Canopy Density, but Not Bacterial Titers, Predicts Fruit Yield in Huanglongbing-Affected Sweet Orange Trees
Abstract
:1. Introduction
2. Results
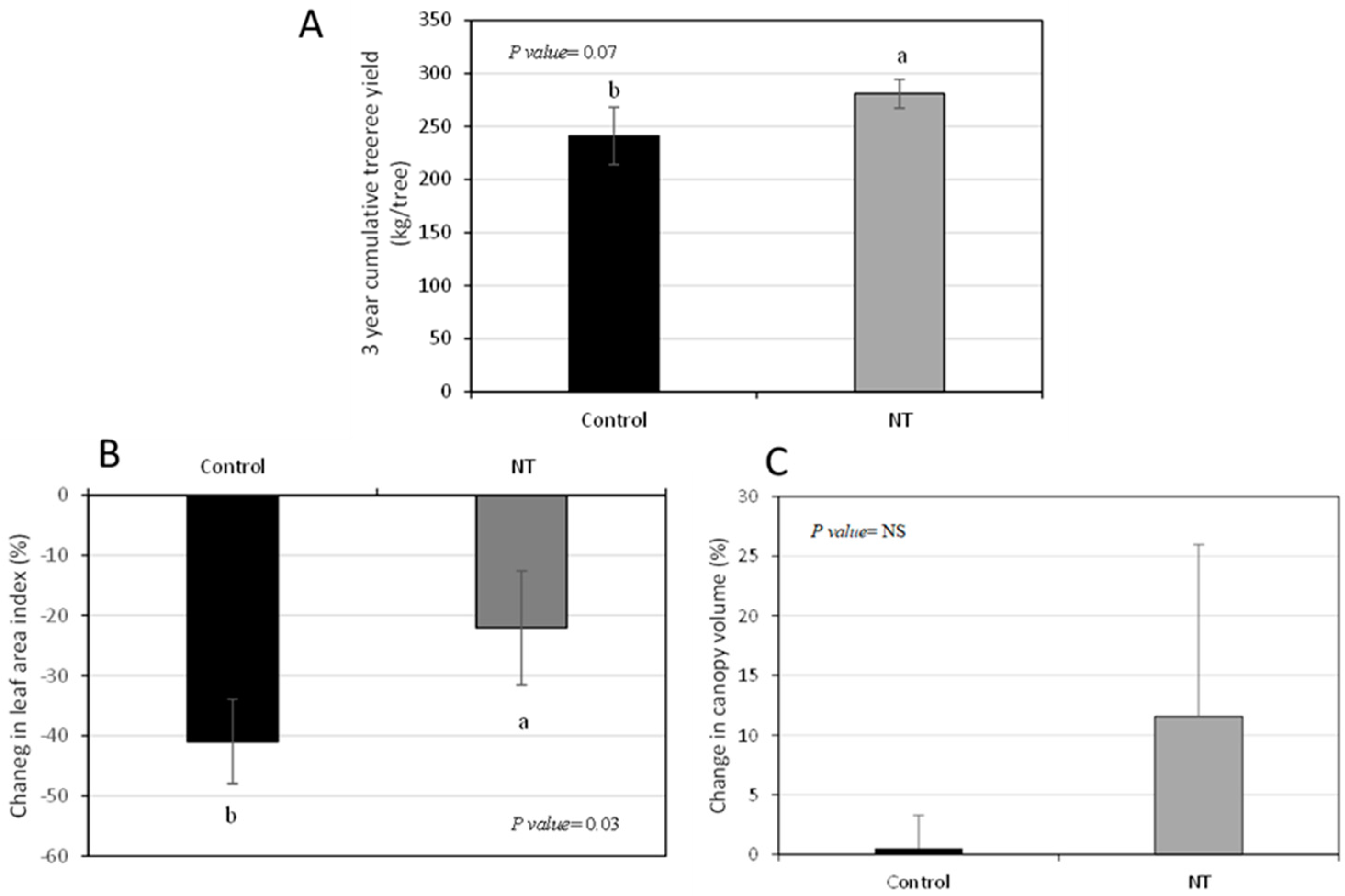
2.1. Canopy Density, but Not Canopy Volume or qPCR Values, Are Related to Yield Increase
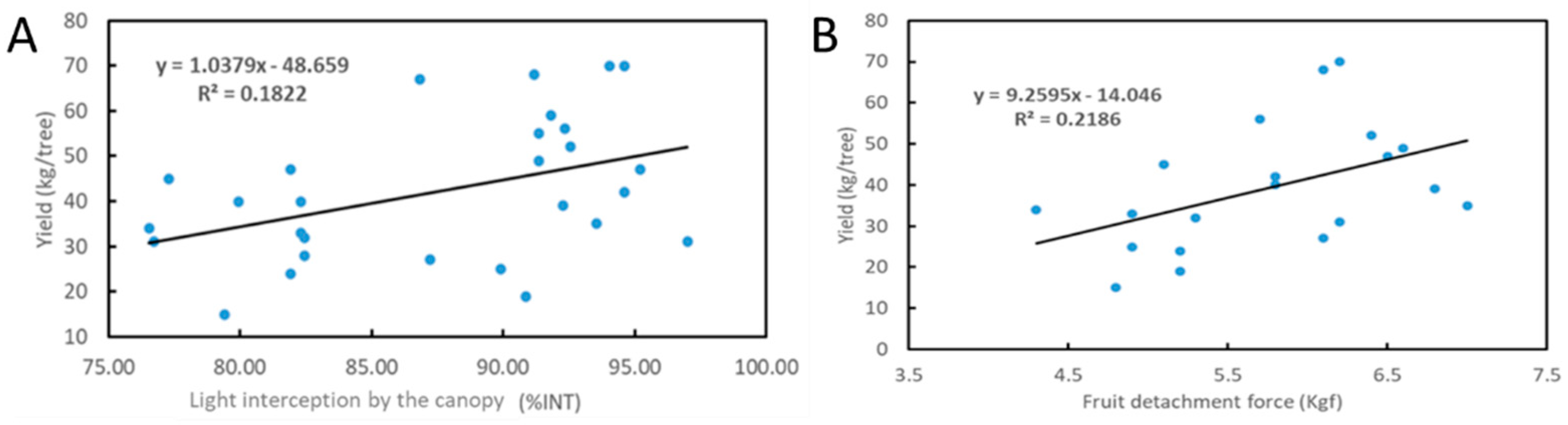
2.2. Evaluation of Trees According to Light Interception (%INT)
2.3. qPCR May Not Always Accurately Detect Live CLas Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Genomic DNA Extraction and Ca. L. asiaticus Titers
4.3. Disease Index Rating
4.4. Canopy Measurements
4.5. Root Density
4.6. Leaf Nutrient Analysis
4.7. SPAD Index
4.8. Yield, Fruit Size and Fruit Detachment Force (FDF)
4.9. HLB Inoculation for TEM
4.10. Transmission Electron Microscopy (TEM)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bove, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Achor, D.; Etxeberria, E.; Wang, N.; Folimonova, S.; Chung, K.; Albrigo, L. Sequence of Anatomical Symptom Observations in Citrus Affected with Huanglongbing Disease. Plant Pathol. J. 2010, 9, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Sagaram, U.S.; Burns, J.K.; Li, J.-L.; Wang, N. Response of Sweet Orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ Infection: Microscopy and Microarray Analyses. Phytopathology 2009, 99, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Vashisth, T.; Grosser, J. Comparison of Controlled Release Fertilizer (CRF) for Newly Planted Sweet Orange Trees under Huanglongbing Prevalent Conditions. J. Hortic. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for Managing Candidatus Liberibacter spp. (Huanglongbing Disease Pathogen): Current Control Measures and Future Directions. Phytopathology 2018, 108, 424–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashisth, T.; Livingston, T. Efficacy of In-field Thermotherapy in Comparison and Combination of Defoliation for Mitigating Huanglongbing in Sweet Orange. HortScience 2020, 55, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.-C.; Xia, Y.-L.; Lin, X.-J.; Hu, H.-Q.; Wang, X.-D.; Ruan, C.-Q.; Lu, L.-M.; Liu, B. Evaluation of thermotherapy against Huanglongbing (citrus greening) in the greenhouse. J. Integr. Agric. 2016, 15, 111–119. [Google Scholar] [CrossRef]
- Zambon, F.T.; Kadyampakeni, D.M.; Grosser, J.W. Ground Application of Overdoses of Manganese Have a Therapeutic Effect on Sweet Orange Trees Infected with Candidatus Liberibacter asiaticus. HortScience 2019, 54, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Duan, Y.; Zhou, L.; Turechek, W.W.; Stover, E.; Powell, C.A. Screening Molecules for Control of Citrus Huanglongbing Using an Optimized Regeneration System for ‘Candidatus Liberibacter asiaticus’-Infected Periwinkle (Catharanthus roseus) Cuttings. Phytopathology 2010, 100, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.A.; Jones, D.; Kolawole, O.O.; Goss, E.M.; van Bruggen, A.H.C. Effects and side effects of penicillin injection in huanglongbing affected grapefruit trees. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Zhang, M.; Powell, C.A.; Guo, Y.; Benyon, L.; Duan, Y. Characterization of the microbial community structure in Candidatus Liberibacter asiaticus-infected citrus plants treated with antibiotics in the field. BMC Microbiol. 2013, 13, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Powell, C.A.; Zhou, L.; He, Z.; Stover, E.; Duan, Y. Chemical Compounds Effective Against the Citrus Huanglongbing Bacterium ‘Candidatus Liberibacter asiaticus’ In Planta. Phytopathology 2011, 101, 1097–1103. [Google Scholar] [CrossRef] [Green Version]
- Valdés, R.A.; Ortiz, J.C.D.; Beache, M.B.; Cabello, J.A.; Chávez, E.C.; Pagaza, Y.R.; Fuentes, Y.M.O. A review of techniques for detecting Huanglongbing (greening) in citrus. Can. J. Microbiol. 2016, 62, 803–811. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Montesino, L.H.; Gasparoto, M.C.G.; Filho, A.B.; Amorim, L. Yield loss caused by huanglongbing in different sweet orange cultivars in São Paulo, Brazil. Eur. J. Plant Pathol. 2011, 130, 577–586. [Google Scholar] [CrossRef]
- Vashisth, T.; Livingston, T. Assessment of Pruning and Controlled-release Fertilizer to Rejuvenate Huanglongbing-affected Sweet Orange. HortTechnology 2019, 29, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Spyke, P.; Sherrod, J.; Grosse, R. Controlled-Release Fertilizer Boosts Health of HLB Trees; Citrus Ind.: Newberry, FL, USA, 2017. [Google Scholar]
- Vasconcelos, F.N.C.; Li, J.; Pang, Z.; Vincent, C.; Wang, N. The Total Population Size of ‘Candidatus Liberibacter asiaticus’ Inside the Phloem of Citrus Trees and the Corresponding Metabolic Burden Related to Huanglongbing Disease Development. Phytopathology 2021, 111, 1122–1128. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the Responses of Different Genotypes of Citrus to Huanglongbing (Citrus Greening) Under Different Conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Etxeberria, E.; Gonzalez, P.; Vincent, C.; Schumann, A. Extended persistence of Candidatus Liberibacter asiaticus (CLas) DNA in Huanglongbing-affected citrus tissue after bacterial death. Physiol. Mol. Plant Pathol. 2019, 106, 204–207. [Google Scholar] [CrossRef]
- Achor, D.; Welker, S.; Ben-Mahmoud, S.; Wang, C.; Folimonova, S.Y.; Dutt, M.; Gowda, S.; Levy, A. Dynamics of Candidatus Liberibacter asiaticus Movement and Sieve-Pore Plugging in Citrus Sink Cells. Plant Physiol. 2019, 182, 882–891. [Google Scholar] [CrossRef]
- Tang, L.; Chhajed, S.; Vashisth, T. Preharvest Fruit Drop in Huanglongbing-affected ‘Valencia’ Sweet Orange. J. Am. Soc. Hortic. Sci. 2019, 144, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Mccollum, G.; Baldwin, E.; Bai, J. Impacts of Huanglongbing Symptom Severity on Fruit Detachment Force and Mechanical Properties of Sweet Oranges (Citrus sinensis). HortScience 2016, 51, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Vashisth, T. New insight in Huanglongbing-associated mature fruit drop in citrus and its link to oxidative stress. Sci. Hortic. 2020, 265, 109246. [Google Scholar] [CrossRef]
- Deng, H.; Achor, D.; Exteberria, E.; Yu, Q.; Du, D.; Stanton, D.; Liang, G.; Gmitter, F.G., Jr. Phloem Regeneration Is a Mechanism for Huanglongbing-Tolerance of “Bearss” Lemon and “LB8-9” Sugar Belle® Mandarin. Front. Plant Sci. 2019, 10, 277. [Google Scholar] [CrossRef]
- Clark, K.J.; Pang, Z.; Trinh, J.; Wang, N.; Ma, W. Sec-Delivered Effector 1 (SDE1) of ‘Candidatus Liberibacter asiaticus’ Promotes Citrus Huanglongbing. Mol. Plant-Microbe Interact. 2020, 33, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.D.B.; Moreira, A.S.; Stuchi, E.S.; Bassanezi, R.B.; Laranjeira, F.F.; Girardi, E.A. Huanglongbing incidence, canopy volume, and sprouting dynamics of ‘Valencia’ sweet orange grafted onto 16 rootstocks. Trop. Plant Pathol. 2020, 45, 611–619. [Google Scholar] [CrossRef]
- Ghimire, L.; Kadyampakeni, D.; Vashisth, T. Effect of Irrigation Water pH on the Performance of Healthy and Huanglongbing-affected Citrus. J. Am. Soc. Hortic. Sci. 2020, 145, 318–327. [Google Scholar] [CrossRef]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar Applications of Essential Nutrients on Growth and Yield of ‘Valencia’ Sweet Orange Infected with Huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Morgan, K.T.; Graham, J.H. Nutrient Status and Root Density of Huanglongbing-Affected Trees: Consequences of Irrigation Water Bicarbonate and Soil pH Mitigation with Acidification. Agronomy 2019, 9, 746. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Twieg, E.; Hartung, J.S.; Levy, L. Optimized Quantification of Unculturable Candidatus Liberibacter Spp. in Host Plants Using Real-Time PCR. Plant Dis. 2008, 92, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Slinski, S. Evaluation of HLB Treatments; Citrus Ind.: Newberry, FL, USA, 2016. [Google Scholar]
- Barritt, B.H.; Rom, C.R.; Konishi, B.J.; Dilley, M.A. Light Level Influences Spur Quality and Canopy Development and Light Interception Influence Fruit Production in Apple. HortScience 1991, 26, 993–999. [Google Scholar] [CrossRef]
- Singh, S.; Livingston, T.; Tang, L.; Vashisth, T. Effects of Exogenous Gibberellic Acid in Huanglongbing-affected Sweet Orange Trees under Florida Conditions—II. Fruit Production and Tree Health. HortScience 2022, 57, 353–359. [Google Scholar] [CrossRef]
- Malladi, A.; Burns, J.K. CsPLDα1 and CsPLDγ1 are differentially induced during leaf and fruit abscission and diurnally regulated in Citrus sinensis. J. Exp. Bot. 2008, 59, 3729–3739. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Block | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Control | 1 | 26.40 | 35.46 | 25.41 | 25.13 |
| Control | 2 | 25.98 | 32.77 | 31.56 | 26.89 |
| Control | 3 | 29.84 | 30.06 | 27.66 | 29.01 |
| Enhanced Nutritional Treatment | 1 | 28.01 | 31.26 | 32.01 | 27.41 |
| Enhanced Nutritional Treatment | 2 | 26.49 | 31.70 | 32.63 | 28.46 |
| Enhanced Nutritional Treatment | 3 | 26.87 | 29.13 | 35.73 | 26.30 |
| p value | 0.85 | 0.2 | 0.13 | 0.83 |
| Tree Description | Visual Symptoms | Canopy m3 | DI | Root Den. | SPAD | %INT | Fruit Size | FDF | Yield kg/tree | Ct | CLas Cells/gram |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-year old Valencia trees | Severe | 6.63 ± 0.83 | 25.17 ± 3.04 | 0.68 ± 0.14 | 61.03 ± 1.35 | 85.28 ± 1.91 | 63.79 ± 0.8 | 5.25 ± 0.18 | 23.67 ± 2.4 | 30.03 ± 0.4 | 4.87 × 109 ± 1.17 × 109 |
| Mild | 7.09 ± 1.08 | 20.83 ± 1.37 | 0.85 ± 1.17 | 58.72 ± 0.77 | 94.18 ± 0.72 | 65.78 ± 0.6 | 6.45 ± 0.17 | 41.00 ± 3.14 | 29.27 ± 0.3 | 7.82 × 109 ± 1.81 × 109 | |
| p value | ns | ns | ns | ns | 0.0008 | 0.0229 | 0.015 | 0.0056 | ns | ns | |
| 18-year old Valencia trees | Severe | 22.97 ± 1.84 | 34.75 ± 2 | 0.56 ± 0.06 | 51.11 ± 4.08 | 80.83 ± 1.38 | 61.50 ± 0.85 | 36.50 ± 4.33 | 30.81 ± 0.18 | 2.50 × 109 ± 2.84 × 108 | |
| Mild | 24.64 ± 2.1 | 27.50 ± 0.86 | 0.64 ± 0.19 | 57.52 ± 2.5 | 91.14 ± 1.6 | 64.13 ± 1 | 62.75 ± 3.47 | 30.86 ± 0.08 | 2.37 × 109 ± 1.48 × 108 | ||
| p value | ns | 0.0662 | ns | ns | 0.0005 | 0.0484 | 0.0435 | ns | ns | ||
| 19-year old Hamlin trees | Severe | 20.04 ± 0.74 | 30.00 ± 4.5 | 0.40 ± 0.12 | 59.00 ± 1.9 | 79.01 ± 1.3 | 57.43 ± 0.93 | 5.59 ± 0.3 | 38.00 ± 2.8 | 29.72 ± 0.6 | 8.34 × 109 ± 2.32 × 109 |
| Mild | 18.37 ± 2.3 | 22.00 ± 1.3 | 0.70 ± 1.14 | 57.99 ± 1.76 | 92.23 ± 0.65 | 61.50 ± 0.44 | 6.15 ± 0.18 | 60.75 ± 4.98 | 30.20 ± 0.42 | 4.15 × 109 ± 1.29 × 109 | |
| p value | ns | ns | ns | ns | 0.0003 | 0.0402 | 0.075 | 0.0613 | ns | ns |
| Variety | Plant | Ct Value 1 | SE 2 | CLas Cells 3 |
|---|---|---|---|---|
| C. mac | 1 | 32.09 | 10 | 8 |
| C. mac | 2 | 31.2 | 25 | 0 |
| C. mac | 3 | 32.75 | 14 | 0 |
| Grapefruit | 1 | 34.9 | 45 | 0 |
| Grapefruit | 2 | 34.7 | 31 | 0 |
| Grapefruit | 3 | 28.5 | 31 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, A.; Livingston, T.; Wang, C.; Achor, D.; Vashisth, T. Canopy Density, but Not Bacterial Titers, Predicts Fruit Yield in Huanglongbing-Affected Sweet Orange Trees. Plants 2023, 12, 290. https://doi.org/10.3390/plants12020290
Levy A, Livingston T, Wang C, Achor D, Vashisth T. Canopy Density, but Not Bacterial Titers, Predicts Fruit Yield in Huanglongbing-Affected Sweet Orange Trees. Plants. 2023; 12(2):290. https://doi.org/10.3390/plants12020290
Chicago/Turabian StyleLevy, Amit, Taylor Livingston, Chunxia Wang, Diann Achor, and Tripti Vashisth. 2023. "Canopy Density, but Not Bacterial Titers, Predicts Fruit Yield in Huanglongbing-Affected Sweet Orange Trees" Plants 12, no. 2: 290. https://doi.org/10.3390/plants12020290





