Physiological and Full-Length Transcriptome Analyses Reveal the Dwarfing Regulation in Trifoliate Orange (Poncirus trifoliata L.)
Abstract
:1. Introduction
2. Results
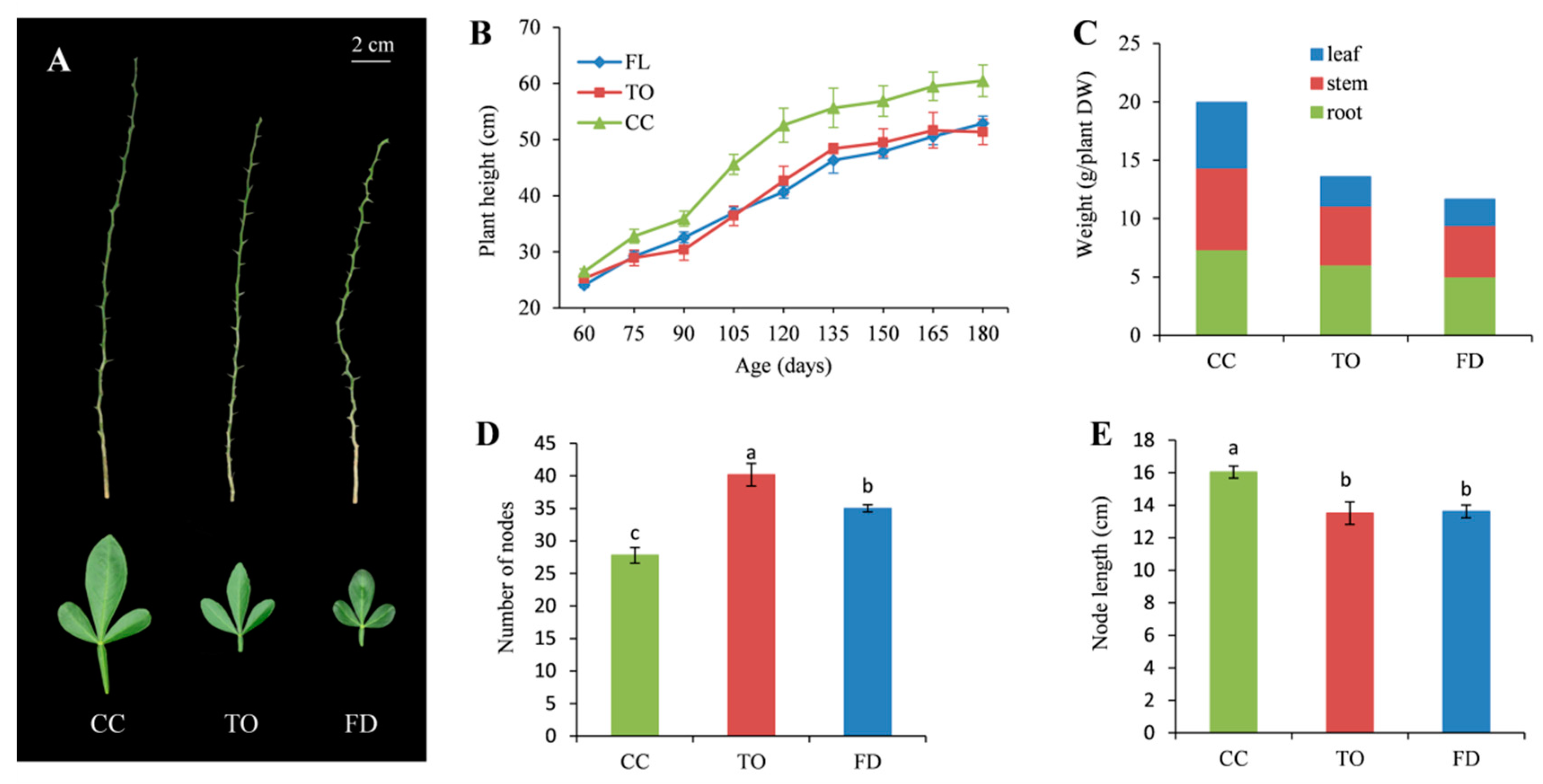
2.1. Comparison of Phenotypic Characteristics among Three Rootstocks
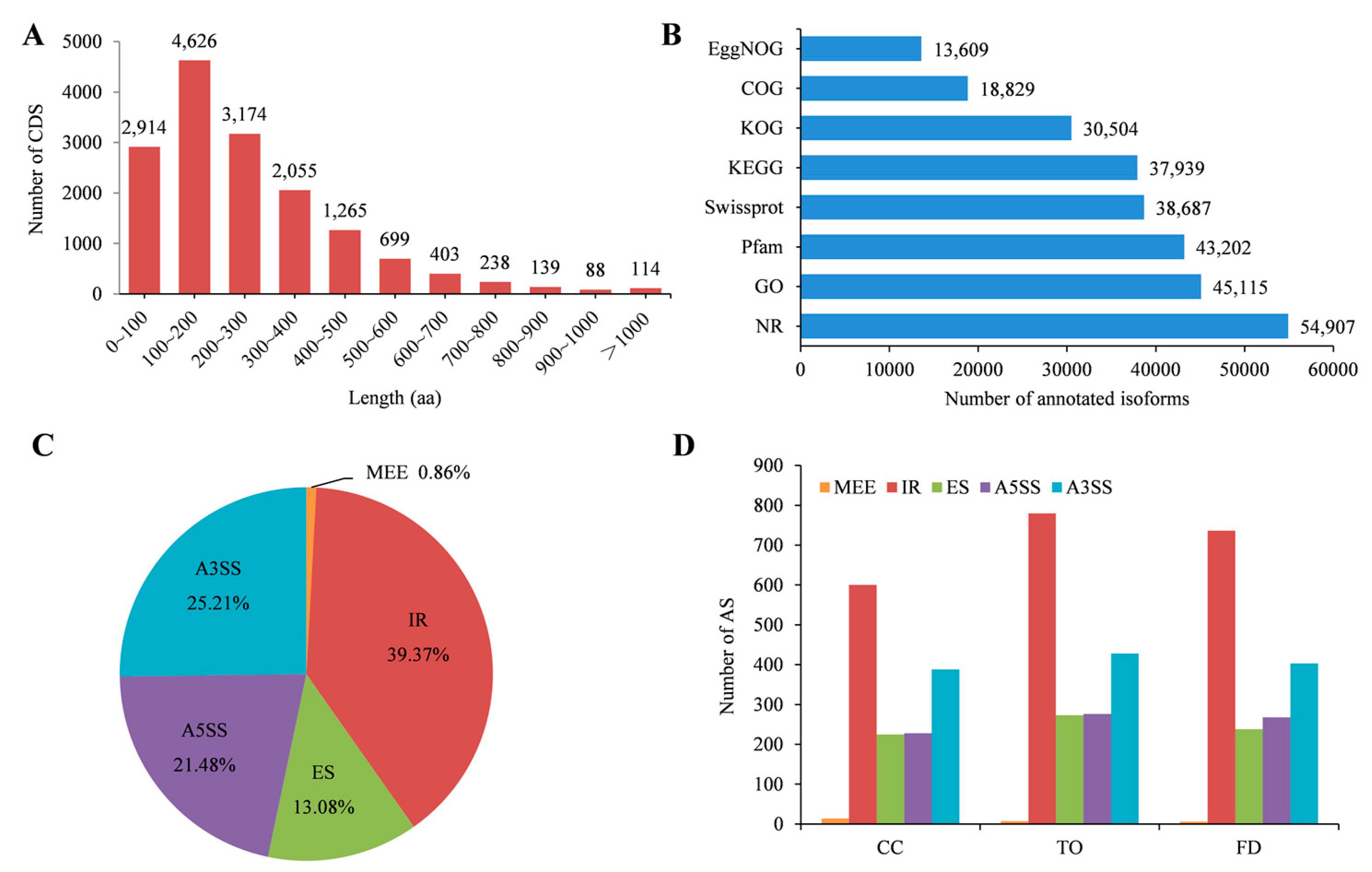
2.2. Summary of Full-Length RNA-Seq and Characterization of Transcripts and Alternative Splicing Events
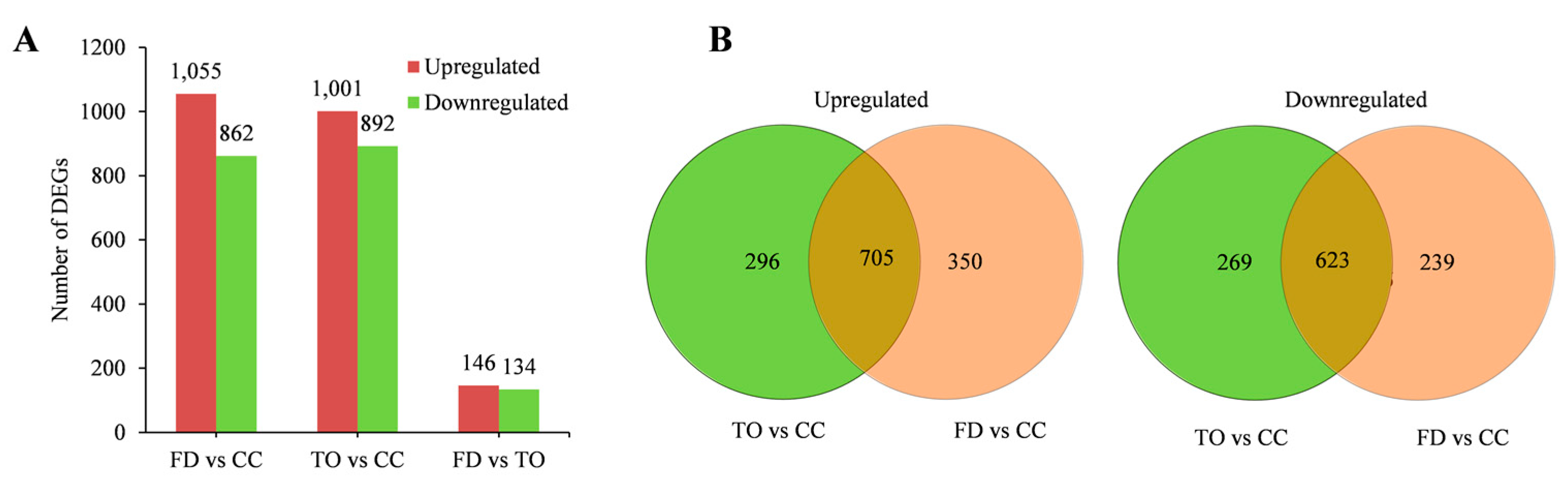
2.3. Comparative Analysis of DEGs
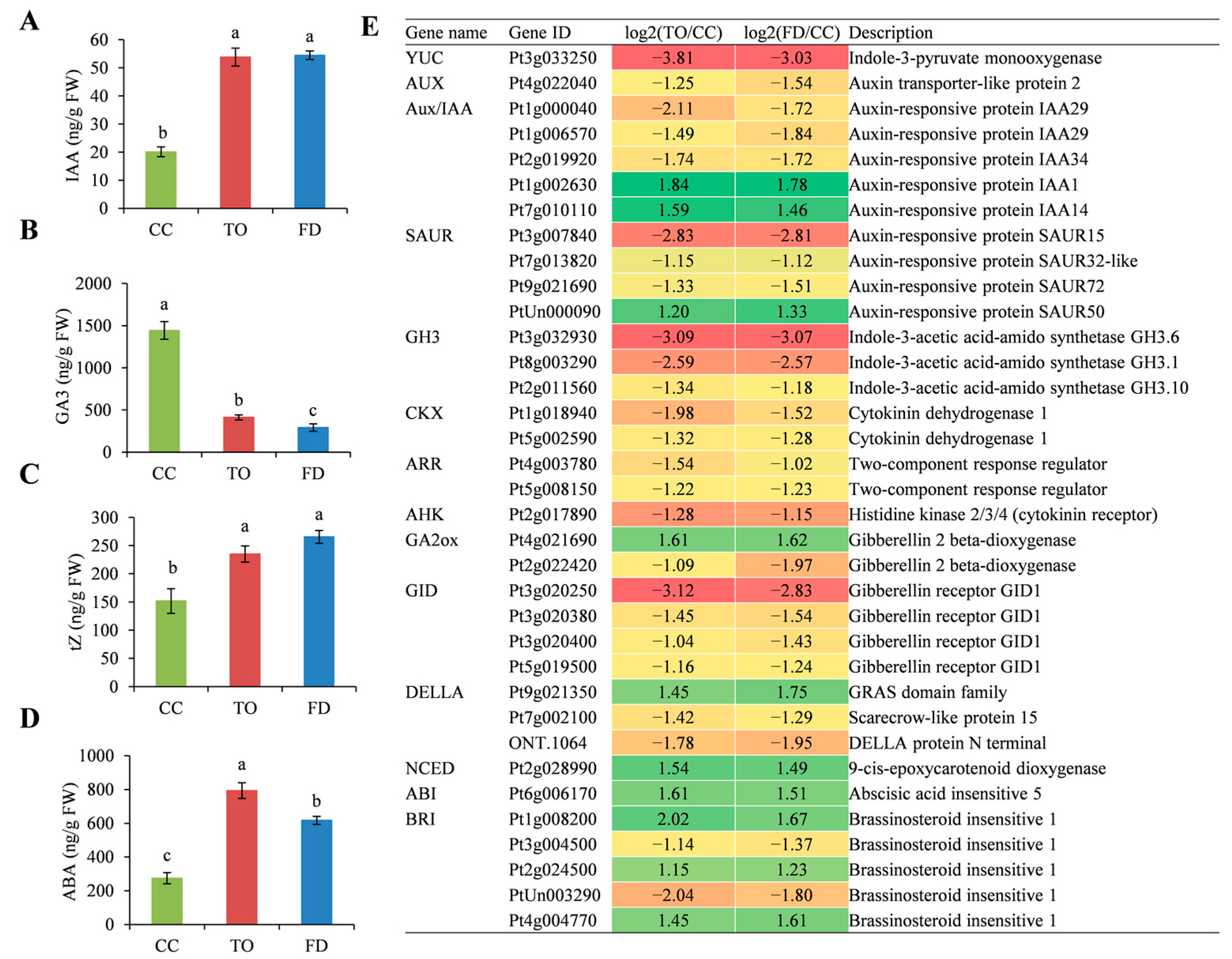
2.4. Analysis of Phytohormone Contents and the Related Gene Expression
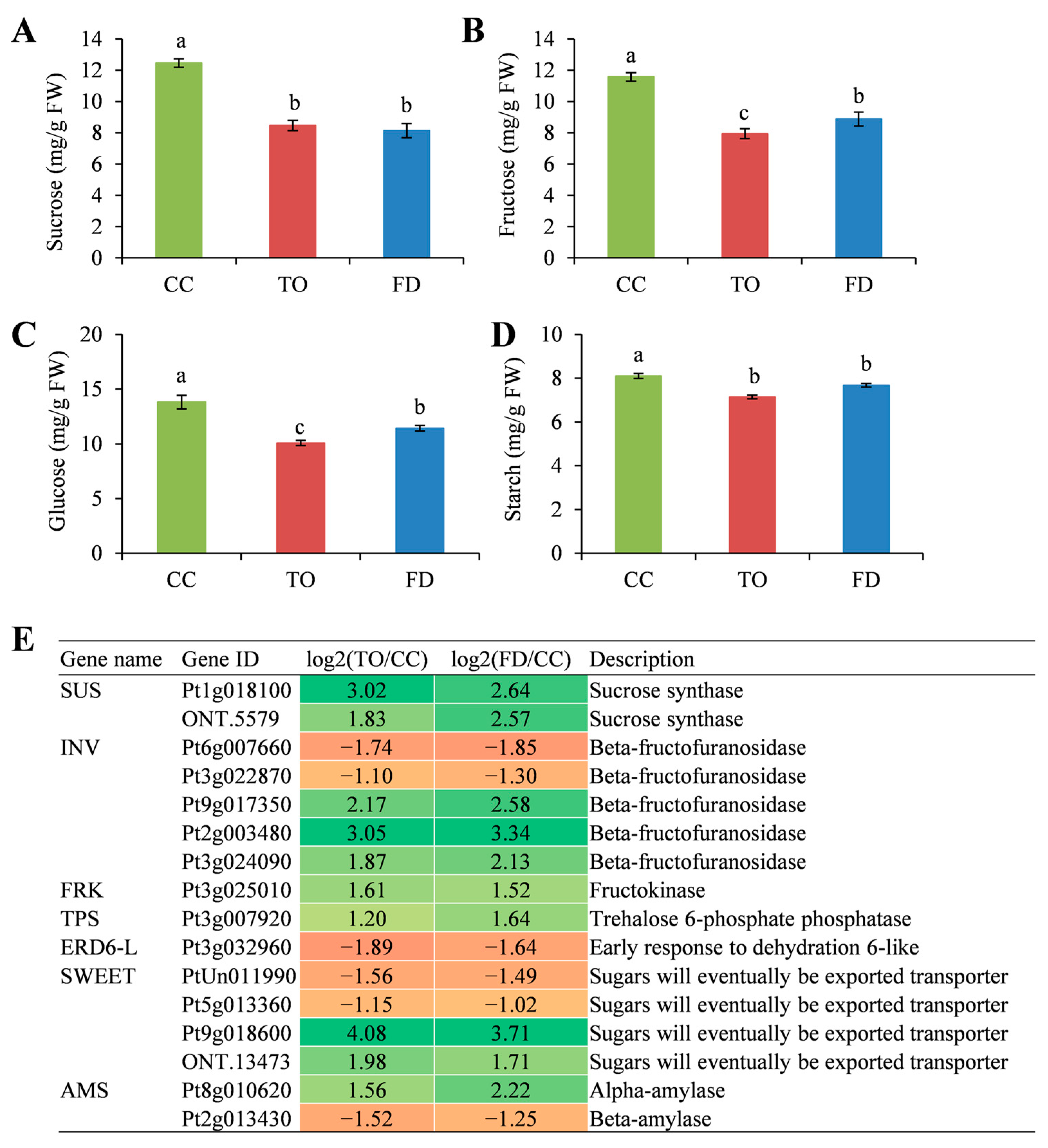
2.5. Analysis of Sugar Contents and the Related Gene Expression
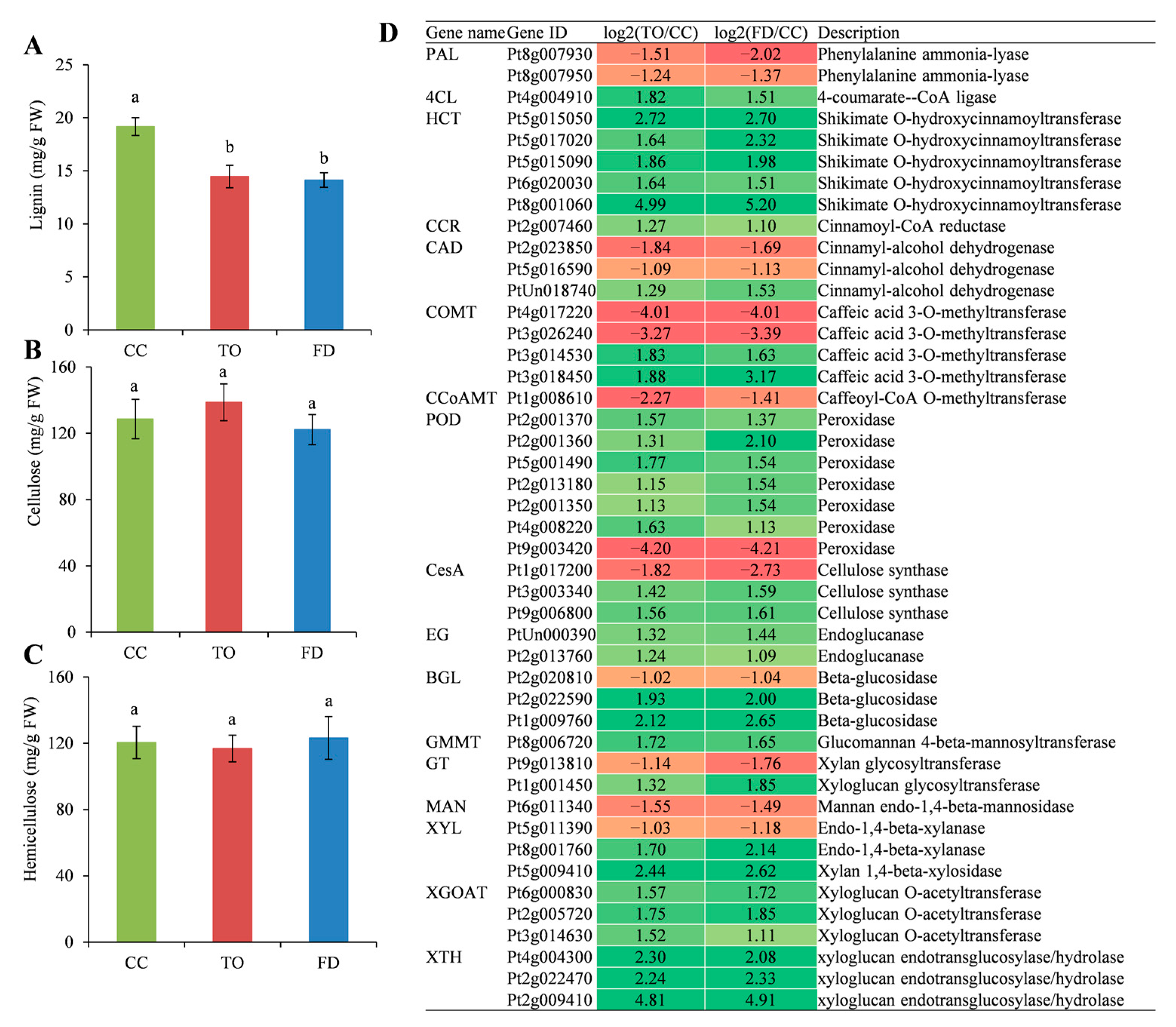
2.6. Analysis of Cell Wall Components and the Related Gene Expression
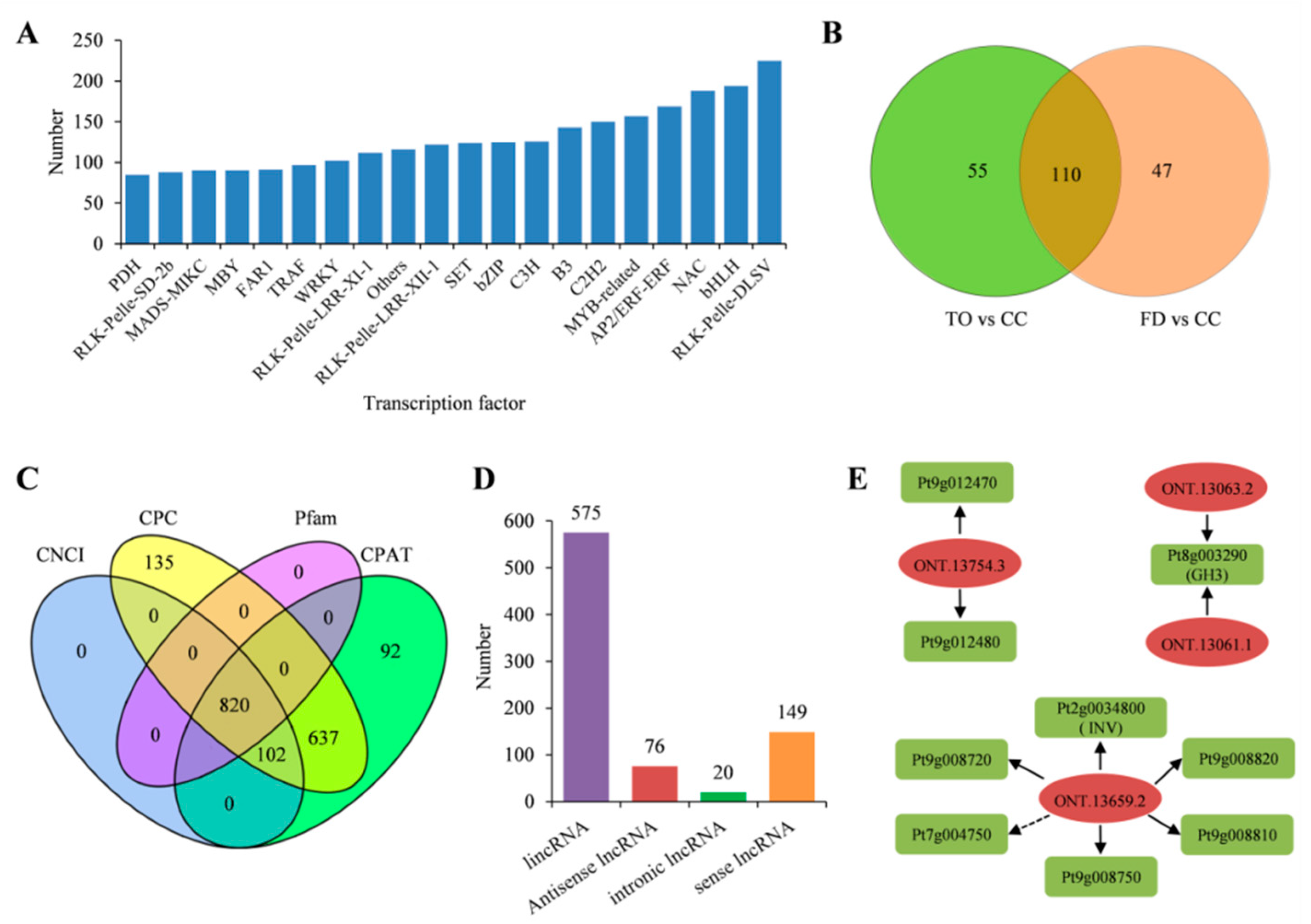
2.7. Characterization of the Transcription Factors and Long Noncoding RNAs
3. Discussion
3.1. Phytohormones Influence Stem Elongation
3.2. Involvement of Carbohydrate Metabolism in Dwarfing Regulation
3.3. Identification of Regulatory Factors Associated with Plant Growth
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurement of Phytohormone
4.3. Quantification of Non-Structural Carbohydrates
4.4. Determination of Lignin, Cellulose, and Hemicellulose Contents
4.5. RNA Extraction, Library Construction, and Transcriptome Sequencing
4.6. Differentially Expressed Transcripts (DEGs) Identification and Functional Enrichment Annotation
4.7. Identification of Alternative Splicing Events
4.8. TF Prediction and lncRNAs Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vidalakis, G.; Pagliaccia, D.; Bash, J.; Afunian, M.; Semancik, J. Citrus dwarfing viroid: Effects on tree size and scion performance specific to Poncirus trifoliata rootstock for high-density planting. Ann. Appl. Biol. 2011, 158, 204–217. [Google Scholar] [CrossRef]
- Hayat, F.; Li, J.; Iqbal, S.; Peng, Y.; Hong, L.; Balal, R.M.; Khan, M.N.; Nawaz, M.A.; Khan, U.; Farhan, M.A.; et al. A Mini Review of Citrus Rootstocks and Their Role in High-Density Orchards. Plants 2022, 11, 2876. [Google Scholar] [CrossRef] [PubMed]
- Fariborz, H.; Liu, T.; Kevin, F.; Ali, S. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Tworkoski, T.; Miller, S. Rootstock effect on growth of apple scions with different growth habits. Sci. Hortic. 2007, 111, 335–343. [Google Scholar] [CrossRef]
- Hayat, F.; Qiu, C.P.; Xu, X.F.; Wang, Y.; Wu, T.; Zhang, X.Z.; Nawaz, M.A.; Han, Z.H. Rootstocks influence morpho-logical and biochemical changes in young ‘Red Fuji’ apple plants. Int. J. Agric. Biol. 2019, 21, 1097–1105. [Google Scholar]
- Wertheim, S.J. Rootstocks for European pear: A review. Acta Hortic. 2000, 596, 299–309. [Google Scholar] [CrossRef]
- Ou, C.; Jiang, S.; Wang, F.; Tang, C.; Hao, N. An RNA-Seq analysis of the pear (Pyrus communis L.) transcriptome, with a focus on genes associated with dwarf. Plant Gene 2015, 4, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Tombesi, S.; Almehdi, A.; Dejong, T.M. Phenotyping vigour control capacity of new peach rootstocks by xylem ves-sel analysis. Sci. Hortic. 2011, 127, 353–357. [Google Scholar] [CrossRef]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.B.; Shi, T.; Gao, Z.H. An insight into dwarf-ing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Phillips, R.L.; Castle, W.S. Evaluation of Twelve Rootstocks for Dwarfing Citrus1. J. Am. Soc. Hortic. Sci. 1977, 102, 526–528. [Google Scholar] [CrossRef]
- Ignacio, L.; Forner, J.B.; Talón, M. The dwarfing mechanism of citrus rootstocks F&A 418 and #23 is related to com-petition between vegetative and reproductive growth. Tree Physiol. 2004, 24, 225–232. [Google Scholar]
- Forner-Giner, M.A.; Rodriguez-Gamir, J.; Martinez-Alcantara, B.; Quiñones, A.; Iglesias, D.J.; Primo-Millo, E.; Forner, J. Performance of navel orange trees grafted onto two new dwarfing rootstocks (Forner-Alcaide 517 and For-ner-Alcaide 418). Sci. Hortic. 2014, 179, 376–387. [Google Scholar] [CrossRef]
- Hervalejo, A.; Arjona-López, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J. Suitability of two dwarfing citrus rootstocks for ‘Salustiana’ orange trees grown under super-high-density conditions with mechanical harvesting. New Zealand J. Crop. Hortic. Sci. 2022, 50, 1–12. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Li, J.; Liu, M.-M.; Yao, Q.; Chen, J.-Z. Transcriptome Profiling to Understand the Effect of Citrus Rootstocks on the Growth of ‘Shatangju’ Mandarin. PLoS ONE 2017, 12, e0169897. [Google Scholar] [CrossRef] [Green Version]
- Sykes, S.R. Characterisation of citrus rootstock germplasm introduced as seeds to Australia from the people’s repub-lic of China. Sci. Hortic. 2011, 127, 298–304. [Google Scholar] [CrossRef]
- Nesom, G. Citrus trifoliata (Rutaceae): Review of biology and distribution in the USA. Phytoneuron 2014, 46, 1–14. [Google Scholar]
- Guo, T.C.; Chen, Q.Y.; Ye, Y.M.; Du, Y.X. The germplasm of Poncirus trifoliata (L.) Raf. South China Fruit 1966, 25, 8–10. (In Chinese) [Google Scholar]
- Dong, C.C. Study on Related Parameters of Dwarfing Traits of Three Kinds of Citrus Rootstocks and Their Grafted Seedlings. Master’s Thesis, Southwest University, Chongqing, China, 2017. (In Chinese). [Google Scholar]
- Peng, Z.; Bredeson, J.V.; Wu, G.A.; Shu, S.; Rawat, N.; Du, D.; Parajuli, S.; Yu, Q.; You, Q.; Rokhsar, D.S.; et al. A chromosome-scale reference genome of trifoliate orange (Poncirus trifoliata) provides insights into dis-ease resistance, cold tolerance and genome evolution in citrus. Plant J. 2020, 104, 1215–1232. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Y.T.; Jiang, X.L.; Yu, H.W.; Jia, H.H.; Tan, C.M.; Hu, G.; Hu, Y.B.; Rao, M.J.; Deng, X.X.; et al. Ge-nome of a citrus rootstock and global DNA demethylation caused by heterografting. Hortic. Res. 2021, 8, 69. [Google Scholar] [CrossRef]
- Noda, K.; Okuda, H.; Iwagaki, I. Indole acetic acid and abscisic acid levels in new shoots and fibrous roots of citrus scion-rootstock combinations. Sci. Hortic. 2000, 84, 245–254. [Google Scholar] [CrossRef]
- Cheng, F.S.; Roose, M.L. Origin and Inheritance of Dwarfing by the Citrus Rootstock Poncirus trifoliata ‘Flying Dragon’. J. Am. Soc. Hortic. Sci. 1995, 120, 286–291. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Rodriguez-Gamir, J.; Martínez-Cuenca, M.R.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Relationship between hydraulic conductance and citrus dwarfing by the Flying Dragon rootstock (Poncirus trifoliata L. Raft var. monstruosa). Trees 2013, 27, 629–638. [Google Scholar] [CrossRef]
- Long, C.R.; Zhou, D.G.; Wang, S.H. Effect of a rootstock on plant dwarfing and fruit quality of Lemon trees. Fujian J. Agr. Sci. 2016, 31, 360–365. (In Chinese) [Google Scholar]
- Mademba-Sy, F. Use of flying dragon trifoliate orange as dwarfing rootstock for citrus under tropical climatic con-ditions. Hort. Sci. 2012, 47, 11–17. [Google Scholar]
- Hayat, F.; Li, J.; Liu, W.; Li, C.; Song, W.; Iqbal, S.; Khan, U.; Javed, H.U.; Altaf, M.A.; Tu, P.; et al. Influence of citrus rootstocks on scion growth, hormone levels, and metabolites profile of ‘Shatangju’ mandarin (Citrus reticulata Blan-co). Horticulturae 2022, 8, 608. [Google Scholar] [CrossRef]
- Chung, Y.; Choe, S. The Regulation of Brassinosteroid Biosynthesis in Arabidopsis. Crit. Rev. Plant Sci. 2013, 32, 396–410. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Wang, X.; Liang, L.; Yue, J.; Li, L. Comparative Analyses of Anatomical Structure, Phytohormone Levels, and Gene Expression Profiles Reveal Potential Dwarfing Mechanisms in Shengyin Bamboo (Phyllostachys edulis f. tubaeformis). Int. J. Mol. Sci. 2018, 19, 1697. [Google Scholar] [CrossRef] [Green Version]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Relationships between xylem vessel characteristics, calculated axial hydraulic conductance and size controlling capacity of peach rootstocks. Ann. Bot. 2010, 105, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wang, C.; Tian, Y.; Chu, Q.; Hu, C. Anatomical characteristics of young stems and mature leaves of dwarf pear. Sci. Hortic. 2015, 186, 172–179. [Google Scholar] [CrossRef]
- Zorić, L.; Ljubojević, M.; Merkulov, L.; Luković, J.; Ognjanov, V. Anatomical Characteristics of Cherry Rootstocks as Possible Preselecting Tools for Prediction of Tree Vigor. J. Plant Growth Regul. 2011, 31, 320–331. [Google Scholar] [CrossRef]
- Foster, T.M.; McAtee, P.A.; Waite, C.N.; Boldingh, H.L.; McGhie, T.K. Apple dwarfing rootstocks exhibit an imbalance in carbohydrate allocation and reduced cell growth and metabolism. Hortic. Res. 2017, 4, 17009. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Bi, F.; Liu, J.; He, W.; Li, C.; Dong, T.; Yang, Q.; Gao, H.; Dou, T.; Zhong, X.; et al. Transcriptome and metabolome profiling provide insights into molecular mechanism of pseudostem elongation in banana. BMC Plant Biol. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Galarneau, E.R.; Yang, Y.; Li, D.; Song, J.; Ma, C.; Zhang, S.; Wang, R. Metabolome analysis reveals important compounds related to dwarfing effect of interstock on scion in pear. Ann. Appl. Biol. 2021, 179, 108–122. [Google Scholar] [CrossRef]
- Tong, H.; Liu, L.; Jin, Y.; Du, L.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF and LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 2012, 24, 2562–2577. [Google Scholar] [CrossRef]
- Lan, J.; Lin, Q.; Zhou, C.; Ren, Y.; Liu, X.; Miao, R.; Jing, R.; Mou, C.; Nguyen, T.; Zhu, X.; et al. Small grain and semi-dwarf 3, a WRKY transcription factor, negatively regulates plant height and grain size by stabilizing SLR1 expression in rice. Plant Mol. Biol. 2020, 104, 429–450. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Ma, C.; Chen, B.; Sun, Z.; Tian, Y.; Wang, C. A mutation in the promoter of the arabinogalactan protein 7-like gene PcAGP7-1 affects cell morphogenesis and brassinolide content in pear (Pyrus communis L.) stems. Plant J. 2022, 109, 47–63. [Google Scholar] [CrossRef]
- Cui, J.; Shen, N.; Lu, J.; Xu, G.; Wang, Y.; Jin, B. Analysis and comprehensive comparison of PacBio and na-nopore-based RNA sequencing of the Arabidopsis transcriptome. Ann. Appl. Biol. 2020, 16, 85. [Google Scholar]
- Yang, S.; Zhang, K.; Zhu, H.; Zhang, X.; Yan, W.; Xu, N.; Liu, D.; Hu, J.; Wu, Y.; Weng, Y.; et al. Melon short in-ternode (CmSi) encodes an ERECTA-like receptor kinase regulating stem elongation through auxin signaling. Hortic. Res. 2020, 7, 202. [Google Scholar] [CrossRef]
- Tworkoski, T.J.; Fazio, G. Effects of size-controlling apple rootstocks on growth, abscisic acid, and hydraulic conduc-tivity of scion of different vigor. Int. J. Fruit. Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Vieten, A.; Sauer, M.; Brewer, P.B.; Friml, J. Molecular and cellular aspects of auxin-transport- mediated develop-ment. Trends Plant Sci. 2007, 12, 160–168. [Google Scholar] [CrossRef]
- Dreher, K.A.; Brown, J.; Saw, R.E.; Callis, J. The Arabidopsis Aux/IAA Protein Family Has Diversified in Degradation and Auxin Responsiveness. Plant Cell 2006, 18, 699–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Ara-bidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.G.; Phillips, A.L.; Hedden, P. Molecular cloning and functional expression of gibberellin 2-oxidases, mul-tifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 1999, 96, 4698–4703. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhuang, W.; Tu, X.; Gao, Z.; Xiong, A.; Yu, X.; Li, X.; Li, F.; Qu, S. Transcriptomic analysis of inter-stock-induced dwarfism in Sweet Persimmon (Diospyros kaki thunb.). Hortic. Res. 2019, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2007, 59, 75–83. [Google Scholar] [CrossRef]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Halford, N.; Curtis, T.; Muttucumaru, N.; Postles, J.; Mottram, D. Sugars in crop plants. Ann. Appl. Biol. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Riou-Khamlichi, C.; Menges, M.; Healy, J.M.S.; Murray, J.A.H. Sugar control of the plant cell cycle: Differential regu-lation of Arabidopsis D-type cyclin gene expression. Mol. Cell Biol. 2000, 20, 4513–4521. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, R.; Sauer, N.; Neuhaus, H.E. Sugar transport across the plant vacuolar membrane: Nature and regulation of carrier proteins. Curr. Opin. Plant Biol. 2015, 25, 63–70. [Google Scholar] [CrossRef]
- Muro-Villanueva, F.; Mao, X.; Chapple, C. Linking phenylpropanoid metabolism, lignin deposition, and plant growth inhibition. Curr. Opin. Biotechnol. 2019, 56, 202–208. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Andrew, C.; Specht, C.D. Understanding plant cellulose synthases through a comprehensive investigation of the cellulose synthase family sequences. Front. Plant Sci. 2011, 2, 5. [Google Scholar]
- Beguin, P. Molecular biology of cellulose degradation. Annu. Rev. Microbiol. 1990, 44, 219–248. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Ye, Z.-H. Xyloglucan O-acetyltransferases from Arabidopsis thaliana and Populus trichocarpa catalyze acetylation of fucosylated galactose residues on xyloglucan side chains. Planta 2018, 248, 1159–1171. [Google Scholar] [CrossRef]
- Zhang, D.; Li, R.; Yue, J.; Shi, Y.; Zhuo, L.; Wang, L.; Shen, X. RNA-seq-based transcriptome analysis of stem devel-opment and dwarfing regulation in Agapanthus praecox ssp orientalis (Leighton) Leighton. Gene 2015, 565, 252–267. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, C.; Tian, Y.; Yang, S.; Shen, J.; Liu, Q.; Zhang, H. Candidates responsible for dwarf pear phenotype as revealed by comparative transcriptome analysis. Mol. Breed. 2019, 39, 1. [Google Scholar] [CrossRef]
- Makkena, S.; Lamb, R.S. The bHLH transcription factor SPATULA is a key regulator of organ size in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e24140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wu, S.; Wang, X.; Mao, T.; Bao, M.; Zhang, J.; Zhang, J. Genome-wide identification and characterization of the bHLH gene family in an ornamental woody plant Prunus mume. Hortic. Plant J. 2022, 8, 531–544. [Google Scholar] [CrossRef]
- Chao, Q.; Gao, Z.F.; Zhang, D.; Zhao, B.G.; Dong, F.Q.; Fu, C.X.; Liu, L.J.; Wang, B.C. The developmental dynamics of the Populus stem transcriptome. Plant Biotechnol. J. 2019, 17, 206–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, G.; Sharma, S.; Upadhyay, S.K.; Singh, K. Long Non-coding RNAs Coordinate Developmental Transitions and Other Key Biological Processes in Grapevine. Sci. Rep. 2019, 9, 3552. [Google Scholar] [CrossRef] [Green Version]
- Staiger, D.; Brown, J.W. Alternative Splicing at the Intersection of Biological Timing, Development, and Stress Responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Shen, Y.; Wen, S.; Zong, Y.; Li, H. Alternative Splicing Enhances the Transcriptome Complexity of Liriodendron chinense. Front. Plant Sci. 2020, 11, 578100. [Google Scholar] [CrossRef]
- Yang, R.; Yang, T.; Zhang, H.; Qi, Y.; Xing, Y.; Zhang, N.; Li, R.; Weeda, S.; Ren, S.; Ouyang, B.; et al. Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. 2014, 77, 23–34. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, Q.; Zhou, G.; Liu, X.; Ma, Z.; Gu, Q. Identification of genes associated with soluble sugar and organic acid accumulation in ‘Huapi’ kumquat (Fortunella crassifolia Swingle) via transcriptome analysis. J. Sci. Food Agric. 2021, 101, 4321–4331. [Google Scholar] [CrossRef]
- Papa, G.; Varanasi, P.; Sun, L.; Cheng, G.; Stavila, V.; Holmes, B.; Simmons, B.A.; Adani, F.; Singh, S. Exploring the effect of different plant lignin content and composition on ionic liquid pretreatment efficiency and enzymatic saccharifica-tion of Eucalyptus globulus L. mutants. Bioresour Technol. 2012, 117, 352–359. [Google Scholar] [CrossRef]
- Zhou, X.; Lindsay, H.; Robinson, M.D. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 2014, 42, e91. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. iTAK: A Program for Genome-wide Prediction and Classification of Plant Transcription Factors, Transcriptional Regulators, and Protein Kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Wei, Q.; Hu, Y.; Chen, M.; Chen, Z.; Zheng, S.; Ma, Q.; Luo, Z. Physiological and Full-Length Transcriptome Analyses Reveal the Dwarfing Regulation in Trifoliate Orange (Poncirus trifoliata L.). Plants 2023, 12, 271. https://doi.org/10.3390/plants12020271
Gu Q, Wei Q, Hu Y, Chen M, Chen Z, Zheng S, Ma Q, Luo Z. Physiological and Full-Length Transcriptome Analyses Reveal the Dwarfing Regulation in Trifoliate Orange (Poncirus trifoliata L.). Plants. 2023; 12(2):271. https://doi.org/10.3390/plants12020271
Chicago/Turabian StyleGu, Qingqing, Qingjiang Wei, Yongwei Hu, Mengru Chen, Ziwen Chen, Shuang Zheng, Qiaoli Ma, and Zhengrong Luo. 2023. "Physiological and Full-Length Transcriptome Analyses Reveal the Dwarfing Regulation in Trifoliate Orange (Poncirus trifoliata L.)" Plants 12, no. 2: 271. https://doi.org/10.3390/plants12020271
APA StyleGu, Q., Wei, Q., Hu, Y., Chen, M., Chen, Z., Zheng, S., Ma, Q., & Luo, Z. (2023). Physiological and Full-Length Transcriptome Analyses Reveal the Dwarfing Regulation in Trifoliate Orange (Poncirus trifoliata L.). Plants, 12(2), 271. https://doi.org/10.3390/plants12020271





