity in the legume—Rhizobial mutualism. Cell. Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lorite, M.J.; Estrella, M.J.; Escaray, F.J.; Sannazzaro, A.; Castro, I.; Monza, J.; Sanjuan, J.; Leon-Barrios, M. The rhizobia-Lotus symbioses: Deeply specific and widely diverse. Front. Microbiol. 2018, 9, 2055. [Google Scholar] [CrossRef] [PubMed]
- van der Drift, K.M.; Spaink, H.P.; Bloemberg, G.V.; van Brussel, A.A.; Lugtenberg, B.J.; Haverkamp, J.; Thomas-Oates, J.E. Rhizobium leguminosarum bv. trifolii produces lipo-chitin oligosaccharides with nodE-dependent highly unsaturated fatty acyl moieties. An electrospray ionization and collision-induced dissociation tandem mass spectrometric study. J. Biol. Chem. 1996, 271, 22563–22569. [Google Scholar] [CrossRef]
- Pueppke, S.G.; Broughton, W.J. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Interact. MPMI 1999, 12, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Toro, A. Nodulation competitiveness in the Rhizobium-legume symbiosis. World J. Microbiol. Biotechnol. 1996, 12, 157–162. [Google Scholar] [CrossRef]
- Frey, S.D.; Blum, L.K. Effect of pH on competition for nodule occupancy by type I and type II strains of Rhizobium leguminosarum bv. phaseoli. Plant Soil 1994, 163, 157–164. [Google Scholar] [CrossRef]
- Verástegui-Valdés, M.M.; Zhang, Y.J.; Rivera-Orduna, F.N.; Cheng, H.P.; Sui, X.H.; Wang, E.T. Microsymbionts of Phaseolus vulgaris in acid and alkaline soils of Mexico. Syst. Appl. Microbiol. 2014, 37, 605–612. [Google Scholar] [CrossRef]
- Ji, Z.J.; Yan, H.; Cui, Q.G.; Wang, E.T.; Chen, W.F.; Chen, W.X. Competition between rhizobia under different environmental conditions affects the nodulation of a legume. Syst. Appl. Microbiol. 2017, 40, 114–119. [Google Scholar] [CrossRef]
- Mason, M.L.; Tabing, B.L.; Yamamoto, A.; Saeki, Y. Influence of flooding and soil properties on the genetic diversity and distribution of indigenous soybean-nodulating bradyrhizobia in the Philippines. Heliyon 2018, 4, e00921. [Google Scholar] [CrossRef]
- Yang, S.H.; Chen, W.H.; Wang, E.T.; Chen, W.F.; Yan, J.; Han, X.Z.; Tian, C.F.; Sui, X.H.; Singh, R.P.; Jiang, G.M.; et al. Rhizobial biogeography and inoculation application to soybean in four regions across China. J. Appl. Microbiol. 2018, 125, 853–866. [Google Scholar] [CrossRef]
- Beckers, B.; Michiel, O.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.; Berkum, P.; Chen, W.; Nour, S.; Fernandez, M.; Cleyetmarel, J.; Gillis, M. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. Int. J. Syst. Bacteriol. 1997, 47, 895–898. [Google Scholar] [CrossRef]
- Nour, S.; Cleyet-Marel, C.; Normand, P.; Fernandez, M. Genomic heterogeneity of strains nodulating chickpeas (Cicer arietinum L.) and description of Rhizobium mediterraneum sp. nov. Int. J. Syst. Bacteriol. 1995, 45, 640–648. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Chen, W.; Wang, E.; Sui, X.; Zhang, X.; Li, Y.; Chen, W. Mesorhizobium muleiense sp. nov., nodulating with Cicer arietinum L. in Xinjiang, China. Int. J. Syst. Evol. Microbiol. 2012, 162, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, C.; Chen, W.; Lajudie, P.d.; Zhang, Z.; Shang, Y.; Wang, E. Mesorhizobium wenxiniae sp. nov., isolated from chickpea (Cicer arietinum L.) in China. Int. J. Syst. Evol. Microbiol. 2018, 68, 1930–1936. [Google Scholar] [CrossRef]
- Laranjo, M.; Machado, J.; Young, J.; Oliveira, S. High diversity of chickpea Mesorhizobium species isolated in a Portuguese agricultural region. FEMS Microbiol. Ecol. 2004, 48, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.; Laranjo, M.; Oliveira, S. Natural populations of chickpea rhizobia evaluated by antibiotic resistance profiles and molecular methods. Microb. Ecol. 2006, 51, 128–136. [Google Scholar] [CrossRef]
- Tena, W.; Wolde-Meskel, E.; Degefu, T.; Walley, F. Genetic and phenotypic diversity of rhizobia nodulating chickpea (Cicer arietinum L.) in soils from southern and central Ethiopia. Can. J. Microbiol. 2017, 63, 690–707. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Ikeda, S.; Rallos, L.E.E.; Okubo, T.; Eda, S.; Inaba, S.; Mitsui, H.; Minamisawa, K. Microbial community analysis of field-grown soybeans with different nodulation phenotypes. Appl. Environ. Microbiol. 2008, 74, 5704–5709. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Ueda, Y.; Zushi, T.; Takase, H.; Yazaki, K. Changes in the bacterial community of soybean rhizospheres during growth in the Field. PLoS ONE 2014, 9, e100709. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, W.; Zong, L.; Yang, J.; Jiao, S.; Lin, Y.; Wang, E.; Wei, G. Two cultivated legume plants reveal the enrichment process of the microbiome in the rhizocompartments. Mol. Ecol. 2017, 26, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Ruben, G.O.; Thomas, N.R.; Nina, D.; Ka-Wai, M.; Mchardy, A.C.; Paul, S.L. Modular traits of the rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe 2018, 24, 155–167.e155. [Google Scholar]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; Van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.; Bohlool, B.; Singleton, P. Environmental effects on competition for nodule occupancy between introduced and indigenous rhizobia and among introduced strains. Can. J. Microbiol. 1992, 38, 493–500. [Google Scholar] [CrossRef][Green Version]
- Romdhane, S.; Aouani, M.; Mhamdi, R. Inefficient nodulation of chickpea (Cicer arietinum L.) in the arid and Saharan climates in Tunisia by Sinorhizobium meliloti biovar medicaginis. Ann. Microbiol. 2007, 57, 15–19. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, K.; Jin, X.; Mao, P.; Wang, E.; Tian, C.; Sui, X.; Chen, W.; Chen, W. Distinctive Mesorhizobium populations associated with Cicer arietinum L. in alkaline soils of Xinjiang, China. Plant Soil 2012, 353, 123–134. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Wang, N.; Chen, W.; Feng, X.; Jia, B.; Zhao, Y.; Yang, T.; Zong, X. The introduced strain Mesorhizobium ciceri USDA3378 is more competitive than an indigenous strain in nodulation of chickpea in newly introduced areas of China. Lett. Appl. Microbiol. 2022, 75, 1171–1181. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yu, T.; Lou, K.; Mao, P.H.; Wang, E.T.; Chen, W.F.; Chen, W.X. Genotypic alteration and competitive nodulation of Mesorhizobium muleiense against exotic chickpea rhizobia in alkaline soils. Syst. Appl. Microbiol. 2014, 37, 520–524. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Chen, W.; Schlaeppi, K.; Erb, M.; Stirling, E.; Hu, L.; Wang, E.; Zhang, Y.; Zhao, K.; et al. Rhizobium symbiotic capacity shapes root-associated microbiomes in soybean. Front. Microbiol. 2021, 12, 709012. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Heijden, M.v.d.; Roussely-Provent, V.; JC, W.; Schlaeppi, K. Deciphering composition and function of the root microbiome of a legume plant. Microbiome 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, J.; Choi, Y.; Weon, H.; Song, J. Bacterial core community in soybean rhizosphere. Korean J. Microbiol. 2015, 51, 347–354. [Google Scholar] [CrossRef]
- Shen, B.; Hong, X.; Cao, Y.; Han, C.; Liu, B.; Zhong, W. Effects of glyphosate-resistant transgenic soybean on soil rhizospheric bacteria and rhizobia. J. Appl. Ecol. 2018, 29, 2988–2996. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, J.; Shi, F.; Su, L.; Liu, K.; Xiang, M.; Liu, X. Rhizosphere bacterial communities associated with healthy and Heterodera glycines-infected soybean roots. Eur. J. Soil Biol. 2013, 58, 32–37. [Google Scholar] [CrossRef]
- Dai, L.; Kang, T.; Ci, D.; Ding, H.; Xu, Y.; Zhang, Z.; Zhang, D.; Li, W. Comparison of the microbial community in the rhizosphere of peanuts between saline-alkali and non-saline soil at different soil depths and intercropping cultivation in the Yellow River Delta. Acta Ecol. Sin. 2019, 39, 7169–7178. [Google Scholar] [CrossRef]
- Islam, M.; Bhattacharya, R.; Sarkar, B.; Maiti, P.; Mahanty, S.; Chaudhuri, P.; Biswas, S.; Mandal, S. Different soil salinity imparts clear alteration in rhizospheric bacterial community dynamics in rice and peanut. Arch. Microbiol. 2021, 204, 36. [Google Scholar] [CrossRef]
- Wang, X.; Hsu, C.; Dubeux, J.; Mackowiak, C.; Blount, A.; Han, X.; Liao, H. Effects of rhizoma peanut cultivars (Arachis glabrata Benth.) on the soil bacterial diversity and predicted function in nitrogen fixation. Ecol. Evol. 2019, 9, 12676–12687. [Google Scholar] [CrossRef]
- Suyal, D.; Yadav, A.; Shouche, Y.; Goel, R. Bacterial diversity and community structure of Western Indian Himalayan red kidney bean (Phaseolus vulgaris) rhizosphere as revealed by 16S rRNA gene sequences. Biologia 2015, 70, 305–313. [Google Scholar] [CrossRef]
- Suyal, D.; Kumar, S.; Joshi, D.; Yadav, A.; Shouche, Y.; Goel, R. Comparative overview of red kidney bean (Phaseolus valgaris) rhizospheric bacterial diversity in perspective of altitudinal variations. Biologia 2019, 74, 1405–1413. [Google Scholar] [CrossRef]
- Sousa, R.; Mendes, L.; Antunes, J.; Oliveira, L.; Sousa, A.; Gomes, R.; Lopes, A.; Arajo, F.; Melo, V.; Araujo, A. Diversity and structure of bacterial community in rhizosphere of lima bean. Appl. Soil Ecol. 2020, 150, 103490. [Google Scholar] [CrossRef]
- Monika, S.; Kamlesh, C. Characterization of multi-trait plant growth promoting Pseudomonas aeruginosa from chickpea (Cicer arietinum) rhizosphere. J. Appl. Nat. Sci. 2021, 13, 1003–1010. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Prakash, B.; Sathya, A.; Vijayabharathi, R. Plant growth-promoting traits of Pseudomonas geniculata isolated from chickpea nodules. 3 Biotech 2015, 5, 653–661. [Google Scholar] [CrossRef]
- Nautiyal, C. Rhizosphere competence of Pseudomonas sp. NBRI9926 and Rhizobium sp. NBRI9513 involved in the suppression of chickpea (Cicer arietinum L.) pathogenic fungi. FEMS Microbiol. Ecol. 1997, 23, 145–158. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M. Glyphosate induced toxicity to chickpea plants and stress alleviation by herbicide tolerant phosphate solubilizing Burkholderia cepacia PSBB1 carrying multifarious plant growth promoting activities. 3 Biotech 2018, 8, 131. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S.; Ramawat, N. Unravelling the potential of microbes isolated from rhizospheric soil of chickpea (Cicer arietinum) as plant growth promoter. 3 Biotech 2019, 9, 277–286. [Google Scholar] [CrossRef]
- Batool, S.; Asghar, H.; Shehzad, M.; Yasin, S.; Sohaib, M.; Nawaz, F.; Akhtar, G.; Mubeen, K.; Zahir, Z.; Uzair, M. Zinc-solubilizing bacteria-mediated enzymatic and physiological regulations confer zinc biofortification in chickpea (Cicer arietinum L.). J. Soil Sci. Plant Nut. 2021, 21, 2456–2471. [Google Scholar] [CrossRef]
- Alemneh, A.; Zhou, Y.; Ryder, M.; Denton, M. Large-scale screening of rhizobacteria to enhance the chickpea-Mesorhizobium symbiosis using a plant-based strategy. Rhizosphere 2021, 18, 100361. [Google Scholar] [CrossRef]
- Amjad, A.; Rabia, K.; Safdar, A.; Zahid, A.; Rifat, H. Characterization of plant growth promoting rhizobacteria isolated from chickpea (Cicer arietinum). Br. Microbiol. Res. J. 2015, 6, 32–40. [Google Scholar] [CrossRef]
- Marschner, P.; Neumann, G.; Kania, A.; Weiskopf, L.; Lieberei, R. Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant Soil 2002, 246, 167–174. [Google Scholar] [CrossRef]
- Rainey, P. Adaptation of Pseudomonas fluorescens to the plant rhizosphere. Environ. Microbiol. 1999, 1, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Mohidin, F.; Khan, U.; Ahamad, F. Native Pseudomonas spp. suppressed the root-knot nematode in in vitro and in vivo, and promoted the nodulation and grain yield in the field grown mungbean. Biol. Control 2016, 101, 159–168. [Google Scholar] [CrossRef]
- Noreen, R.; Ali, S.; Hasan, K.; Habiba; Urooj, F.; Tariq, A.; Ara, J.; Ehteshamul-Haque, S. Role of fluorescent Pseudomonas associated with root nodules of Mungbean in the induction of nodulation by the rhizobia in Mungbean. Pak. J. Bot. 2019, 51, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, D.; Mahmoudi, M.; Radl, V.; Brachmann, A.; Schloter, M.; Kemen, E.; Marn, M. Microbiome profiling reveals that Pseudomonas antagonises parasitic nodule colonisation of cheater rhizobia in Lotus. New Phytol. 2022, 234, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Bueis, R.; Jimenez-Gomez, A.; Barquero, M.; Mateos, P.F.; Gonzalez-Andres, F. Yield response of common bean to co-inoculation with Rhizobium and Pseudomonas endophytes and microscopic evidence of different colonised spaces inside the nodule. Eur. J. Agron. 2021, 122, 126187. [Google Scholar] [CrossRef]
- Zhao, L.F.; Xu, Y.J.; Ma, Z.Q.; Deng, Z.S.; Shan, C.J.; Wei, G.H. Colonization and plant growth promoting characterization of endophytic Pseudomonas chlororaphis strain Zong1 isolated from Sophora alopecuroides root nodules. Braz. J. Microbiol. 2013, 44, 629–637. [Google Scholar] [CrossRef]
- Berggren, I.; Alstrom, S.; van Vuurde, J.W.L.; Martensson, A.M. Rhizoplane colonisation of peas by Rhizobium leguminosarum bv. viceae and a deleterious Pseudomonas putida. Fems Microbiol. Ecol. 2005, 52, 71–78. [Google Scholar] [CrossRef][Green Version]
- Samavat, S.; Samavat, S.; Besharati, H.; Behboudi, K. Interactions of rhizobia cultural filtrates with Pseudomonas fluorescens on bean damping-off control. J. Agric. Sci. Technol. 2011, 13, 965–976. [Google Scholar]
- Egamberdieva, D.; Berg, G.; Lindstrom, K.; Rasanen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Malik, D.K.; Sindhu, S.S. Production of indole acetic acid by Pseudomonas sp.: Effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2011, 17, 25–32. [Google Scholar] [CrossRef]
- Zhang, J.J.; Shang, Y.M.; Wang, E.T.; Chen, W.F.; de Lajudie, P.; Li, B.Y.; Guo, C.; Yang, X.; Zheng, J.Q.; Liu, C.Z. Mesorhizobium jarvisii sv. astragali as predominant microsymbiont for Astragalus sinicus L. in acidic soils, Xinyang, China. Plant Soil 2018, 433, 201–212. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, H.; Xie, W.; Yang, Z.; Lv, Q. Long-term effects of maize straw return and manure on the microbial community in cinnamon soil in Northern China using 16S rRNA sequencing. PLoS ONE 2021, 16, e0249884. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Schloss, P.; Westcott, S.; Ryabin, T.; Hall, J.; Hartmann, M.; Hollister, E.; Lesniewski, R.; Oakley, B.; Parks, D.; Robinson, C. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Sofware for Canonical Community Ordination (version 4.5); Canoco: Ithaca, NY, USA, 2002. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
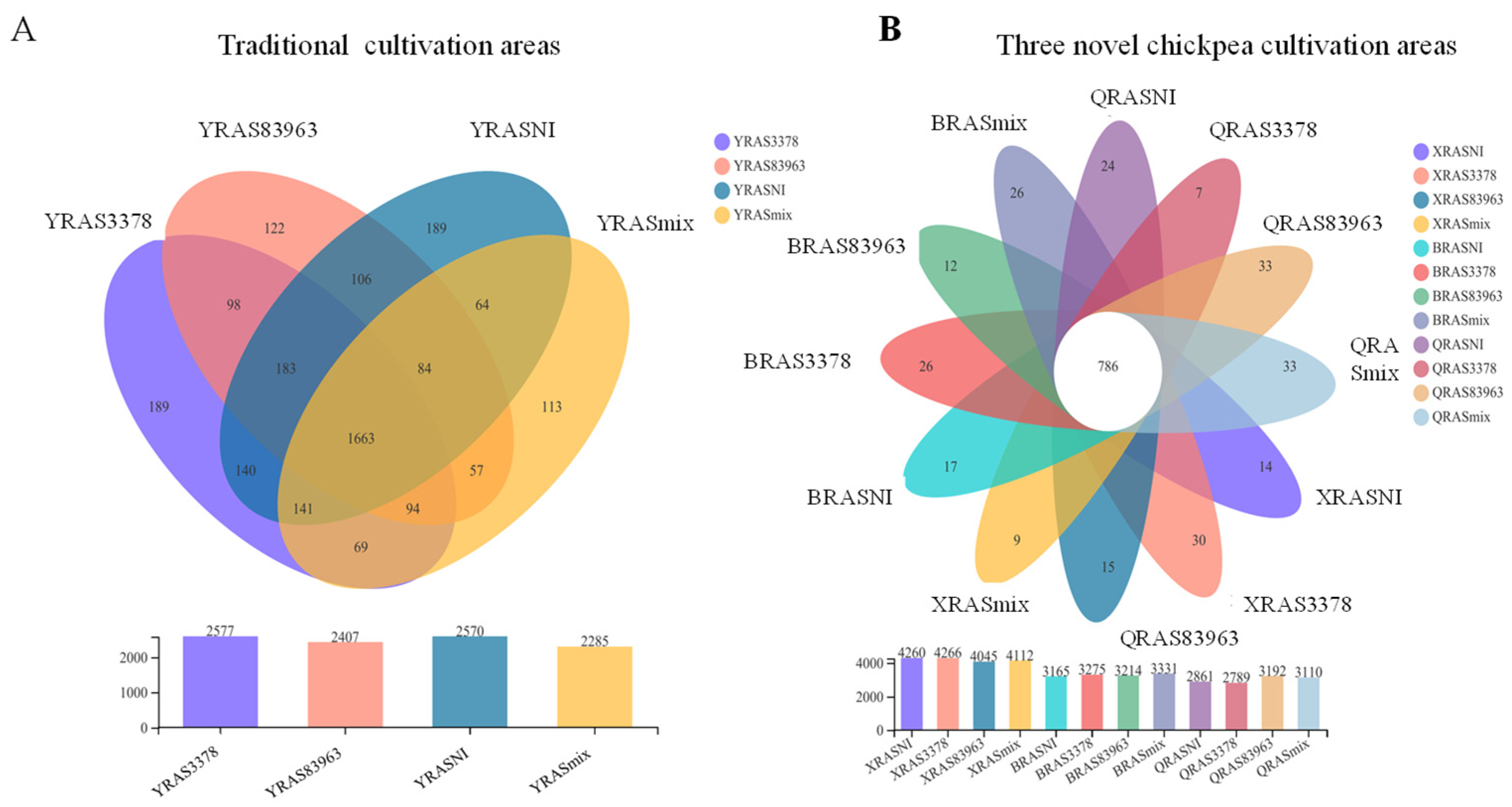



| Sample No. | Sample Size | Base NO. | Mean Read | OTU No. | OTUseq |
|---|---|---|---|---|---|
| Total | 1265838 | 526583331 | 415 | ||
| YRASNI | 51,312.00 ± 1666.87 | 21251445 | 414.1700 | 2577 | 35930 |
| YRAS3378 | 49,144.66 ± 1712.33 | 20366835 | 414.4113 | 2407 | 35332 |
| YRAS83963 | 45,687.33 ± 3925.27 | 18951169 | 414.7993 | 2570 | 32341 |
| YRASmix | 51,525.33 ± 6203.75 | 21358247 | 414.4623 | 2285 | 37639 |
| XRASNI | 58,066.33 ± 2758.87 | 24132554 | 415.613 | 4260 | 49386 |
| XRAS3378 | 60,827.00 ± 435.95 | 25336443 | 416.5310 | 4266 | 51263 |
| XRAS83963 | 48,320.00 ± 3928.64 | 20114175 | 416.2629 | 4045 | 42153 |
| XYRAmix | 45,707.33 ± 2526.44 | 19032936 | 416.4048 | 4112 | 38020 |
| BRASNI | 48,381.66 ± 1579.35 | 20112274 | 415.7096 | 3165 | 42205 |
| BRAS3378 | 50,884.33 ± 1446.52 | 21166839 | 415.9785 | 3275 | 43872 |
| BRAS83963 | 54,192.66 ± 4336.47 | 22533585 | 415.8094 | 3214 | 46036 |
| BRASmix | 55,566.66 ± 5986.33 | 23098968 | 415.6967 | 3331 | 47569 |
| QRASNI | 65,260.66 ± 3544.78 | 27242438 | 417.4568 | 2861 | 60863 |
| QRAS3378 | 68,681.66 ± 4046.47 | 28731629 | 418.2565 | 2789 | 63750 |
| QRAS83963 | 71,741.66 ± 7174.03 | 29905753 | 416.8529 | 3192 | 65771 |
| QRASmix | 67,219.00 ± 1500.52 | 28021370 | 416.8775 | 3110 | 62046 |
| Bacterial Genus | Average Relative Abundance (%) in Rhizosphere of Treatment * | |||
|---|---|---|---|---|
| YRASmix | XRASmix | BRASmix | QRASmix | |
| Rhizobium | 16.70 ± 1.82 | 1.9 ± 0.18 | 2.35 ± 0.25 | 6.64 ± 0.76 |
| Pseudomonas | 4.04 ± 0.28 | 0.24 ± 0.04 | 0.20 ± 0.07 | 0.02 ±0.02 |
| Arthrobacter | 3.52 ± 0.62 | 2.45 ± 0.17 | 6.02 ± 0.88 | 9.19 ± 1.24 |
| Sphingomonas | 3.41 ± 0.16 | 3.01 ± 0.27 | 4.50 ± 0.72 | 4.62 ± 0.25 |
| JG30-KF-CM45 | 2.88 ± 0.14 | 0.72 ± 0.03 | 3.07 ± 0.14 | 1.10 ± 0.12 |
| Xanthomonas | 2.55 ± 0.28 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sphingobacterium | 2.48 ± 0.23 | 1.49 ± 0.36 | 1.48 ± 0.50 | 0.00 ± 0.00 |
| Pantoea | 2.23 ± 0.27 | 0.38 ± 0.03 | 0.01 ± 0.00 | 0.02 ± 0.01 |
| f__Caulobacteraceae | 1.97 ± 0.07 | 0.21 ± 0.02 | 1.13 ± 0.11 | 0.08 ± 0.01 |
| Stenotrophomonas | 1.88 ± 0.28 | 0.63 ± 0.48 | 0.81 ± 0.16 | 0.03 ± 0.02 |
| Nocardioides | 1.61 ± 0.41 | 2.25 ± 0.08 | 2.01 ± 0.25 | 1.08 ± 0.14 |
| Novosphingobium | 1.21 ± 0.10 | 2.07 ± 0.32 | 1.07 ± 0.15 | 0.12 ± 0.00 |
| Streptomyces | 0.77 ± 0.02 | 1.75 ± 0.17 | 2.41 ± 0.59 | 2.15 ± 0.18 |
| Rubrobacter | 0.70 ± 0.97 | 0.03 ± 0.00 | 2.20 ± 0.20 | 0.36 ± 0.05 |
| o__Vicinamibacterales | 0.67 ± 0.14 | 3.72 ± 0.69 | 2.55 ± 0.26 | 1.01 ± 0.25 |
| 67–14 | 0.67 ± 0.02 | 2.42 ± 0.08 | 1.21 ± 0.15 | 1.93 ± 0.14 |
| o__Gaiellales | 0.59 ± 0.02 | 3.56 ± 0.21 | 2.32 ± 0.05 | 5.66 ± 0.22 |
| Gaiella | 0.57 ± 0.01 | 1.27 ± 0.16 | 1.07 ± 0.04 | 3.07 ± 0.27 |
| c__KD4-96 | 0.57 ± 0.11 | 1.55 ± 0.20 | 2.23 ± 0.14 | 0.38 ± 0.08 |
| Bacillus | 0.37 ± 0.09 | 2.01 ± 0.08 | 1.56 ± 0.19 | 1.02 ± 0.33 |
| Burkholderia | 0.03 ± 0.00 | 0.70 ± 0.10 | 4.07 ± 0.24 | 7.19 ± 0.41 |
| Enterobacter | 0.00 ± 0.00 | 1.20 ± 0.07 | 0.10 ± 0.02 | 3.64 ± 4.39 |
| Sampling Sites | Soil Trait # | |||||
|---|---|---|---|---|---|---|
| OM g/kg | TN g/kg | AP mg/kg | AK mg/kg | pH | TS g/kg | |
| Xinyang | 26.40 ± 0.44 a $ | 1.36 ± 0.02 a | 7.47 ± 0.31 a | 127.33 ± 2.31 a | 7.47 ± 0.15 b | 1.53 ± 0.06 bc |
| Qiubei | 23.33 ± 0.70 b | 1.06 ± 0.02 b | 9.53 ± 0.42 b | 226.67 ± 1.15 b | 5.87 ± 0.21 a | 1.07 ± 0.15 a |
| Yinggebu | 14.6 ± 0.53 d | 0.83 ± 0.02 d | 7.20 ± 0.70 a | 146.33 ± 1.53 c | 8.20 ± 0.10 c | 1.37 ± 0.06 b |
| Baicheng | 21.9 ± 1.05 c | 0.94 ± 0.07 c | 10.13 ± 0.23 b | 94.00 ± 1.73 d | 8.23 ± 0.15 c | 1.63 ± 0.06 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, N.; Li, S.; Wang, J.; Feng, Y.; Wang, E.; Li, Y.; Yang, T.; Chen, W. The Effect of Different Rhizobial Symbionts on the Composition and Diversity of Rhizosphere Microorganisms of Chickpea in Different Soils. Plants 2023, 12, 3421. https://doi.org/10.3390/plants12193421
Zhang J, Wang N, Li S, Wang J, Feng Y, Wang E, Li Y, Yang T, Chen W. The Effect of Different Rhizobial Symbionts on the Composition and Diversity of Rhizosphere Microorganisms of Chickpea in Different Soils. Plants. 2023; 12(19):3421. https://doi.org/10.3390/plants12193421
Chicago/Turabian StyleZhang, Junjie, Nan Wang, Shuo Li, Jingqi Wang, Yufeng Feng, Entao Wang, Youguo Li, Tao Yang, and Wenfeng Chen. 2023. "The Effect of Different Rhizobial Symbionts on the Composition and Diversity of Rhizosphere Microorganisms of Chickpea in Different Soils" Plants 12, no. 19: 3421. https://doi.org/10.3390/plants12193421
APA StyleZhang, J., Wang, N., Li, S., Wang, J., Feng, Y., Wang, E., Li, Y., Yang, T., & Chen, W. (2023). The Effect of Different Rhizobial Symbionts on the Composition and Diversity of Rhizosphere Microorganisms of Chickpea in Different Soils. Plants, 12(19), 3421. https://doi.org/10.3390/plants12193421







