Effect of Soil Environment on Species Diversity of Desert Plant Communities
Abstract
:1. Introduction
2. Results
2.1. Community Species Diversity and Species Composition
2.2. Species Diversity Index and Distribution
2.3. Relationship between Species Diversity and Soil Abiotic Factors
3. Discussion
3.1. Species Composition
3.2. Species Diversity
3.3. Impact of Soil Factors on Community Species Diversity
4. Materials and Methods
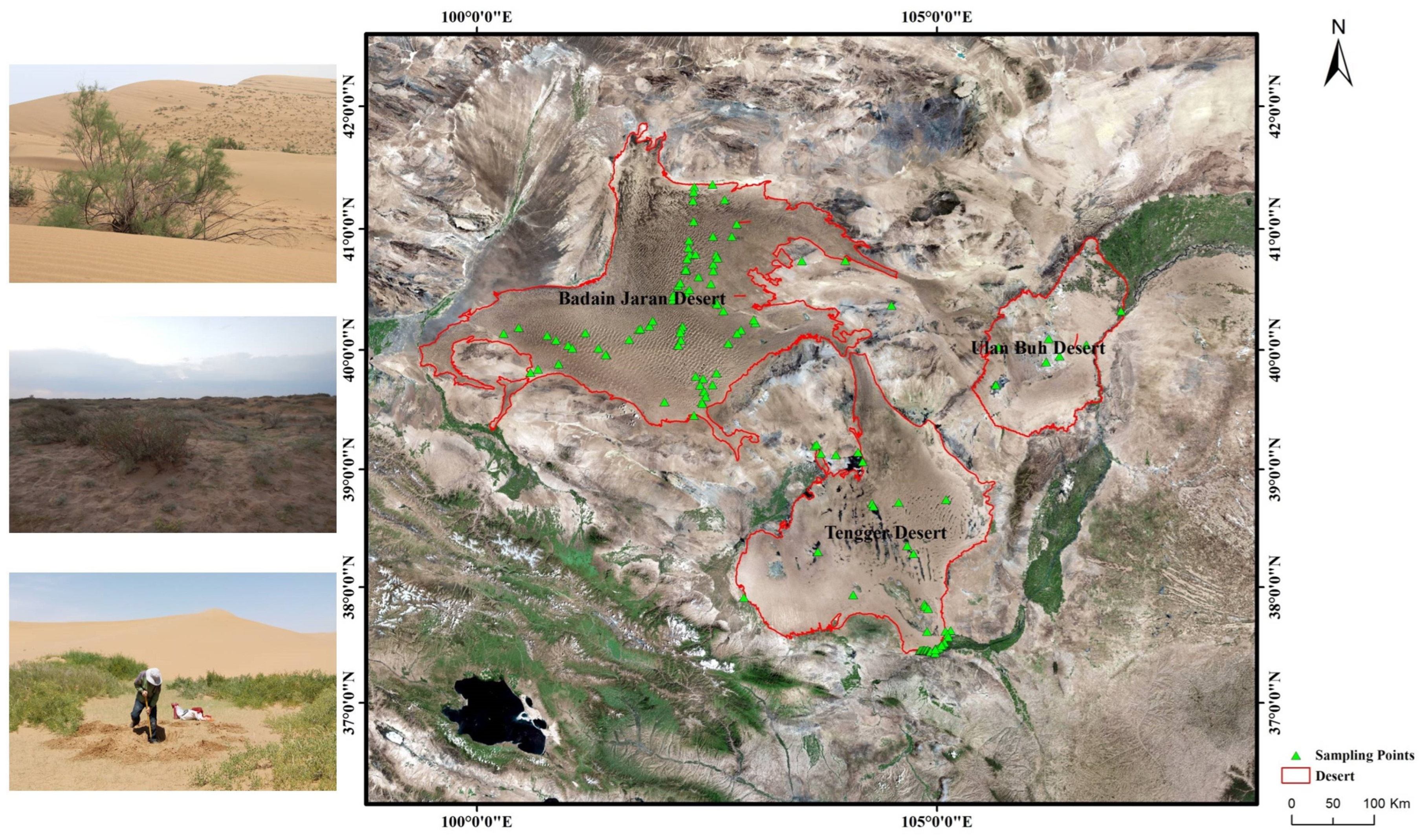
4.1. Study Area
4.2. Plant Community Survey
4.3. Investigation and Measurement of Soil Properties
4.4. Biodiversity Index
4.4.1. Importance Value of Species (IV)
4.4.2. α Species Diversity Index
4.4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burke, A. Classification and Ordination of Plant Communities of the Naukluft Mountains, Namibia. J. Veg. Sci. 2001, 12, 53–60. [Google Scholar] [CrossRef]
- Li, S.; Su, P.; Zhang, H.; Zhou, Z.; Xie, T.; Shi, R. Distribution patterns of desert plant diversity and relationship to soil properties in the heihe river basin, China. Ecosphere 2018, 9, e02355. [Google Scholar] [CrossRef]
- Hamberg, L.J.; Fraser, R.A.; Robinson, D.T.; Trant, A.J.; Murphy, S.D. Surface temperature as an indicator of plant species diversity and restoration in oak woodland. Ecol. Indic. 2020, 113, 106249. [Google Scholar] [CrossRef]
- Sun, S.S.; Zhao, S.L.; Liu, X.P.; Lv, P.; Liang, M.W.; Li, Y.L.; Zuo, X.A. Grazing impairs ecosystem stability through changes in species asynchrony and stability rather than diversity across spatial scales in desert steppe, Northern China. Agric. Ecosyst. Environ. 2023, 346, 108343. [Google Scholar] [CrossRef]
- Isbell, F.; Calcagno, V.; Hector, A.; Connolly, J.; Harpole, W.S.; Reich, P.B.; Scherer-Lorenzen, M.; Schmid, B.; Tilman, D.; Van Ruijven, J.; et al. High plant diversity is needed to maintain ecosystem services. Nature 2011, 477, 7363. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gong, Y. α Diversity of Desert Shrub Communities and Its Relationship with Climatic Factors in Xinjiang. Forests 2023, 14, 178. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Guo, A.; Yue, P.; Guo, X.; Lv, P.; Zhao, S.; Zuo, X. Species diversity is a strong predictor of ecosystem multifunctionality under altered precipitation in desert steppes. Ecol. Indic. 2022, 137, 108762. [Google Scholar] [CrossRef]
- Zuo, X.; Zhao, S.; Cheng, H.; Hu, Y.; Wang, S.; Yue, P.; Liu, R.; Knapp, A.K.; Smith, M.D.; Yu, Q.; et al. Functional diversity response to geographic and experimental precipitation gradients varies with plant community type. Funct. Ecol. 2021, 35, 2119–2132. [Google Scholar] [CrossRef]
- Xu, D.; Yu, X.; Yang, J.; Zhao, X.; Bao, Y. High-Throughput Sequencing Reveals the Diversity and Community Structure in Rhizosphere Soils of Three Endangered Plants in Western Ordos, China. Curr. Microbiol. 2020, 77, 2713–2723. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Fang, Z.; Yu, H.; Huang, J. Plant species diversity is driven by soil base cations under acid deposition in desert coal-mining region in northwestern China. Ecol. Indic. 2022, 145, 109682. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Farrer, E.C.; Spasojevic, M.J.; Bueno de Mesquita, C.P.; Sartwell, S.A.; Smith, J.G.; White, C.T.; King, A.J.; Suding, K.N.; Schmidt, S.K. Plant diversity and density predict belowground diversity and function in an early successional alpine ecosystem. Ecology 2018, 99, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Q.; Buyantuev, A.; Liu, Q.; Niu, J. Plant functional β diversity is an important mediator of effects of aridity on soil multifunctionality. Sci. Total Environ. 2020, 726, 138529. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Summary for Policymakers. Available online: https://www.ipcc.ch/site/assets/uploads/2019/08/Fullreport-1.pdf (accessed on 7 August 2019).
- Wang, X.; Geng, X.; Liu, B.; Cai, D.; Li, D.; Xiao, F.; Zhu, B.; Hua, T.; Lu, R.; Liu, F. Desert ecosystems in China: Past, present, and future. Earth-Sci. Rev. 2022, 234, 104206. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Yang, X.; Liu, G.; Cui, Q.; Indree, T.; Ye, X.; Huang, Z. The Relationship and Influencing Factors between Endangered Plant Tetraena mongolica and Soil Microorganisms in West Ordos Desert Ecosystem, Northern China. Plants 2023, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Nick, M. Rangeland management and climate hazards in drylands: Dust storms, desertification and the overgrazing debate. Nat. Hazards 2018, 92 (Suppl. S1), 57–70. [Google Scholar]
- Wu, C.; Lin, Z.; Shao, Y.; Liu, X.; Li, Y. Drivers of recent decline in dust activity over East Asia. Nat. Commun. 2022, 13, 7105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ge, Q.; Geng, X.; Wang, Z.; Gao, L.; Bryan, B.A.; Chen, S.; Su, Y.; Cai, D.; Ye, J.; et al. Unintended consequences of combating desertification in China. Nat. Commun. 2023, 14, 1139. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.M.; Pettorelli, N.; Bashir, S.; Woodroffe, R.; Wacher, T.; De Ornellas, P.; Ransom, C.; Abáigar, T.; Abdelgadir, M.; El Alqamy, H.; et al. Forgotten Biodiversity in Desert Ecosystems. Science 2012, 336, 1379–1380. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, W.; Mao, Y.; Yang, T.; Chen, Y. Biochar Addition Alters C: N: P Stoichiometry in Moss Crust-Soil Continuum in Gurbantünggüt Desert. Plants 2022, 11, 814. [Google Scholar] [CrossRef]
- Tongway, D.J.; Valentin, C.; Seghieri, J. Banded Vegetation Patterning in Arid and Semiarid Environments: Ecological Processes and Consequences for Management; Springer: New York, NY, USA, 2001. [Google Scholar]
- Schlesinger, W.H.; Pilmanis, A.M. Plant-soil interactions in the desert. Biogeochemistry 1998, 42, 169–187. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Reynolds, J.F.; Cunningham, G.L.; Huenneke, L.F.; Jarrell, W.M.; Virginia, R.A.; Whitford, W.G. Biological Feedbacks in Global Desertification. Science 1990, 247, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhao, X.; Zhao, H.; Zhang, T.; Li, Y.; Wang, S.; Li, W.; Powers, R. Scale dependent effects of environmental factors on vegetation pattern and composition in Horqin Sandy Land, Northern China. Geoderma 2012, 173-174, 1–9. [Google Scholar] [CrossRef]
- Ma, K.; Huang, J.; Yu, S.; Chen, L. Plant community diversity in onglingmountain, Beijing, China: II. species richness, evenness and species diversities. Acta Ecol. Sin. 1995, 15, 268–277. [Google Scholar] [CrossRef]
- Li, X.; Tan, H.; He, M.; Wang, X.; Li, X. Patterns of shrub species richness and abundance in relation to environmental factors on the Alxa Plateau: Prerequisites for conserving shrub diversity in extreme arid desert regions. Sci. China Ser. D Earth Sci. 2009, 52, 669–680. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.A.; Li, R.; Ning, K.; Cheng, H.; Zhao, L. Indication of millennial-scale moisture changes by the temporal distribution of Holocene calcareous root tubes in the deserts of the Alashan Plateau, Northwest China. Palaeogeogr. Palaeoclimatol. Palaeoecol. Int. J. Geo-Sci. 2015, 440, 496–505. [Google Scholar] [CrossRef]
- Meng, N.; Wang, N.; Cheng, H.; Liu, X.; Niu, Z. Impacts of climate change and anthropogenic activities on the normalized difference vegetation index of desertified areas in northern China. J. Geogr. Sci. 2023, 33, 483–507. [Google Scholar] [CrossRef]
- Meng, W.; Dong, Z.; Lu, J.; Li, J.; Luo, W.; Cui, X.; Zhang, Y.; Liu, Z.; Jiao, Y.; Yang, L. Vegetation characteristics and species diversity around the Badain Jaran desert. J. Desert Res. 2015, 35, 1226–1233. [Google Scholar]
- Li, Z.; Gao, Y.; Han, L. Holocene vegetation signals in the Alashan Desert of northwest China revealed by lipid molecular proxies from calcareous root tubes. Quat. Res. 2017, 88, 60–70. [Google Scholar] [CrossRef]
- Li, X.R.; Xiao, H.L.; Liu, L.C.; Zhang, J.G.; Wang, X.P. Long-term effect of sand-fixed vegetation on restoration of biodiversity in Shapotou region in Tengger Desert. J. Desert Res. 2005, 25, 173–181. [Google Scholar]
- Huang, Z.; Selvalakshmi, S.; Vasu, D.; Liu, Q.; Cheng, H.; Guo, F.; Ma, X. Identification of indicators for evaluating and monitoring the effects of chinese fir monoculture plantations on soil quality. Ecol. Indic. 2018, 93, 547–554. [Google Scholar]
- Wang, J.; Ding, J.; Yu, D.; Ma, X.; Zhang, Z.; Ge, X.; Teng, D.; Li, X.; Liang, J.; Guo, Y.; et al. Machine learning-based detection of soil salinity in an arid desert region, Northwest China: A comparison between Landsat-8 OLI and Sentinel-2 MSI. Sci. Total. Environ. 2020, 707, 136092. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Anwar, E.; Zhou, J.; He, D.; Gao, Y.-C.; Lv, G.-H.; Cao, Y.-E. Higher association and integration among functional traits in small tree than shrub in resisting drought stress in an arid desert. Environ. Exp. Bot. 2022, 201, 104993. [Google Scholar] [CrossRef]
- Souahi, H.; Gacem, R.; Chenchouni, H. Variation in Plant Diversity along a Watershed in the Semi-Arid Lands of North Africa. Diversity 2022, 14, 450. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Lv, G.; Wang, J.; Jiang, L.; Wang, H.; Yang, X. Predicting spatial variability of species diversity with the minimum data set of soil properties in an arid desert riparian forest. Front. Plant Sci. 2022, 13, 1014643. [Google Scholar] [CrossRef] [PubMed]
- Ownley, B.H.; Duffy, B.K.; Weller, D.M. Identification and Manipulation of Soil Properties to Improve the Biological Control Performance of Phenazine-Producing Pseudomonas fluorescens. Appl. Environ. Microbiol. 2003, 69, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Yerima, B.; Ranst, E.V. Major Soil Classification Systems Used in the Tropics: Soils of Cameroon; Trafford Publishing: Victoria, BC, Canada, 2005. [Google Scholar]
- Qiu, K.; Xie, Y.; Xu, D.; Pott, R. Ecosystem functions including soil organic carbon, total nitrogen and available potassium are crucial for vegetation recovery. Sci. Rep. 2018, 8, 7607. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Huang, J.; Xu, Y.; Li, J.; Zang, R. Disentangling environmental effects on the tree species abundance distribution and richness in a subtropical forest. Front. Plant Sci. 2021, 12, 622043. [Google Scholar] [CrossRef] [PubMed]
- DeMalach, N.; Zaady, E.; Kadmon, R. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett. 2017, 20, 60–69. [Google Scholar] [CrossRef]
- Dingaan, M.N.; Tsubo, M.; Walker, S.; Newby, T. Soil chemical properties and plant species diversity along a rainfall gradient in semi-arid grassland of south Africa. Plant Ecol. Evol. 2017, 150, 35–44. [Google Scholar] [CrossRef]
- Oijen, M.V.; Bellocchi, G.; Hglind, M. Effects of climate change on grassland biodiversity 2 and productivity: The need for a diversity of models. Agronomy 2018, 8, 14. [Google Scholar] [CrossRef]
- López-Angulo, J.; Pescador, D.S.; Sánchez, A.M.; Luzuriaga, A.L.; Cavieres, L.A.; Escudero, A. Impacts of climate, soil and biotic interactions on the interplay of the different facets of alpine plant diversity. Sci. Total Environ. 2020, 698, 133960. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yuan, Q.; Ren, P. Coupled effect of climate change and human activities on the restoration/degradation of the Qinghai-Tibet Plateau grassland. J. Geogr. Sci. 2021, 31, 1299–1327. [Google Scholar] [CrossRef]
- Wang, N.; Li, Z.; Cheng, H.; Li, Y.; Huang, Y. High lake levels on Alxa Plateau during the Late Quaternary. Chin. Sci. Bull. 2011, 56, 1799–1808. [Google Scholar] [CrossRef]
- Zhu, Z. Desertification of Sandy Land in China; Science Press: Beijing, China, 1994; pp. 69–72. [Google Scholar]
- Chen, X. Natural Geography of Arid Areas in China; Science Press: Beijing, China, 2010; pp. 495–534. [Google Scholar]
- Yang, C. Ecology Experiment and Practice; Higher Education Press: Beijing, China, 2003; pp. 26–38. [Google Scholar]
- Chen, J.; Yang, S. Terrestrial Ecology Research Methods; Higher Education Press: Beijing, China, 2014. [Google Scholar]
- Cui, X.; Dong, Z.; Lu, J. Relationship Between Vegetation Feature and Physiognomy Morphology of Mega-dunes in Badain Jaran Desert. Bull. Soil Water Conserv. 2014, 34, 278–283. [Google Scholar] [CrossRef]
- Shannon, C.E. The mathematical theory of communication. In The Mathematical Theory of Communication; Shannon, C.E., Weaver, W., Eds.; University of Illinois Press: Urbana, IL, USA, 1948; pp. 29–125. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. J. Cardiothorac. Vasc. Anesth. 1997, 11, 812. [Google Scholar] [CrossRef]
- Lloyd, M.; Ghelardi, J.R. A Table for Calculating the ‘Equitability’ Component of Species Diversity. J. Anim. Ecol. 1964, 33, 217–225. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; pp. 24–25. [Google Scholar]
- Gao, H.; Zhang, T.; Wu, Y.; Jiang, L.; Zhan, J.; Li, J.; Yang, R. Erratum: Multiple-trait genome-wide association study based on principal component analysis for residual covariance matrix. Heredity 2014, 113, 526–532. [Google Scholar] [CrossRef]


| Families | Genera | Species | Percentage of Total Species (%) |
|---|---|---|---|
| Compositae | 21 | 40 | 21.86 |
| Chenopodiaceae | 13 | 29 | 15.85 |
| Poaceae | 17 | 24 | 13.11 |
| Leguminosae | 8 | 19 | 10.38 |
| Zygophyllaceae | 5 | 10 | 5.46 |
| Polygonaceae | 4 | 5 | 2.73 |
| Liliaceae | 2 | 4 | 2.19 |
| Apocynaceae | 3 | 4 | 2.19 |
| Salicaceae | 2 | 4 | 2.19 |
| Asclepiadaceae | 2 | 4 | 2.19 |
| Ephedraceae | 1 | 3 | 1.64 |
| Cyperaceae | 2 | 3 | 1.64 |
| Boraginaceae | 2 | 3 | 1.64 |
| Plumbaginaceae | 2 | 2 | 1.09 |
| Tamaricaceae | 2 | 2 | 1.09 |
| Verbenaceae | 2 | 2 | 1.09 |
| Rosaceae | 2 | 2 | 1.09 |
| Solanaceae | 1 | 2 | 1.09 |
| Caryophyllaceae | 2 | 2 | 1.09 |
| Convolvulaceae | 1 | 2 | 1.09 |
| Iridaceae | 1 | 2 | 1.09 |
| Primulaceae | 1 | 1 | 0.55 |
| Labiatae | 1 | 1 | 0.55 |
| Elaeagnaceae | 1 | 1 | 0.55 |
| Geraniaceae | 1 | 1 | 0.55 |
| Ranunculaceae | 1 | 1 | 0.55 |
| Casuarinaceae | 1 | 1 | 0.55 |
| Vitaceae | 1 | 1 | 0.55 |
| Umbelliferae | 1 | 1 | 0.55 |
| Cruciferae | 1 | 1 | 0.55 |
| Juncaginaceae | 1 | 1 | 0.55 |
| Asparagaceae | 1 | 1 | 0.55 |
| Papaveraceae | 1 | 1 | 0.55 |
| Ulmaceae | 1 | 1 | 0.55 |
| Bignoniaceae | 1 | 1 | 0.55 |
| All | 109 | 183 | 100 |
| Number of Plots | H′ | D | S | E | |
|---|---|---|---|---|---|
| Badain Jaran Desert | 101 | 0.71 ± 0.52 | 0.36 ± 0.24 | 4.41 ± 3.84 | 0.5 ± 0.29 |
| Tengger Desert | 56 | 0.83 ± 0.6 | 0.4 ± 0.27 | 6.41 ± 4.67 | 0.47 ± 0.28 |
| Ulan Buh Desert | 17 | 0.85 ± 0.61 | 0.4 ± 0.25 | 6.41 ± 7.1 | 0.51 ± 0.26 |
| Alxa Deserts | 174 | 0.76 ± 0.56 | 0.38 ± 0.25 | 5.25 ± 4.59 | 0.49 ± 0.28 |
| Average Value | Tree | Shrub | Shrub–Herb | Herb |
|---|---|---|---|---|
| E | 0.57 | 0.56 | 0.48 | 0.57 |
| S | 3.64 | 5.40 | 5.97 | 4.50 |
| H′ | 0.55 | 0.79 | 0.79 | 0.71 |
| D | 0.28 | 0.39 | 0.39 | 0.36 |
| Average Value | Badain Jaran Desert | Tengger Desert | Ulan Buh Desert |
|---|---|---|---|
| SW (%) | 2.28 ± 1.49 b | 4.61 ± 6.17 ab | 6.68 ± 5.88 a |
| Cly (%) | 1.21 ± 0.69 b | 2.03 ± 1.72 b | 4.81 ± 4.27 a |
| Sit (%) | 5.99 ± 4.96 b | 12.03 ± 11.15 b | 34.06 ± 33.35 a |
| Sad (%) | 92.79 ± 34.49 a | 85.93 ± 42.92 a | 61.13 ± 47.77 a |
| Median size (μm) | 186 ± 76 a | 141.35 ± 76.91 a | 143.55 ± 116.97 a |
| TN (g/kg) | 0.01 ± 0.01 a | 0.23 ± 0.16 a | 0.16 ± 0.05 a |
| AP (ppm) | 0.33 ± 0.23 b | 1.02 ± 0.61 a | 1.16 ± 0.56 a |
| SOM (g/kg) | 0.15 ± 0.05 b | 0.35 ± 0.26 ab | 0.22 ± 0.07 a |
| EC (μS/cm) | 0.07 ± 0.04 a | 0.08 ± 0.03 a | 0.08 ± 0.01 a |
| AK (mg/kg) | 176.42 ± 68.48 b | 141.76 ± 73.88 ab | 103.53 ± 20.08 a |
| RZ (g/cm3) | 1.39 ± 0.44 a | 1.5 ± 0.1 a | 1.55 ± 0.78 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, N.; Niu, Z. Effect of Soil Environment on Species Diversity of Desert Plant Communities. Plants 2023, 12, 3465. https://doi.org/10.3390/plants12193465
Sun J, Wang N, Niu Z. Effect of Soil Environment on Species Diversity of Desert Plant Communities. Plants. 2023; 12(19):3465. https://doi.org/10.3390/plants12193465
Chicago/Turabian StyleSun, Jie, Nai’ang Wang, and Zhenmin Niu. 2023. "Effect of Soil Environment on Species Diversity of Desert Plant Communities" Plants 12, no. 19: 3465. https://doi.org/10.3390/plants12193465
APA StyleSun, J., Wang, N., & Niu, Z. (2023). Effect of Soil Environment on Species Diversity of Desert Plant Communities. Plants, 12(19), 3465. https://doi.org/10.3390/plants12193465





