Disease Forecasting for the Rational Management of Grapevine Mildews in the Chianti Bio-District (Tuscany)
Abstract
:1. Introduction
2. Results
2.1. Season 2020
2.1.1. Weather Conditions
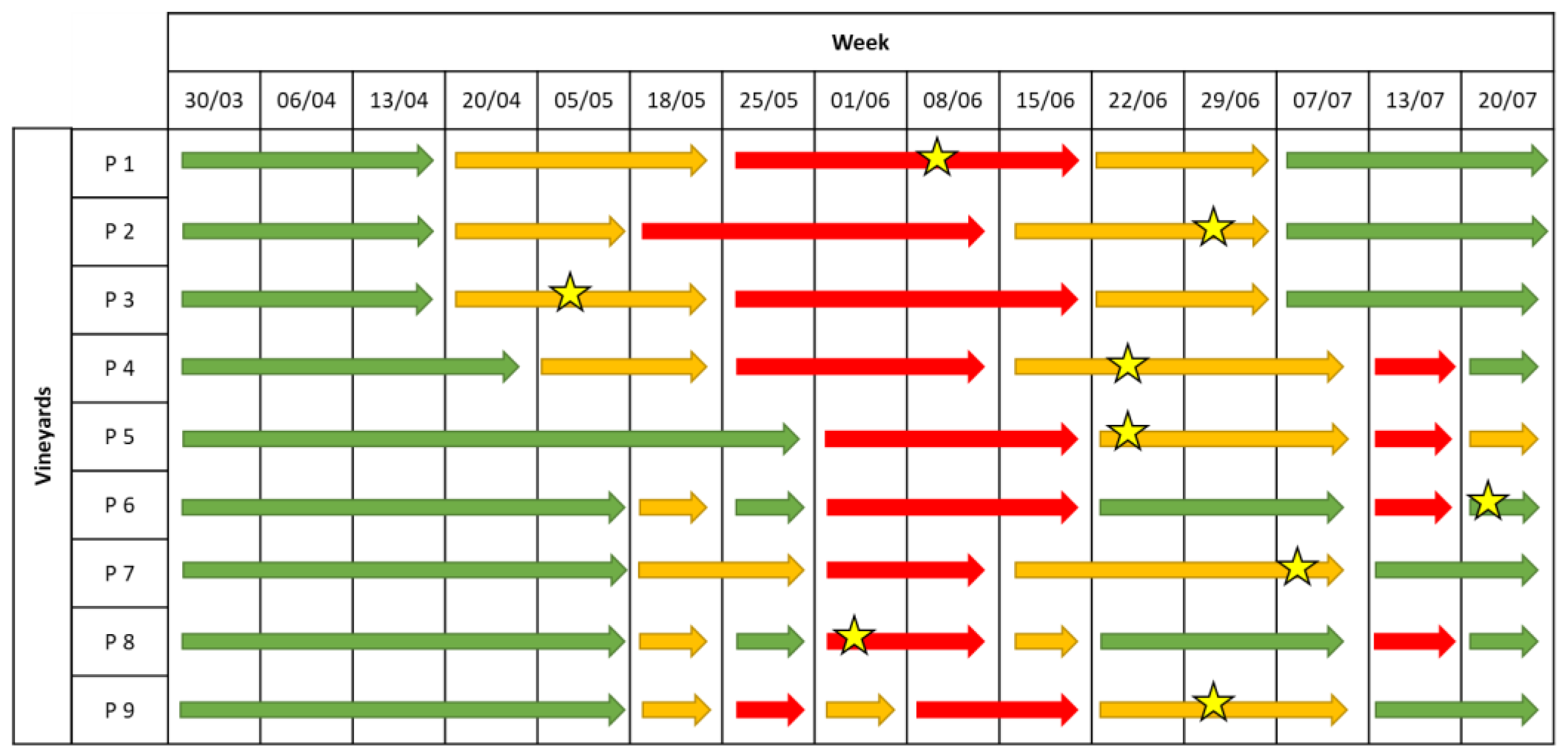
2.1.2. Downy Mildew
2.1.3. Powdery Mildew
2.2. Season 2021
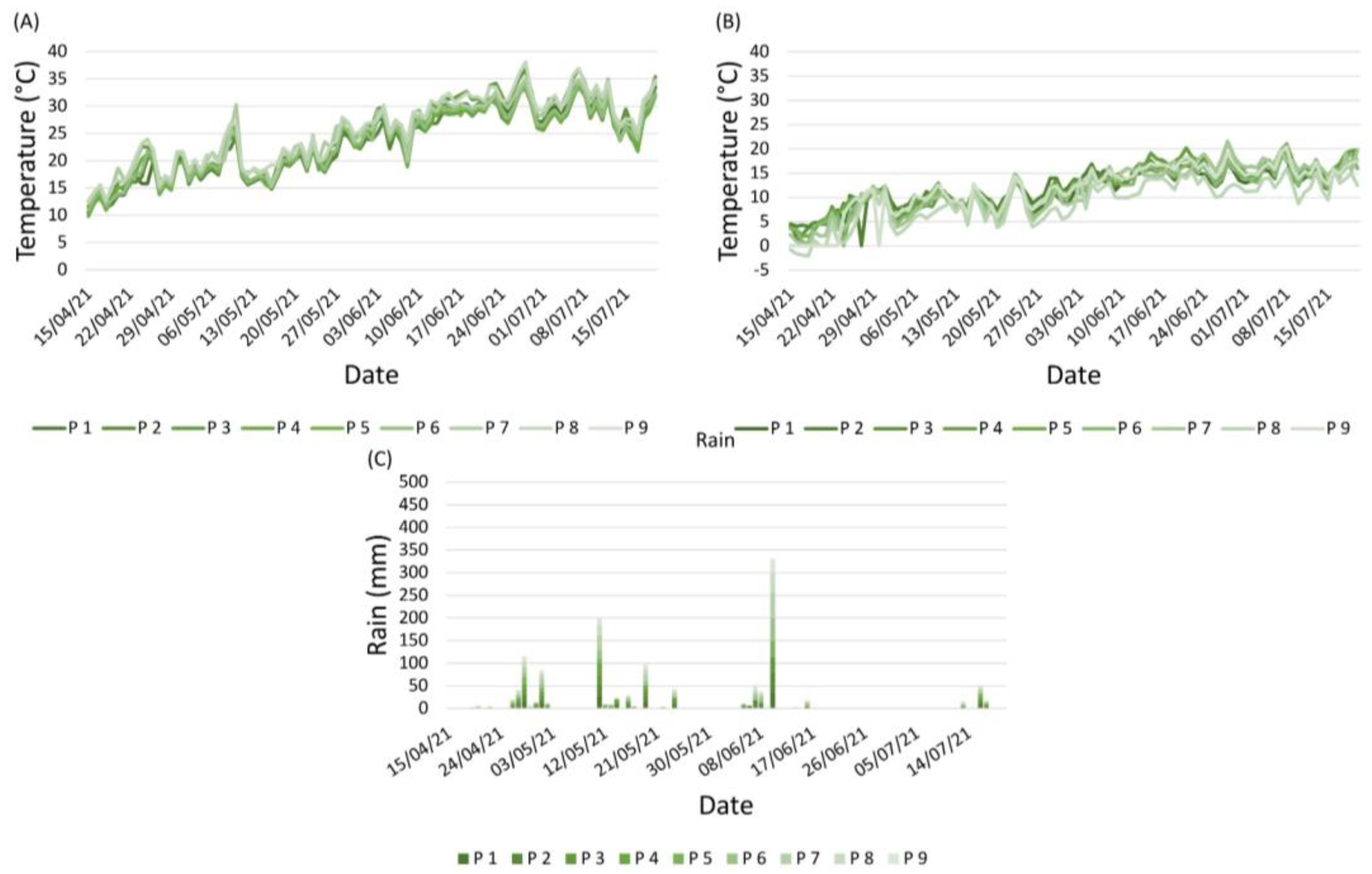
2.2.1. Weather Conditions
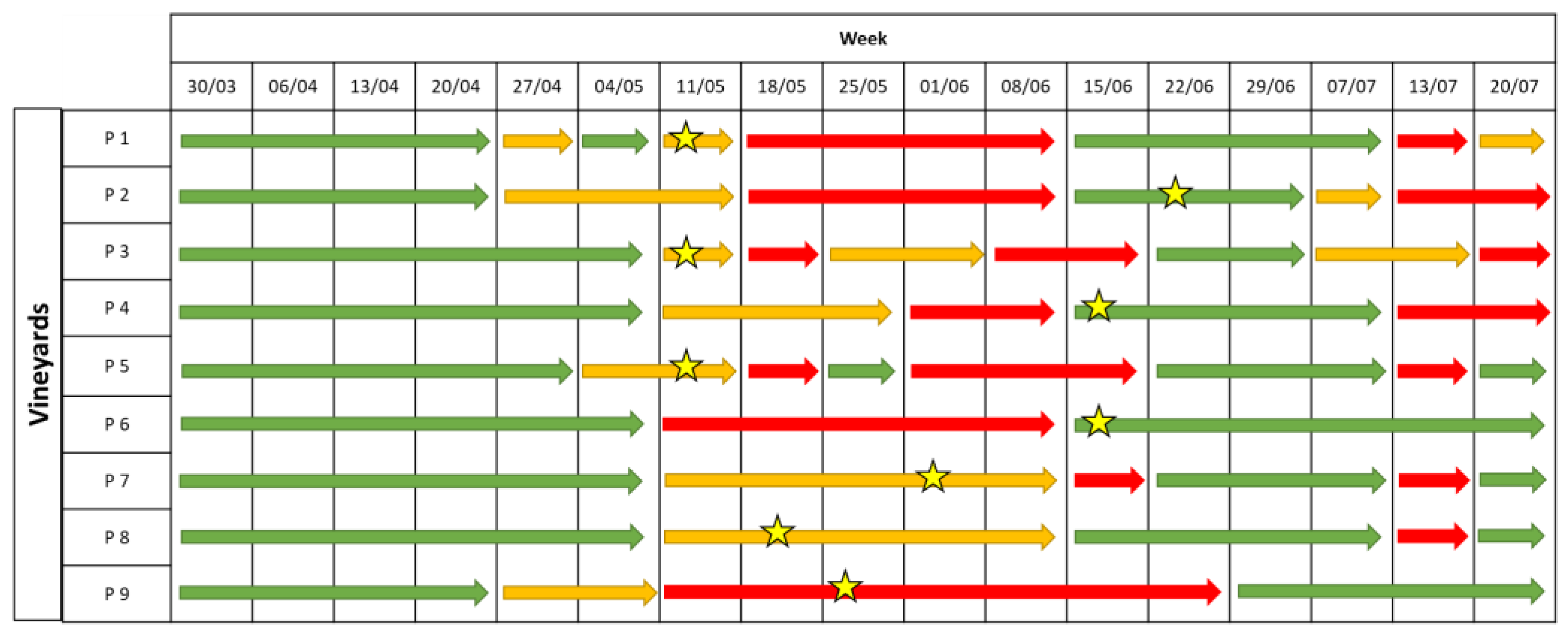
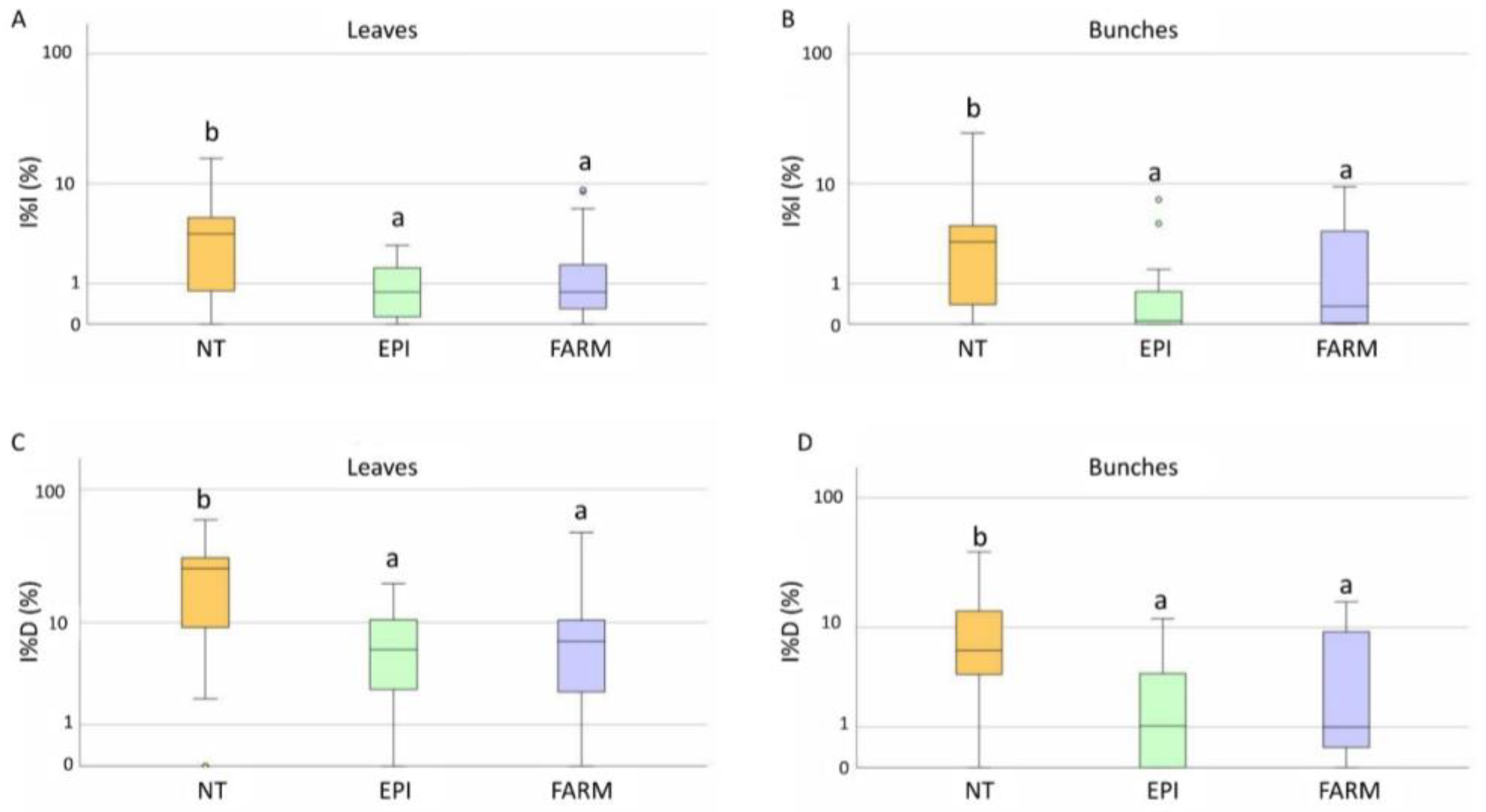
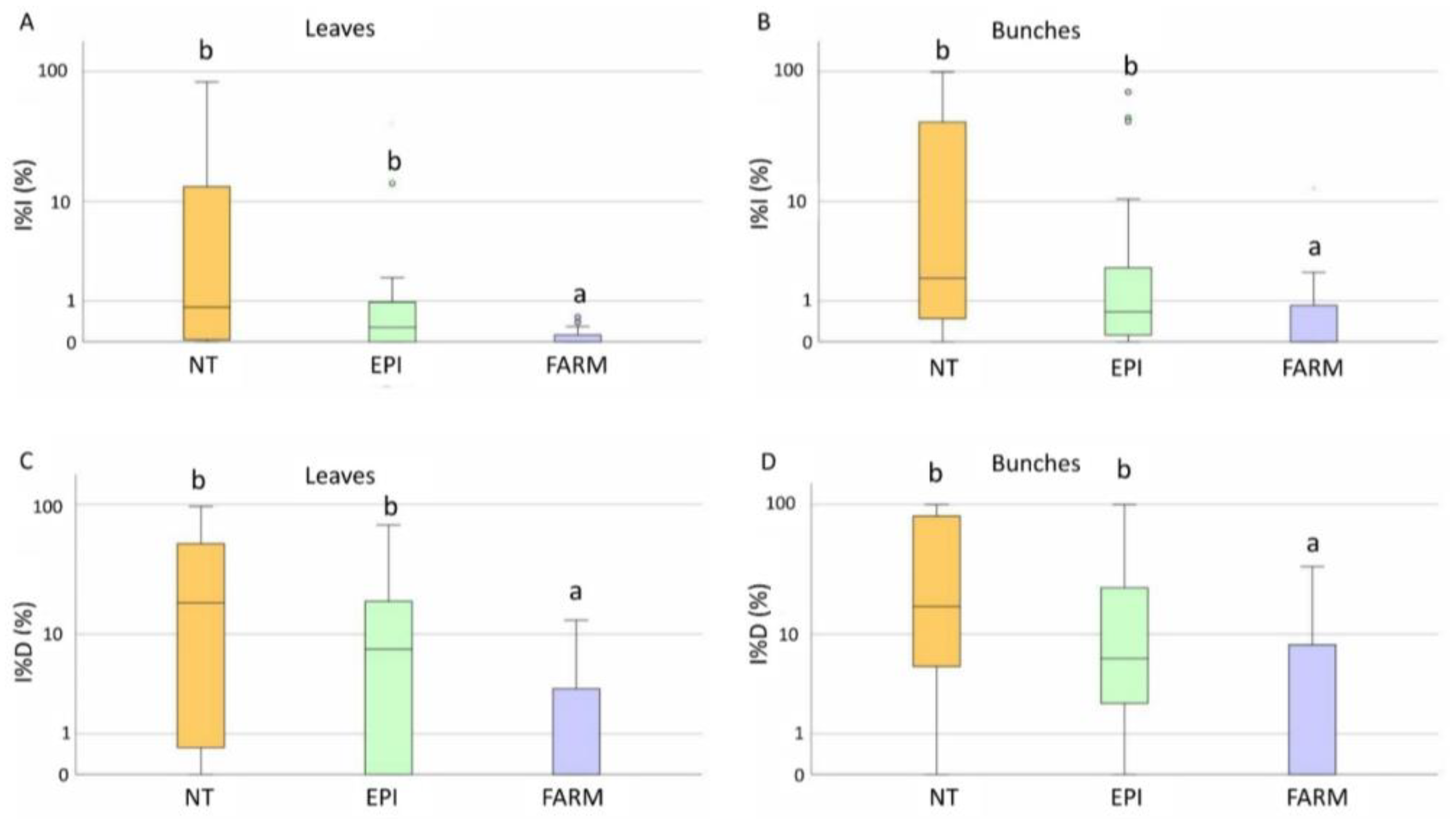
2.2.2. Downy Mildew
2.2.3. Powdery Mildew
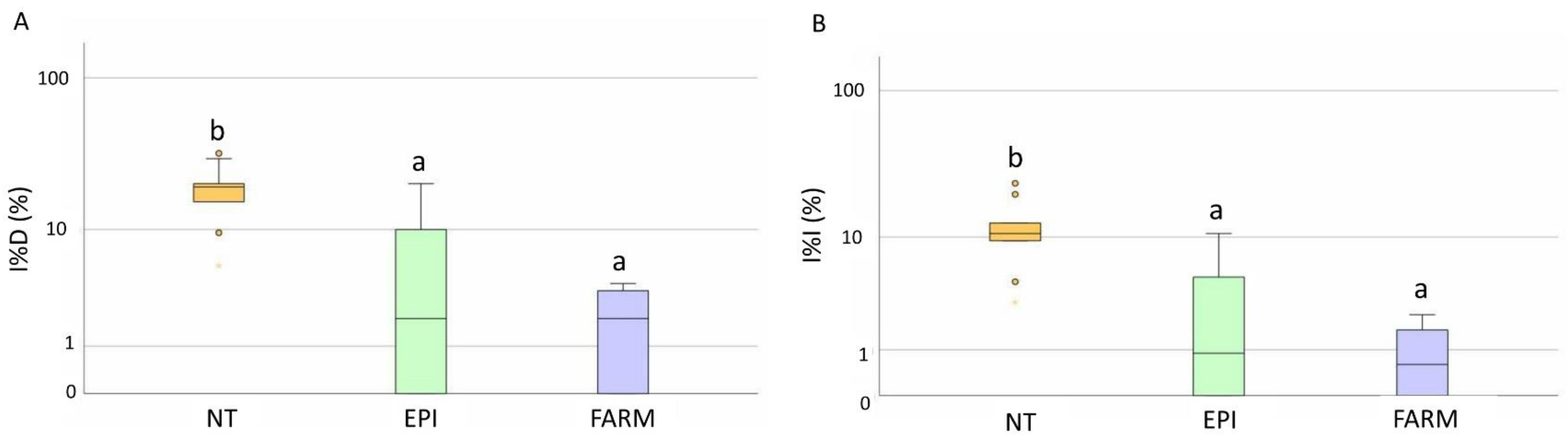
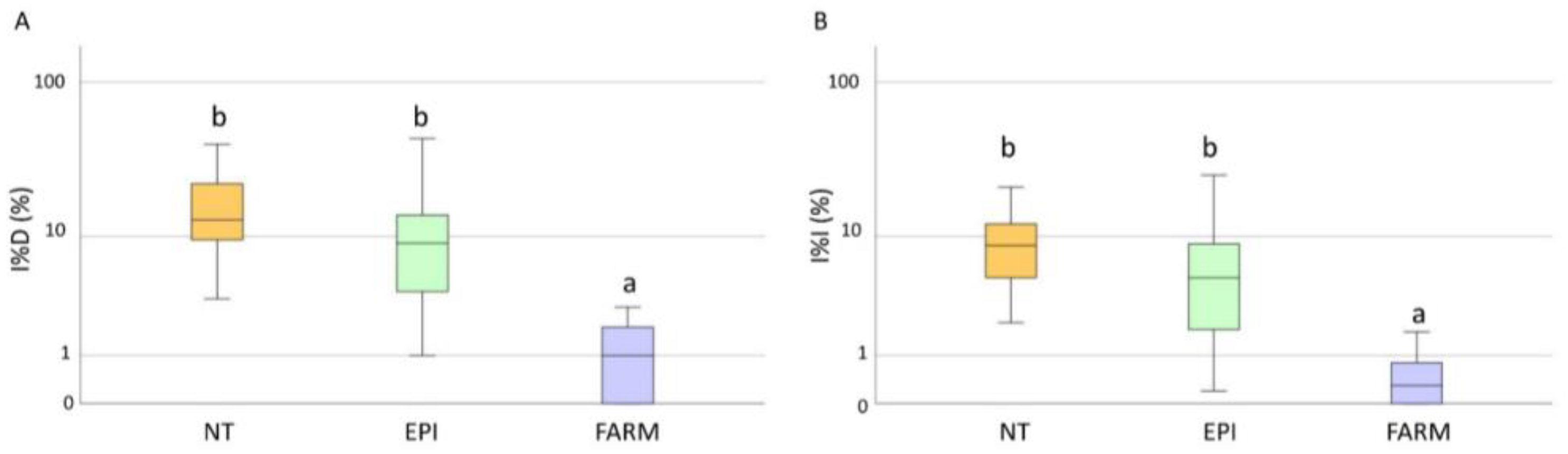
2.3. Amounts and Costs of Fungicides
3. Discussion
4. Materials and Methods
4.1. Vineyards and Meteorological Data Collection
4.2. EPI Model Simulation
4.3. Field Trials
4.4. Disease Assessment
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Storchi, P.; Perria, R.; Carella, G.; Mugnai, L.; Landi, S.; Binazzi, F.; Mocali, S.; Fabiani, A.; Cucu, M.A.; Valentini, P.; et al. Soil management and plant protection strategies with reduced use of copper: Productive and environmental aspects in a Sangiovese vineyard. BIO Web Conf. 2022, 44, 03002. [Google Scholar] [CrossRef]
- Fermaud, M.; Smits, N.; Merot, A.; Roudet, J.; Thiéry, D.; Wery, J.; Delbac, L. New multipest damage indicator to assess protection strategies in grapevine cropping systems. Aust. J. Grape Wine Res. 2016, 22, 450–461. [Google Scholar] [CrossRef]
- Sambucci, O.; Alston, J.M.; Fuller, K.B.; Lusk, J. The Pecuniary and Nonpecuniary Costs of Powdery Mildew and the Potential Value of Resistant Grape Varieties in California. Am. J. Enol. Vitic. 2019, 70, 177–187. [Google Scholar] [CrossRef]
- Buonassisi, D.; Colombo, M.; Migliaro, D.; Dolzani, C.; Peressotti, E.; Mizzotti, C.; Velasco, R.; Masiero, S.; Perazzolli, M.; Vezzulli, S. Breeding for grapevine downy mildew resistance: A review of omics approaches. Euphytica 2017, 213, 103. [Google Scholar] [CrossRef]
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine Merlot (Vitis vinifera). Vitis 2010, 49, 77–85. [Google Scholar]
- Calonnec, A.; Cartolaro, P.; Poupot, C.; Dubourdieu, D.; Darriet, P. Effects of Uncinula necator on the yield and quality of grapes (Vitis vinifera) and wine. Plant Pathol. 2004, 53, 434–445. [Google Scholar] [CrossRef]
- Ough, C.S.; Berg, H.W. Research note powdery mildew sensory effect on wine. Am. J. Enol. Vitic. 1979, 30, 321. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Pearson, R.C. Initiation, Development, Dispersal, and Survival of Cleistothecia of Uncinula necator in New York Vineyards. Phytopathology 1988, 78, 1413–1421. [Google Scholar] [CrossRef]
- Vercesi, A.; Tornaghi, R.; Sant, S.; Burruano, S.; Faoro, F. A cytological and ultrastructural study on the maturation and germination of oospores of Plasmopara viticola from overwintering vine leaves. Mycol. Res. 1999, 103, 193–202. [Google Scholar] [CrossRef]
- Maddalena, G.; Russo, G.; Toffolatti, S.L. The Study of the Germination Dynamics of Plasmopara viticola Oospores Highlights the Presence of Phenotypic Synchrony with the Host. Front. Microbiol. 2021, 12, 698586. [Google Scholar] [CrossRef] [PubMed]
- Csikós, A.; Németh, M.Z.; Frenkel, O.; Kiss, L.; Zoltán Váczy, K. A Fresh Look at Grape Powdery Mildew (Erysiphe necator) A and B Genotypes Revealed Frequent Mixed Infections and Only B Genotypes in Flag Shoot Samples. Plants 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, P.; Pizzatti, C.; Bertocchi, D.; Milgroom, M.G. Persistence and spatial autocorrelation of clones of Erysiphe necator overwintering as mycelium in dormant buds in an isolated vineyard in northern Italy. Phytopathology 2008, 98, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Volpi, I.; Guidotti, D.; Mammini, M.; Marchi, S. Predicting Symptoms of Downy Mildew, Powdery Mildew, and Gray Mold Diseases of Grapevine Through Machine Learning. Ital. J. Agrometeorol. 2021, 2, 57–69. [Google Scholar] [CrossRef]
- Chen, M.; Ois Brun, F.; Raynal, M.; Makowski, D. Forecasting severe grape downy mildew attacks using machine learning. PLoS ONE 2020, 15, e0230254. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Newlands, N.K.; Carisse, O.; Atkinson, D.E.; Cannon, A.J. Disease risk forecasting with Bayesian learning networks: Application to grape powdery mildew (Erysiphe necator) in vineyards. Agronomy 2020, 10, 622. [Google Scholar] [CrossRef]
- Molitor, D.; Baus, O.; Hoffmann, L.; Beyer, M. Meteorological conditions determine the thermal-temporal position of the annual Botrytis bunch rot epidemic on Vitis vinifera L. cv. Riesling grapes. OENO One 2016, 50, 231–244. [Google Scholar] [CrossRef] [Green Version]
- International Organization of Vine and Wine Intergovernmental Organization. 2019 Statistical Report on World Vitiviniculture; International Organization of Vine and Wine Intergovernmental Organization: Paris, France, 2019; p. 23. [Google Scholar]
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; Vázquez González, Y.; Ragazzo-Sánchez, J.A. Application of essential oils and polyphenols as natural antimicrobial agents in postharvest treatments: Advances and challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural Organic Compounds for Application in Organic Farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising Natural Products in Crop Protection and Food Preservation: Basis, Advances, and Future Prospects. Int. J. Agron. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Cha, B.; Kim, J.C. Recent Trends in Studies on Botanical Fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanazzi, G.; Mancini, V.; Foglia, R.; Marcolini, D.; Kavari, M.; Piancatelli, S. Use of Chitosan and Other Natural Compounds Alone or in Different Strategies with Copper Hydroxide for Control of Grapevine Downy Mildew. Plant Dis. 2021, 105, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Prodorutti, D.; Frizzi, A.; Gessler, C.; Pertot, I. Development and evaluation of a warning model for the optimal use of copper in organic viticulture. J. Plant Pathol. 2010, 92, 43–55. [Google Scholar]
- Rusjan, D.; Strlič, M.; Pucko, D.; Korošec-Koruza, Z. Copper accumulation regarding the soil characteristics in Sub-Mediterranean vineyards of Slovenia. Geoderma 2007, 141, 111–118. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, P.; Soto-Gómez, D.; De La Calle, I.; López-Periago, J.E.; Paradelo, M. Rainfall-induced removal of copper-based spray residues from vines. Ecotoxicol. Environ. Saf. 2016, 132, 304–310. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace elements in terrestrial environments: Biogeochemistry, bioavailability and risks of metal. In Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metal; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2001; Volume 860, pp. 223–232. ISBN 9781468495058. [Google Scholar]
- Dent, D.R. Integrated Pest Management; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Gubler, W.D.; Ypema, H.L.; Ouimette, D.G.; Bettiga, L. Occurrence of resistance in Uncinula necator to triadimefon, myclobutanil and fenarimol in California grapevine. Plant Dis. 1996, 80, 902–909. [Google Scholar] [CrossRef]
- Calvert, D.J.; Huffaker, C.B. Predator [Metaseiulus occidentalis]—Prey [Pronematus spp.] interactions under sulfur and cattail pollen applications in a noncommercial vineyard. Entomophaga 1974, 19, 361–369. [Google Scholar] [CrossRef]
- Rutto, L.K.; Mersha, Z.; Nita, M. Evaluation of cultivars and spray programs for organic grape production in Virginia. Horttechnology 2021, 31, 166–173. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2016, 97, 70–84. [Google Scholar] [CrossRef]
- Caffi, T.; Rossi, V.; Legler, S.E.; Bugiani, R. A mechanistic model simulating ascosporic infections by Erysiphe necator, the powdery mildew fungus of grapevine. Plant Pathol. 2011, 60, 522–531. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Caffi, T.; Ciliberti, N.; Rossi, V. A mechanistic model of botrytis cinerea on grapevines that includes weather, vine growth stage, and the main infection pathways. PLoS ONE 2015, 10, e0140444. [Google Scholar] [CrossRef] [PubMed]
- Legler, S.E.; Caffi, T.; Rossi, V. A nonlinear model for temperature-dependent development of Erysiphe necator chasmothecia on grapevine leaves. Plant Pathol. 2012, 61, 96–105. [Google Scholar] [CrossRef]
- Rossi, V.; Caffi, T.; Giosuè, S.; Bugiani, R. A mechanistic model simulating primary infections of downy mildew in grapevine. Ecol. Modell. 2008, 212, 480–491. [Google Scholar] [CrossRef]
- Raynal, M.; Debord, C.; Guittard, S.; Vergnes, M. EPIcure, a geographic information decision support system applied on downy and powdery risks of mildews epidemics on the Bordeaux vineyard. In Proceedings of the 6th International Workshop on Grapevine Downy and Powdery Mildew, Bordeaux, France, 4–9 July 2010; pp. 144–146. [Google Scholar]
- Strizyk, S. Modèle d’Etat Potentiel d’Infection. Application à Plasmopara viticola. ACTA 1983, 1–46. [Google Scholar]
- Fremiot, P.; Parisi, N.; Pinzetta, M.; Tonni, M.; Salvetti, M.; Strizyk, S.; Vercesi, A. Andamento delle epidemie di Plasmopara viticola e valutazione del modello EPI nei vigneti lombardi. In Giornate Fitopatologiche; CLUEB: Bologna, Italy, 2008; Volume 2, pp. 153–160. [Google Scholar]
- Tran Manh Sung, C.; Strizyk, S.; Clerjeau, M. Simulation of the date of maturity of Plasmopara viticola oospores to predict the severity of primary infections in grapevine. Plant Dis. 1990, 74, 120–124. [Google Scholar] [CrossRef]
- Delp, C.J. Effect of temperature and humidity on the Grape powdery mildew fungus. Phytopathology 1954, 44, 615–626. [Google Scholar]
- Merot, A.; Fermaud, M.; Gosme, M.; Smits, N. Effect of Conversion to Organic Farming on Pest and Disease Control in French Vineyards. Agronomy 2020, 10, 1047. [Google Scholar] [CrossRef]
- Madden, L.V.; Ellis, M.A.; Wilson, L.L. Evaluation of a Disease Warning System for Downy Mildew of Grapes. Plant Dis. 2000, 84, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Caffi, T.; Legler, S.E.; Rossi, V.; Bugiani, R. Evaluation of a warning system for early-season control of grapevine powdery mildew. Plant Dis. 2012, 96, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Imperatore, G.; Ghirardelli, A.; Strinna, L.; Baldoin, C.; Pozzebon, A.; Zanin, G.; Otto, S. Evaluation of a fixed spraying system for phytosanitary treatments in heroic viticulture in north-eastern italy. Agriculture 2021, 11, 833. [Google Scholar] [CrossRef]
- Caffi, T.; Legler, S.E.; Bugiani, R.; Rossi, V. Combining sanitation and disease modelling for control of grapevine powdery mildew. Eur. J. Plant Pathol. 2013, 135, 817–829. [Google Scholar] [CrossRef]
- Menesatti, P.; Antonucci, F.; Costa, C.; Mandalà, C.; Battaglia, V.; Torre, A. Multivariate forecasting model to optimize management of grape downy mildew control. Vitis J. Grapevine Res. 2013, 52, 141–148. [Google Scholar] [CrossRef]
- Massetti, L.; Dalla Marta, A.; Orlandini, S. Preliminary economic evaluation of an agrometeorological system for Plasmopara viticola infections management. Acta Hortic. 2008, 803, 43–50. [Google Scholar] [CrossRef]
- Valdés-Gómez, H.; Araya-Alman, M.; Pañitrur-De la Fuente, C.; Verdugo-Vásquez, N.; Lolas, M.; Acevedo-Opazo, C.; Gary, C.; Calonnec, A. Evaluation of a decision support strategy for the control of powdery mildew, Erysiphe necator (Schw.) Burr., in grapevine in the central region of Chile. Pest Manag. Sci. 2017, 73, 1813–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, E.A.C.; Barbetti, R. Environmental and visual impact analysis of viticulture and olive tree cultivation in the province of Siena (Italy). Eur. J. Agron. 2008, 28, 412–426. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Rattalino Edreira, J.I.; Conley, S.P.; Grassini, P. From grid to field: Assessing quality of gridded weather data for agricultural applications. Eur. J. Agron. 2017, 82, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Toffolatti, S.L.; Russo, G.; Campia, P.; Bianco, P.A.; Borsa, P.; Coatti, M.; Torriani, S.F.F.; Sierotzki, H. A time-course investigation of resistance to the carboxylic acid amide mandipropamid in field populations of Plasmopara viticola treated with anti-resistance strategies. Pest Manag. Sci. 2018, 74, 2822–2834. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experi ments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Goidanich, G.; Casarini, B.; Foschi, S. Lotta antiparassitaria e calendario dei trattamenti in viticoltura. G. di Agric. 1957, 13, 11–14. [Google Scholar]





| Week | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14/05 | 25/05 | 01/06 | 08/06 | 15/06 | 22/06 | 29/06 | 07/07 | 13/07 | 20/07 | Total | |||||||||||||
| E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | ||
| Vineyards | P1 | X | X | X | X | X | X | X | X | X | X | X | X | X | 5 | 8 | |||||||
| P2 | X | X | X | X | X | X | X | X | X | X | X | X | 5 | 7 | |||||||||
| P3 | X | X | X | X | X | X | X | X | X | X | X | X | X | 4 | 9 | ||||||||
| P4 | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 7 | ||||||||
| P5 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 8 | 9 | ||||
| P6 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 8 | 7 | ||||||
| P7 | X | X | X | X | X | X | X | X | X | X | X | 5 | 6 | ||||||||||
| P8 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 7 | 8 | ||||||
| P9 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 8 | 8 | |||||
| Average | |||||||||||||||||||||||
| 6a | 8b | ||||||||||||||||||||||
| Week | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14/05 | 25/05 | 01/06 | 08/06 | 15/06 | 22/06 | 29/06 | 07/07 | 13/07 | 20/07 | Total | |||||||||||||
| E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | ||
| Vineyards | P1 | X | X | X | X | X | X | X | X | X | X | X | 3 | 8 | |||||||||
| P2 | X | X | X | X | X | X | X | X | X | 3 | 6 | ||||||||||||
| P3 | X | X | X | X | X | X | X | X | X | X | X | X | 3 | 9 | |||||||||
| P4 | X | X | X | X | X | X | X | X | 2 | 6 | |||||||||||||
| P5 | X | X | X | X | X | X | X | X | X | X | X | X | 3 | 9 | |||||||||
| P6 | X | X | X | X | X | X | X | X | X | X | 3 | 7 | |||||||||||
| P7 | X | X | X | X | X | X | X | X | X | 3 | 6 | ||||||||||||
| P8 | X | X | X | X | X | X | X | X | X | X | 3 | 7 | |||||||||||
| P9 | X | X | X | X | X | X | X | X | X | X | X | X | 4 | 8 | |||||||||
| Average | |||||||||||||||||||||||
| 3a | 7b | ||||||||||||||||||||||
| Week | |||||||||||||||||||||||||
| 27/04 | 04/05 | 10/05 | 18/05 | 24/05 | 01/06 | 08/06 | 15/06 | 22/06 | 29/06 | 06/07 | Total | ||||||||||||||
| E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | ||
| Vineyards | P1 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 8 | 6 | ||||||||
| P2 | X | X | X | X | X | X | X | X | X | X | X | X | X | 8 | 5 | ||||||||||
| P3 | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 7 | ||||||||||
| P4 | X | X | X | X | X | X | X | X | X | X | X | X | 5 | 7 | |||||||||||
| P5 | X | X | X | X | X | X | X | X | X | X | 5 | 5 | |||||||||||||
| P6 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 7 | 7 | |||||||||
| P7 | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 7 | ||||||||||
| P8 | X | X | X | X | X | X | X | X | X | X | X | 4 | 7 | ||||||||||||
| P9 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 8 | |||||||||
| Average | |||||||||||||||||||||||||
| 6a | 7a | ||||||||||||||||||||||||
| Week | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27/04 | 10/05 | 18/05 | 24/05 | 01/06 | 08/06 | 15/06 | 22/06 | 29/06 | 06/07 | Total | |||||||||||||
| E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | E | F | ||
| Vineyards | P1 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 8 | ||||||
| P2 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 5 | 9 | |||||||
| P3 | X | X | X | X | X | X | X | X | X | X | X | X | X | 7 | 6 | ||||||||
| P4 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 8 | 8 | |||||
| P5 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 7 | 9 | |||||
| P6 | X | X | X | X | X | X | X | X | X | X | X | X | 5 | 7 | |||||||||
| P7 | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 6 | |||||||||
| P8 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 8 | |||||||
| P9 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 6 | 8 | |||||||
| Average | |||||||||||||||||||||||
| 6a | 8b | ||||||||||||||||||||||
| Disease | Parameter | Plot | |
|---|---|---|---|
| EPI | Farm | ||
| DM | Copper (Kg/ha) | 2.3 ± 0.5 a | 2.4 ± 0.5 a |
| Costs (EUR/ha) | 368.3 ± 81.4 a | 388.2 ± 74.9 a | |
| PM | Sulphur (Kg/ha) | 19.3 ± 3.3 a | 24.5 ± 3.5 b |
| Costs (EUR/ha) | 454.3 ± 66 a | 558.3 ± 81.1 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddalena, G.; Marone Fassolo, E.; Bianco, P.A.; Toffolatti, S.L. Disease Forecasting for the Rational Management of Grapevine Mildews in the Chianti Bio-District (Tuscany). Plants 2023, 12, 285. https://doi.org/10.3390/plants12020285
Maddalena G, Marone Fassolo E, Bianco PA, Toffolatti SL. Disease Forecasting for the Rational Management of Grapevine Mildews in the Chianti Bio-District (Tuscany). Plants. 2023; 12(2):285. https://doi.org/10.3390/plants12020285
Chicago/Turabian StyleMaddalena, Giuliana, Elena Marone Fassolo, Piero Attilio Bianco, and Silvia Laura Toffolatti. 2023. "Disease Forecasting for the Rational Management of Grapevine Mildews in the Chianti Bio-District (Tuscany)" Plants 12, no. 2: 285. https://doi.org/10.3390/plants12020285
APA StyleMaddalena, G., Marone Fassolo, E., Bianco, P. A., & Toffolatti, S. L. (2023). Disease Forecasting for the Rational Management of Grapevine Mildews in the Chianti Bio-District (Tuscany). Plants, 12(2), 285. https://doi.org/10.3390/plants12020285










