A Model for Changes in Germination Synchrony and Its Implements to Study Weed Population Dynamics: A Case Study of Brassicaceae
Abstract
1. Introduction
2. Results
2.1. Dynamic Synchronization
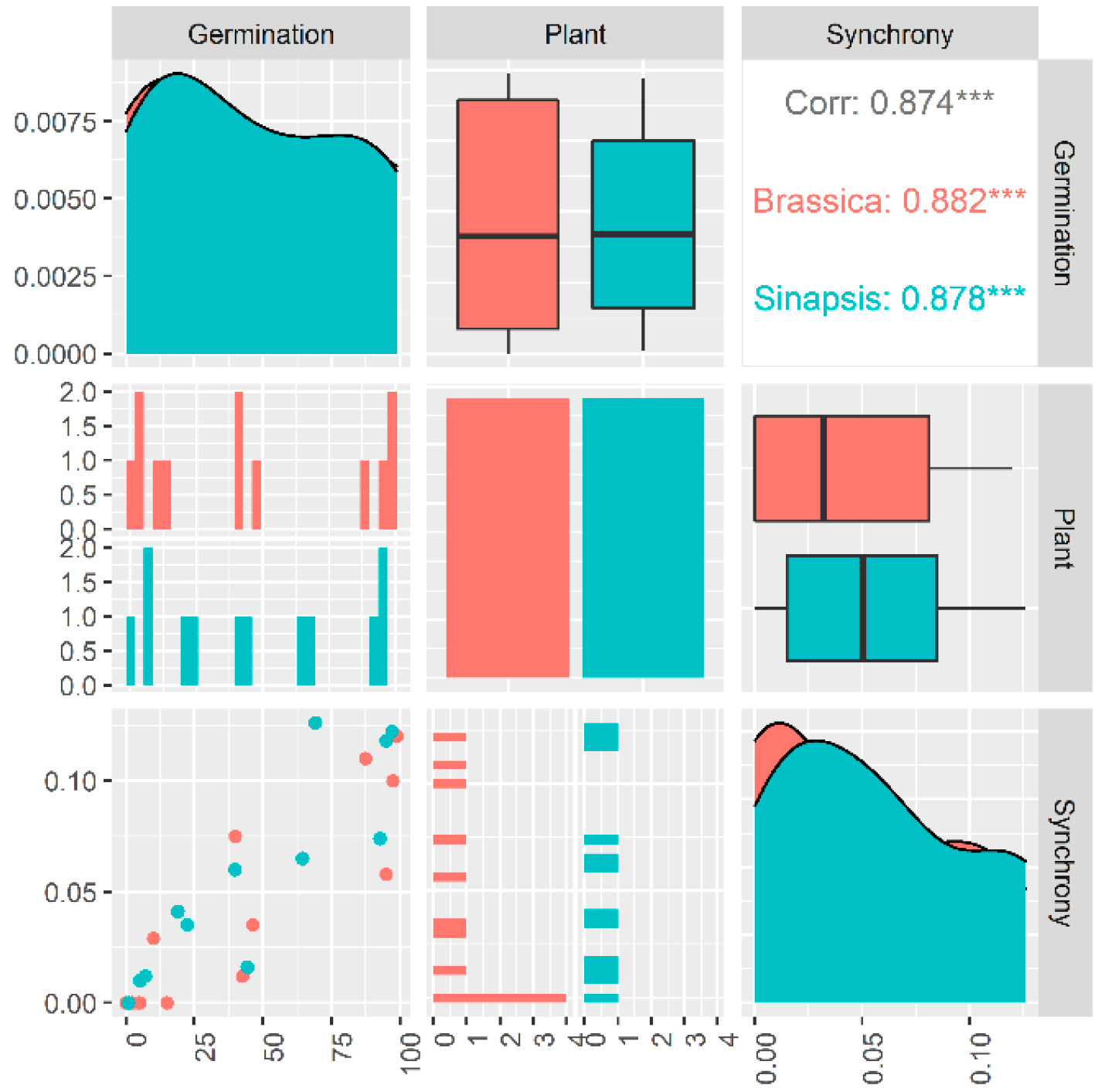
2.2. Germination Synchrony Patterns
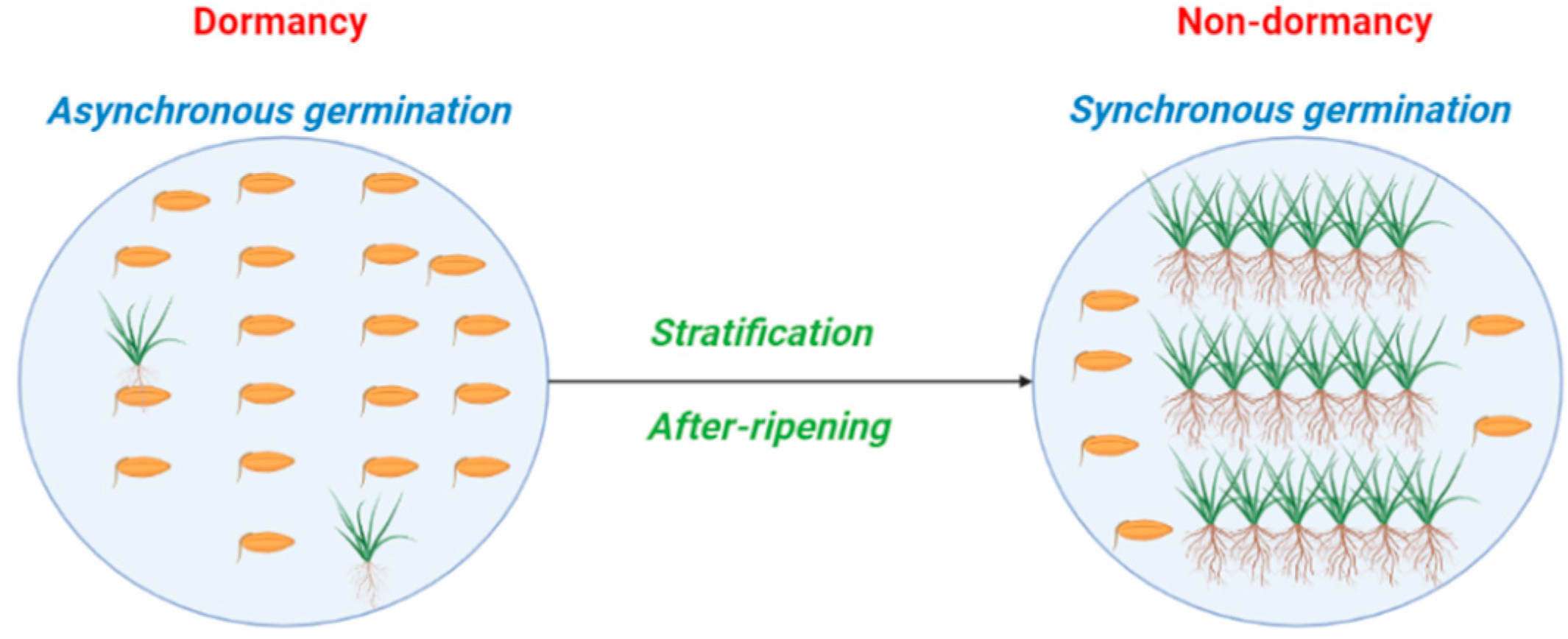
2.3. Dormancy Cycling and Germination Synchrony Patterns
2.4. Germination Synchrony and Dormancy Type
3. Discussion
3.1. After-Ripening and Stratification as a Synchronizing Process
3.2. Are All Seeds Responsive to After-Ripening?
3.3. Stratification
3.4. Seasonal Dormancy Cycling
3.5. Non-Deep Physiological Dormancy as a Determinant of Synchrony
4. Materials and Methods
4.1. Germination Synchrony
4.2. Germination Trials
4.3. Burial Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tørresen, K.S.; Karlsson, L.M.; Gonzalez-Andujar, J.L. Seed biology and population dynamics. In Expanding Horizons in Weed Research; Hatcher, P., Froud-Williams, R.J., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 85–106. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press/Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Gremer, J.R.; Wilcox, C.J.; Chiono, A.; Suglia, E.; Schmitt, J. Germination timing and chilling exposure create contingency in life history and influence fitness in the native wildflower Streptanthus tortuosus. J. Ecol. 2020, 108, 239–255. [Google Scholar] [CrossRef]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Kimball, S.; Angert, A.L.; Huxman, T.E.; Venable, D.L. Contemporary climate change in the Sonoran Desert favors cold-adapted species. Glob. Chang. Biol. 2010, 16, 1555–1565. [Google Scholar] [CrossRef]
- Eckhart, V.M.; Geber, M.A.; Morris, W.F.; Fabio, E.S.; Tiffin, P.; Moeller, D.A. The geography of demography: Long-term demographic studies and species distribution models reveal a species border limited by adaptation. Am. Nat. 2011, 178 (Suppl. S1), S26–S43. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; McEachern, A.K.; Cowan, C. Seasonal timing of first rain storms affects rare plant population dynamics. Ecology 2011, 92, 2236–2247. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Yates, C.J.; Hoyle, G.L.; Nicotra, A.B. Will among-population variation in seed traits improve the chance of species persistence under climate change? Glob. Ecol. Biogeogr. 2015, 24, 12–24. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. Niche construction through germination cueing: Life-history responses to timing of germination in Arabidopsis thaliana. Evolution 2005, 59, 771–785. [Google Scholar] [PubMed]
- Cohen, D.A.N. Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J. Theor. Biol. 1967, 16, 1–14. [Google Scholar] [CrossRef]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Phelps, K. Onion (Allium cepa L.) seedling emergence patterns can be explained by the influence of soil temperature and water potential on seed germination. J. Exp. Bot. 1993, 44, 407–414. [Google Scholar] [CrossRef]
- Ooi, M.K.; Auld, T.D.; Denham, A.J. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: Implications for seed bank persistence under climate change. Plant Soil 2012, 353, 289–303. [Google Scholar] [CrossRef]
- Hoyle, G.L.; Steadman, K.J.; Good, R.B.; McIntosh, E.J.; Galea, L.M.; Nicotra, A.B. Seed germination strategies: An evolutionary trajectory independent of vegetative functional traits. Front. Plant Sci. 2015, 6, 731. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, G.K.; Li, J.; Biddick, M.; Han, K.; Song, D.; Yang, Y.; Liu, B. Mechanisms underpinning the onset of seed coat impermeability and dormancy-break in Astragalus adsurgens. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.; Maleki, K.; Heshmati, S. Application of a process-based model to quantifying dormancy loss in seeds of Parrotia persica CA Meyer. S. Afr. J. Bot. 2022, 144, 97–104. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Gonzalez-Andujar, J.L. An Overview of Environmental Cues That Affect Germination of Nondormant Seeds. Seeds 2022, 1, 146–151. [Google Scholar] [CrossRef]
- Finch, J.; Walck, J.L.; Hidayati, S.N.; Kramer, A.T.; Lason, V.; Havens, K. Germination niche breadth varies inconsistently among three Asclepias congeners along a latitudinal gradient. Plant Biol. 2019, 21, 425–438. [Google Scholar] [CrossRef]
- Crocker, W.M. Mechanics of dormancy in seeds. American Journal of Botany. Am. J. Bot. 1916, 99–120. [Google Scholar] [CrossRef]
- Harper, J.L. Population biology of plants. Popul. Biol. Plants 1977. [Google Scholar]
- Maleki, K.; Soltani, E.; Arabhosseini, A.; Aghili Lakeh, M. A quantitative analysis of primary dormancy and dormancy changes during burial in seeds of Brassica napus. Nord. J. Bot. 2021, 39. [Google Scholar] [CrossRef]
- Arana, M.V.; Gonzalez-Polo, M.; Martinez-Meier, A.; Gallo, L.A.; Benech-Arnold, R.L.; Sánchez, R.A.; Batlla, D. Seed dormancy responses to temperature relate to Nothofagus species distribution and determine temporal patterns of germination across altitudes in Patagonia. New Phytol. 2016, 209, 507–520. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of non-deep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Batlla, D.; Benech-Arnold, R.L. Seed dormancy loss assessed by changes in Polygonum aviculare L. population hydrotime parameters. Development of a predictive model. Seed Sci. Res. 2004, 14, 277–286. [Google Scholar] [CrossRef]
- Maleki, K.; Baskin, C.C.; Baskin, J.M.; Kiani, M.; Alahdadi, I.; Soltani, E. Seed germination thermal niche differs among nine populations of an annual plant: A modeling approach. Ecol. Evol. 2022, 12, e9240. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Breaking seed dormancy during dry storage: A useful tool or major problem for successful restoration via direct seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef]
- Davis, W.E.; Rose, R.C. The effect of external conditions upon the after-ripening of the seeds of Crataegus mollis. Bot. Gaz. 1912, 54, 49–62. [Google Scholar]
- Grushvitzky, I.V. After-ripening of seeds of primitive tribes of angiosperms, conditions and peculiarities. In Physiologie, Okologie und Biochemie der der Keimung; Borris, H., Ed.; Ernst-Moritz-Arnst Universitat: Greifswald, Germany, 1967; Volume 1, pp. 329–336. [Google Scholar]
- Bewley, J.D.; Bradford, K.; Hilhorst, H. Seeds: Physiology of development, germination and dormancy; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Voight, P.W.; Tischler, C.R. Effect of seed treatment on germination and emergence of 3 warm-season grasses. J. Range Manag. 1997, 50, 170–174. [Google Scholar] [CrossRef]
- Kaye, T.N. Seed dormancy in high elevation plants: Implications for ecology and restoration. In Conservation and Management of Native Plants and Fungi; Kaye, T.N., Love, R.M., Luoma, D.L., Meinke, R.J., Wilson, M.V., Eds.; Native Plant Society of Oregon: Corvallis, OR, USA, 1997; pp. 115–120. [Google Scholar]
- Cristaudo, A.; Catara, S.; Mingo, A.; Restuccia, A.; Onofri, A. Temperature and storage time strongly affect the germination success of perennial Euphorbia species in Mediterranean regions. Ecol. Evol. 2019, 9, 10984–10999. [Google Scholar] [CrossRef]
- Carruggio, F.; Onofri, A.; Catara, S.; Impelluso, C.; Castrogiovanni, M.; Lo Cascio, P.; Cristaudo, A. Conditional Seed Dormancy Helps Silene hicesiae Brullo & Signor. Overcome Stressful Mediterranean Summer Conditions. Plants 2021, 10, 2130. [Google Scholar] [PubMed]
- Maleki, K.; Soltani, E.; Seal, C.E.; Pritchard, H.W.; Lamichhane, J.R. The seed germination spectrum of 528 plant species: A global meta-regression in relation to temperature and water potential. bioRxiv 2022. [Google Scholar] [CrossRef]
- Aravind, J.; Vimala Devi, S.; Radhamani, J.; Jacob, S.R.; Srinivasan, K. Germinationmetrics: Seed Germination Indices and Curve Fitting. R package version 0.1.5. 2021. Available online: https://github.com/aravind-j/germinationmetricshttps://cran.r-project.org/package=germinationmetrics (accessed on 28 August 2022).
- Weber, E.A.; Frick, K.; Gruber, S.; Claupein, W. Research and development towards a laboratory method for testing the genotypic predisposition of oilseed rape (Brassica napus L.) to secondary dormancy. Seed Sci. Technol. 2010, 38, 298–310. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.D.; Ferreira, W.R.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Braz. J. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef]



| Species | Mature Seed-Dormant Seed | After-Ripening-Non Dormant | ||
|---|---|---|---|---|
| Asynchrony | Synchrony | Asynchrony | Synchrony | |
| Experiment 1 | ||||
| Brassica napus | 3.14 ± 0.016 | 0.089 ± 0.002 | 2.57 ± 0.042 | 0.116 ± 0.007 |
| Sinapis arvensis | 2.40 ± 0.014 | 0.054 ± 0001 | 2.84 ± 0.072 | 0.132 ± 0.002 |
| Experiment 2 | Mature seed-Dormant seed | Stratification-non dormant | ||
| Asynchrony | Synchrony | Asynchrony | Synchrony | |
| Brassica napus | 2.75 ± 0.022 | 0.023 ± 0.002 | 1.95 ± 0.062 | 0.115 ± 0.002 |
| Sinapis arvensis | 2.25 ± 0.012 | 0.038 ± 0.004 | 2.00 ± 0.009 | 0.140 ± 0.0029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleki, K.; Maleki, K.; Soltani, E.; Oveisi, M.; Gonzalez-Andujar, J.L. A Model for Changes in Germination Synchrony and Its Implements to Study Weed Population Dynamics: A Case Study of Brassicaceae. Plants 2023, 12, 233. https://doi.org/10.3390/plants12020233
Maleki K, Maleki K, Soltani E, Oveisi M, Gonzalez-Andujar JL. A Model for Changes in Germination Synchrony and Its Implements to Study Weed Population Dynamics: A Case Study of Brassicaceae. Plants. 2023; 12(2):233. https://doi.org/10.3390/plants12020233
Chicago/Turabian StyleMaleki, Keyvan, Kourosh Maleki, Elias Soltani, Mostafa Oveisi, and Jose L. Gonzalez-Andujar. 2023. "A Model for Changes in Germination Synchrony and Its Implements to Study Weed Population Dynamics: A Case Study of Brassicaceae" Plants 12, no. 2: 233. https://doi.org/10.3390/plants12020233
APA StyleMaleki, K., Maleki, K., Soltani, E., Oveisi, M., & Gonzalez-Andujar, J. L. (2023). A Model for Changes in Germination Synchrony and Its Implements to Study Weed Population Dynamics: A Case Study of Brassicaceae. Plants, 12(2), 233. https://doi.org/10.3390/plants12020233







