Transcriptome and Metabolome Provide Insights into Fruit Ripening of Cherry Tomato (Solanum lycopersicum var. cerasiforme)
Abstract
:1. Introduction
2. Results
2.1. Metabolic Changes during Fruit Ripening
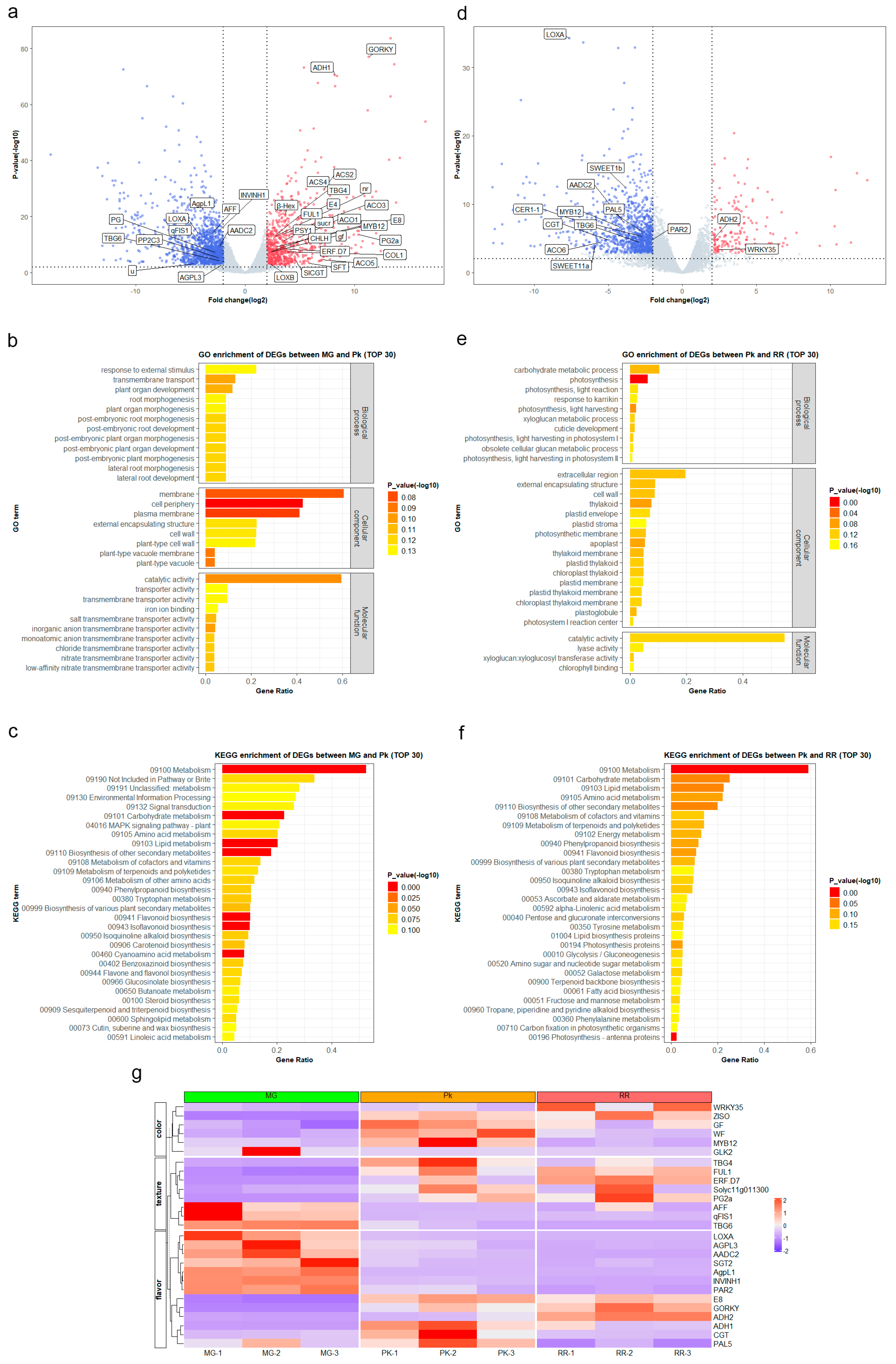
2.2. Gene Expression Changes during Fruit Ripening
2.3. Correlation Analysis between DAMs and DEGs
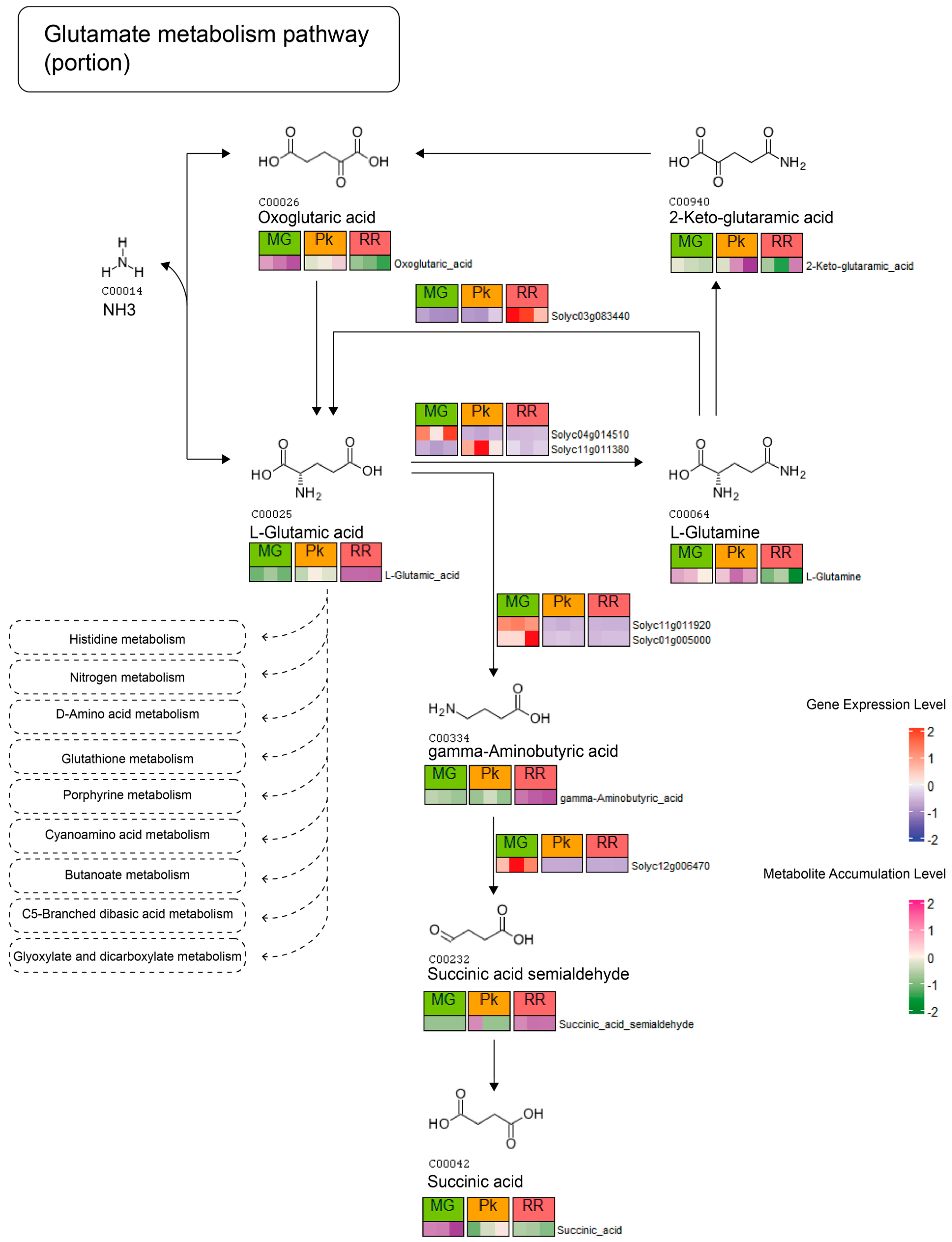
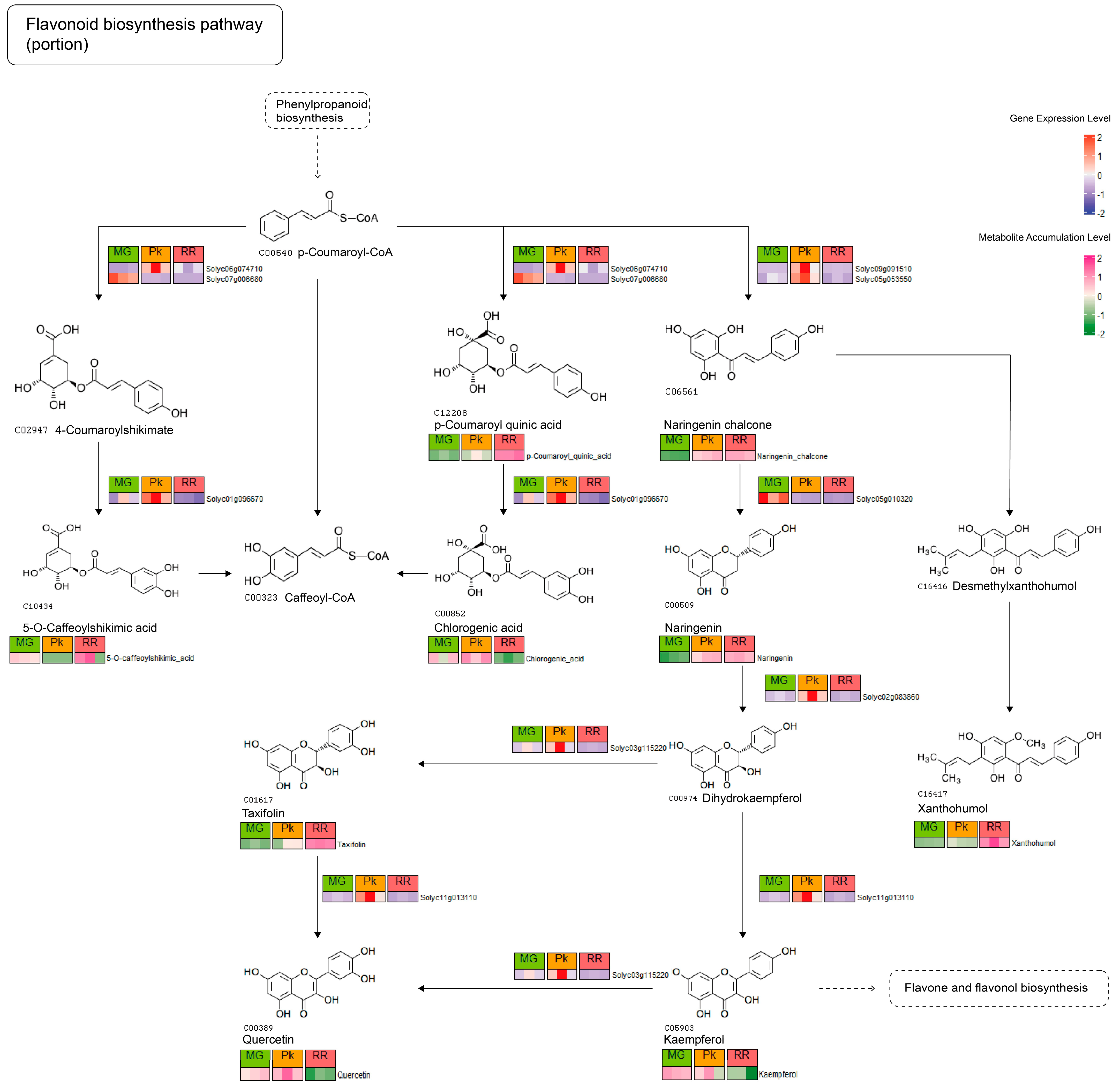
2.4. Integrative Analysis of KEGG Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Metabolome Analysis
4.3. RNA Sequencing and Transcriptome Analysis
4.4. Integrative Analysis of Metabolome and Transcriptome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klee, H.J.; Tieman, D.M. Genetic Challenges of Flavor Improvement in Tomato. Trends Genet. 2013, 29, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A Chemical Genetic Roadmap to Improved Tomato Flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef]
- Causse, M. Genetic Background of Flavour: The Case of the Tomato. In Fruit and Vegetable Flavour; Elsevier: Amsterdam, The Netherlands, 2008; pp. 229–253. ISBN 978-1-84569-183-7. [Google Scholar]
- Tikunov, Y.; Lommen, A.; De Vos, C.H.R.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A Novel Approach for Nontargeted Data Analysis for Metabolomics. Large-Scale Profiling of Tomato Fruit Volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef]
- Hobson, G.E.; Bedford, L. The Composition of Cherry Tomatoes and Its Relation to Consumer Acceptability. J. Hortic. Sci. 1989, 64, 321–329. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-Resolution Spatiotemporal Transcriptome Mapping of Tomato Fruit Development and Ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef]
- Seymour, G.B.; Taylor, J.E.; Tucker, G.A. Biochemistry of Fruit Ripening; Chapman and Hall: London, UK, 1993; ISBN 978-94-010-4689-3. [Google Scholar]
- Alexander, L.; Grierson, D. Ethylene Biosynthesis and Action in Tomato: A Model for Climacteric Fruit Ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell Wall Disassembly in Ripening Fruit. Funct. Plant Biol. 2006, 33, 103. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The Genetics of Fruit Flavour Preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Wang, S.; Qiang, Q.; Xiang, L.; Fernie, A.R.; Yang, J. Targeted Approaches to Improve Tomato Fruit Taste. Hortic. Res. 2023, 10, uhac229. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A Dynamic Interplay between Phytohormones Is Required for Fruit Development, Maturation, and Ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Oetiker, J.H.; Olson, D.C.; Shiu, O.Y.; Yang, S.F. Differential Induction of Seven 1-Aminocyclopropane-1-Carboxylate Synthase Genes by Elicitor in Suspension Cultures of Tomato (Lycopersicon esculentum). Plant Mol. Biol. 1997, 34, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Shiu, O.Y.; Oetiker, J.H.; Yip, W.K.; Yang, S.F. The Promoter of LE-ACS7, an Early Flooding-Induced 1-Aminocyclopropane-1-Carboxylate Synthase Gene of the Tomato, Is Tagged by a Sol3 Transposon. Proc. Natl. Acad. Sci. USA 1998, 95, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Zarembinski, T.I.; Theologis, A. Ethylene Biosynthesis and Action: A Case of Conservation. Plant Mol. Biol. 1994, 26, 1579–1597. [Google Scholar] [CrossRef]
- Manning, K.; Tör, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A Naturally Occurring Epigenetic Mutation in a Gene Encoding an SBP-Box Transcription Factor Inhibits Tomato Fruit Ripening. Nat. Genet. 2006, 38, 948–952. [Google Scholar] [CrossRef]
- Bird, C.R.; Ray, J.A.; Fletcher, J.D.; Boniwell, J.M.; Bird, A.S.; Teulieres, C.; Blain, I.; Bramley, P.M.; Schuch, W. Using Antisense RNA to Study Gene Function: Inhibition of Carotenoid Biosynthesis in Transgenic Tomatoes. Nat. Biotechnol. 1991, 9, 635–639. [Google Scholar] [CrossRef]
- Fray, R.G.; Grierson, D. Identification and Genetic Analysis of Normal and Mutant Phytoene Synthase Genes of Tomato by Sequencing, Complementation and Co-Suppression. Plant Mol. Biol. 1993, 22, 589–602. [Google Scholar] [CrossRef]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An Alternative Pathway to β-Carotene Formation in Plant Chromoplasts Discovered by Map-Based Cloning of Beta and Old-Gold Color Mutations in Tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef]
- DellaPenna, D.; Alexander, D.C.; Bennett, A.B. Molecular Cloning of Tomato Fruit Polygalacturonase: Analysis of Polygalacturonase mRNA Levels during Ripening. Proc. Natl. Acad. Sci. USA 1986, 83, 6420–6424. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the Fruit Cell Wall by the Ripening-Associated Polygalacturonase and Expansin Influences Tomato Cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Wallner, S.J.; Walker, J.E. Glycosidases in Cell Wall-Degrading Extracts of Ripening Tomato Fruits. Plant Physiol. 1975, 55, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell Biology and Prospects for Functional Analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, W.; Xiong, F.; Xian, Z.; Su, D.; Ren, M.; Li, Z. Silencing of SlPL, Which Encodes a Pectate Lyase in Tomato, Confers Enhanced Fruit Firmness, Prolonged Shelf-Life and Reduced Susceptibility to Grey Mould. Plant Biotechnol. J. 2017, 15, 1544–1555. [Google Scholar] [CrossRef]
- Tieman, D.M.; Harriman, R.W.; Ramamohan, G.; Handa, A.K. An Antisense Pectin Methylesterase Gene Alters Pectin Chemistry and Soluble Solids in Tomato Fruit. Plant Cell 1992, 4, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, R.; Nakayasu, M.; Umemoto, N.; Kato, J.; Kobayashi, M.; Lee, H.J.; Sugimoto, Y.; Iijima, Y.; Saito, K.; Muranaka, T.; et al. Tomato E8 Encodes a C-27 Hydroxylase in Metabolic Detoxification of α-Tomatine during Fruit Ripening. Plant Cell Physiol. 2021, 62, 775–783. [Google Scholar] [CrossRef]
- Fridman, E.; Pleban, T.; Zamir, D. A Recombination Hotspot Delimits a Wild-Species Quantitative Trait Locus for Tomato Sugar Content to 484 Bp within an Invertase Gene. Proc. Natl. Acad. Sci. USA 2000, 97, 4718–4723. [Google Scholar] [CrossRef]
- Tieman, D.M.; Loucas, H.M.; Kim, J.Y.; Clark, D.G.; Klee, H.J. Tomato Phenylacetaldehyde Reductases Catalyze the Last Step in the Synthesis of the Aroma Volatile 2-Phenylethanol. Phytochemistry 2007, 68, 2660–2669. [Google Scholar] [CrossRef]
- Seymour, G.B. Genetic Identification and Genomic Organization of Factors Affecting Fruit Texture. J. Exp. Bot. 2002, 53, 2065–2071. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom Metabolic Network: Rewiring Tomato Metabolic Regulatory Network throughout the Growth Cycle. Mol. Plant 2020, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, C.; Dong, W.; Jiang, Q.; Wang, D.; Li, S.; Chen, M.; Liu, C.; Sun, C.; Chen, K. Transcriptome and Metabolome Analyses of Sugar and Organic Acid Metabolism in Ponkan (Citrus reticulata) Fruit during Fruit Maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative Analyses of Metabolome and Genome-wide Transcriptome Reveal the Regulatory Network Governing Flavor Formation in Kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, Y.; Chen, X.; Yan, Q.; Chen, J.; Liu, H.; Shi, F.; Wen, Y.; Cai, C.; Ou, L. Combined Metabolome and Transcriptome Analyses Unveil the Molecular Mechanisms of Fruit Acidity Variation in Litchi (Litchi chinensis Sonn.). IJMS 2023, 24, 1871. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Bai, J.; Lu, J.; Lu, X.; Hu, J.; Pan, C.; He, S.; Yuan, J.; Zhang, Y.; et al. All-Flesh Fruit in Tomato Is Controlled by Reduced Expression Dosage of AFF through a Structural Variant Mutation in the Promoter. J. Exp. Bot. 2021, 73, 123–138. [Google Scholar] [CrossRef]
- Qin, G.; Wang, Y.; Cao, B.; Wang, W.; Tian, S. Unraveling the Regulatory Network of the MADS Box Transcription Factor RIN in Fruit Ripening: Identification of Direct Targets of RIN. Plant J. 2012, 70, 243–255. [Google Scholar] [CrossRef]
- Buker, M.; Schunemann, D.; Borchert, S. Enzymic Properties and Capacities of Developing Tomato (Lycopersicon esculentum L.) Fruit Plastids. J. Exp. Bot. 1998, 49, 681–691. [Google Scholar] [CrossRef]
- Kahlau, S.; Bock, R. Plastid Transcriptomics and Translatomics of Tomato Fruit Development and Chloroplast-to-Chromoplast Differentiation: Chromoplast Gene Expression Largely Serves the Production of a Single Protein. Plant Cell 2008, 20, 856–874. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Anton, M.T.; et al. Tomato Fruit Photosynthesis Is Seemingly Unimportant in Primary Metabolism and Ripening but Plays a Considerable Role in Seed Development. Plant Physiol. 2011, 157, 1650–1663. [Google Scholar] [CrossRef]
- Helyes, L.; Pék, Z.; Lugasi, A. Tomato Fruit Quality and Content Depend on Stage of Maturity. HortScience 2006, 41, 1400–1401. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Schuch, W. Flavor Trivia and Tomato Aroma: Biochemistry and Possible Mechanisms for Control of Important Aroma Components. HortScience 2000, 35, 1013–1022. [Google Scholar] [CrossRef]
- Kader, A.A.; Stevens, M.A.; Albright, M.; Morris, L.L. Amino Acid Composition and Flavor of Fresh Market Tomatoes as Influenced by Fruit Ripeness When Harvested1. J. Amer. Soc. Hort. Sci. 1978, 103, 541–544. [Google Scholar] [CrossRef]
- Kaur, G.; Abugu, M.; Tieman, D. The Dissection of Tomato Flavor: Biochemistry, Genetics, and Omics. Front. Plant Sci. 2023, 14, 1144113. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma, E.; Smith, D.L.; Gross, K.C. Antisense Suppression of a Β-galactosidase Gene (TB G6) in Tomato Increases Fruit Cracking. J. Exp. Bot. 2003, 54, 2025–2033. [Google Scholar] [CrossRef]
- Shima, Y.; Fujisawa, M.; Kitagawa, M.; Nakano, T.; Kimbara, J.; Nakamura, N.; Shiina, T.; Sugiyama, J.; Nakamura, T.; Kasumi, T.; et al. Tomato FRUITFULL Homologs Regulate Fruit Ripening via Ethylene Biosynthesis. Biosci. Biotechnol. Biochem. 2014, 78, 231–237. [Google Scholar] [CrossRef]
- Bird, C.R.; Smith, C.J.S.; Ray, J.A.; Moureau, P.; Bevan, M.W.; Bird, A.S.; Hughes, S.; Morris, P.C.; Grierson, D.; Schuch, W. The Tomato Polygalacturonase Gene and Ripening-Specific Expression in Transgenic Plants. Plant Mol. Biol. 1988, 11, 651–662. [Google Scholar] [CrossRef]
- Li, R.; Sun, S.; Wang, H.; Wang, K.; Yu, H.; Zhou, Z.; Xin, P.; Chu, J.; Zhao, T.; Wang, H.; et al. FIS1 Encodes a GA2-Oxidase That Regulates Fruit Firmness in Tomato. Nat. Commun. 2020, 11, 5844. [Google Scholar] [CrossRef]
- Gambhir, P.; Singh, V.; Parida, A.; Raghuvanshi, U.; Kumar, R.; Sharma, A.K. Ethylene Response Factor ERF.D7 Activates Auxin Response Factor 2 Paralogs to Regulate Tomato Fruit Ripening. Plant Physiol. 2022, 190, 2775–2796. [Google Scholar] [CrossRef]
- Ochoa-Jiménez, V.-A.; Berumen-Varela, G.; Burgara-Estrella, A.; Orozco-Avitia, J.-A.; Ojeda-Contreras, Á.-J.; Trillo-Hernández, E.-A.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional Analysis of Tomato Rhamnogalacturonan Lyase Gene Solyc11g011300 during Fruit Development and Ripening. J. Plant Physiol. 2018, 231, 31–40. [Google Scholar] [CrossRef]
- Speirs, J.; Correll, R.; Cain, P. Relationship between ADH Activity, Ripeness and Softness in Six Tomato Cultivars. Sci. Hortic. 2002, 93, 137–142. [Google Scholar] [CrossRef]
- Yang, J.; Liang, B.; Zhang, Y.; Liu, Y.; Wang, S.; Yang, Q.; Geng, X.; Liu, S.; Wu, Y.; Zhu, Y.; et al. Genome-Wide Association Study of Eigenvectors Provides Genetic Insights into Selective Breeding for Tomato Metabolites. BMC Biol. 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Knollenberg, B.J.; Liu, J.; Yu, S.; Lin, H.; Tian, L. Cloning and Functional Characterization of a P-Coumaroyl Quinate/Shikimate 3′-Hydroxylase from Potato (Solanum tuberosum). Biochem. Biophys. Res. Commun. 2018, 496, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids—Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, M.; Li, Y.; Zhang, D.; Yang, Y.; Li, L. Natural Xanthine Oxidase Inhibitor 5-O-Caffeoylshikimic Acid Ameliorates Kidney Injury Caused by Hyperuricemia in Mice. Molecules 2021, 26, 7307. [Google Scholar] [CrossRef]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Characterization and Elimination of Linkage-Drag Associated with Fusarium Wilt Race 3 Resistance Genes. Theor. Appl. Genet. 2021, 134, 2129–2140. [Google Scholar] [CrossRef]
- Dixon, M.S.; Jones, D.A.; Keddie, J.S.; Thomas, C.M.; Harrison, K.; Jones, J.D.G. The Tomato Cf-2 Disease Resistance Locus Comprises Two Functional Genes Encoding Leucine-Rich Repeat Proteins. Cell 1996, 84, 451–459. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, T.; You, X.; Jiang, J.; Li, J.; Xu, X. Molecular Mapping of the Cf-10 Gene by Combining SNP/InDel-Index and Linkage Analysis in Tomato (Solanum lycopersicum). BMC Plant Biol. 2019, 19, 15. [Google Scholar] [CrossRef]
- Zhi, X.; Shu, J.; Zheng, Z.; Li, T.; Sun, X.; Bai, J.; Cui, Y.; Wang, X.; Huang, Z.; Guo, Y.; et al. Fine Mapping of the Ph-2 Gene Conferring Resistance to Late Blight (Phytophthora infestans) in Tomato. Plant Dis. 2021, 105, 851–858. [Google Scholar] [CrossRef]
- Pei, C.; Wang, H.; Zhang, J.; Wang, Y.; Francis, D.M.; Yang, W. Fine Mapping and Analysis of a Candidate Gene in Tomato Accession PI128216 Conferring Hypersensitive Resistance to Bacterial Spot Race T3. Theor. Appl. Genet. 2012, 124, 533–542. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Jiang, J.; Liu, M.; Liu, Z.; Tan, Y.; Zhao, T.; Zhang, H.; Chen, X.; Li, J.; et al. The Sm Gene Conferring Resistance to Gray Leaf Spot Disease Encodes an NBS-LRR (Nucleotide-Binding Site-Leucine-Rich Repeat) Plant Resistance Protein in Tomato. Theor. Appl. Genet. 2022, 135, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Sullenberger, M.T.; Jia, M.; Gao, S.; Ashrafi, H.; Foolad, M.R. Identification of Late Blight Resistance Quantitative Trait Loci in Solanum pimpinellifolium Accession PI 270441. Plant Genome 2022, 15, e20251. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.L.; Smyth, G.K. From Reads to Genes to Pathways: Differential Expression Analysis of RNA-Seq Experiments Using Rsubread and the edgeR Quasi-Likelihood Pipeline. F1000Research 2016, 5, 1438. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Ballester, A.-R.; Molthoff, J.; De Vos, R.; Hekkert, B.T.L.; Orzaez, D.; Fernaݩndez-Moreno, J.-P.; Tripodi, P.; Grandillo, S.; Martin, C.; Heldens, J.; et al. Biochemical and Molecular Analysis of Pink Tomatoes: Deregulated Expression of the Gene Encoding Transcription Factor SlMYB12 Leads to Pink Tomato Fruit Color. Plant Physiol. 2009, 152, 71–84. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, S.; Liu, X.; Su, L.; Wu, Y.; Zhang, W.; Li, Y.; Jiang, Y.; Wang, H.; Fu, R.; et al. SlWRKY35 Positively Regulates Carotenoid Biosynthesis by Activating the MEP Pathway in Tomato Fruit. New Phytol. 2022, 234, 164–178. [Google Scholar] [CrossRef]
- Galpaz, N.; Ronen, G.; Khalfa, Z.; Zamir, D.; Hirschberg, J. A Chromoplast-Specific Carotenoid Biosynthesis Pathway Is Revealed by Cloning of the Tomato White-Flower Locus. Plant Cell 2006, 18, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; McQuinn, R.P.; Chung, M.-Y.; Besuden, A.; Giovannoni, J.J. Amino Acid Substitutions in Homologs of the STAY-GREEN Protein Are Responsible for the Green-Flesh and Chlorophyll Retainer Mutations of Tomato and Pepper. Plant Physiol. 2008, 147, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.L.T.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernández-Muñoz, R.; Vicente, A.; et al. Uniform Ripening Encodes a Golden 2-like Transcription Factor Regulating Tomato Fruit Chloroplast Development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of Tomato Lycopene Biosynthesis through Virus-Induced Gene Silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Chung, W.-I. Molecular Cloning and Organ-Specific Expression of Three Isoforms of Tomato ADP-Glucose Pyrophosphorylase Gene. Gene 1998, 206, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Petreikov, M.; Shen, S.; Yeselson, Y.; Levin, I.; Bar, M.; Schaffer, A.A. Temporally Extended Gene Expression of the ADP-Glc Pyrophosphorylase Large Subunit (AgpL1) Leads to Increased Enzyme Activity in Developing Tomato Fruit. Planta 2006, 224, 1465–1479. [Google Scholar] [CrossRef]
- Kazachkova, Y.; Zemach, I.; Panda, S.; Bocobza, S.; Vainer, A.; Rogachev, I.; Dong, Y.; Ben-Dor, S.; Veres, D.; Kanstrup, C.; et al. The GORKY Glycoalkaloid Transporter Is Indispensable for Preventing Tomato Bitterness. Nat. Plants 2021, 7, 468–480. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato Aromatic Amino Acid Decarboxylases Participate in Synthesis of the Flavor Volatiles 2-Phenylethanol and 2-Phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef]
- Brisou, G.; Piquerez, S.J.M.; Minoia, S.; Marcel, F.; Cornille, A.; Carriero, F.; Boualem, A.; Bendahmane, A. Induced Mutations in SlE8 and SlACO1 Control Tomato Fruit Maturation and Shelf-Life. J. Exp. Bot. 2021, 72, 6920–6932. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Huang, S.; Hu, J.; Wang, Q.; Tang, Y.; Yang, T.; Asmutola, P.; Wang, J.; Yu, Q. Enhanced Soluble Sugar Content in Tomato Fruit Using CRISPR/Cas9-Mediated SlINVINH1 and SlVPE5 Gene Editing. PeerJ 2021, 9, e12478. [Google Scholar] [CrossRef]
- Moummou, H.; Tonfack, L.B.; Chervin, C.; Benichou, M.; Youmbi, E.; Ginies, C.; Latché, A.; Pech, J.-C.; Van Der Rest, B. Functional Characterization of SlscADH1, a Fruit-Ripening-Associated Short-Chain Alcohol Dehydrogenase of Tomato. J. Plant Physiol. 2012, 169, 1435–1444. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, F.; Zhang, Q.; Zhu, H.; Li, J.; Wen, Q. Transcriptome and Metabolome Provide Insights into Fruit Ripening of Cherry Tomato (Solanum lycopersicum var. cerasiforme). Plants 2023, 12, 3505. https://doi.org/10.3390/plants12193505
Pan F, Zhang Q, Zhu H, Li J, Wen Q. Transcriptome and Metabolome Provide Insights into Fruit Ripening of Cherry Tomato (Solanum lycopersicum var. cerasiforme). Plants. 2023; 12(19):3505. https://doi.org/10.3390/plants12193505
Chicago/Turabian StylePan, Feng, Qianrong Zhang, Haisheng Zhu, Junming Li, and Qingfang Wen. 2023. "Transcriptome and Metabolome Provide Insights into Fruit Ripening of Cherry Tomato (Solanum lycopersicum var. cerasiforme)" Plants 12, no. 19: 3505. https://doi.org/10.3390/plants12193505



