Isoprene-Emitting Tobacco Plants Are Less Affected by Moderate Water Deficit under Future Climate Change Scenario and Show Adjustments of Stress-Related Proteins in Actual Climate
Abstract
:1. Introduction
2. Results
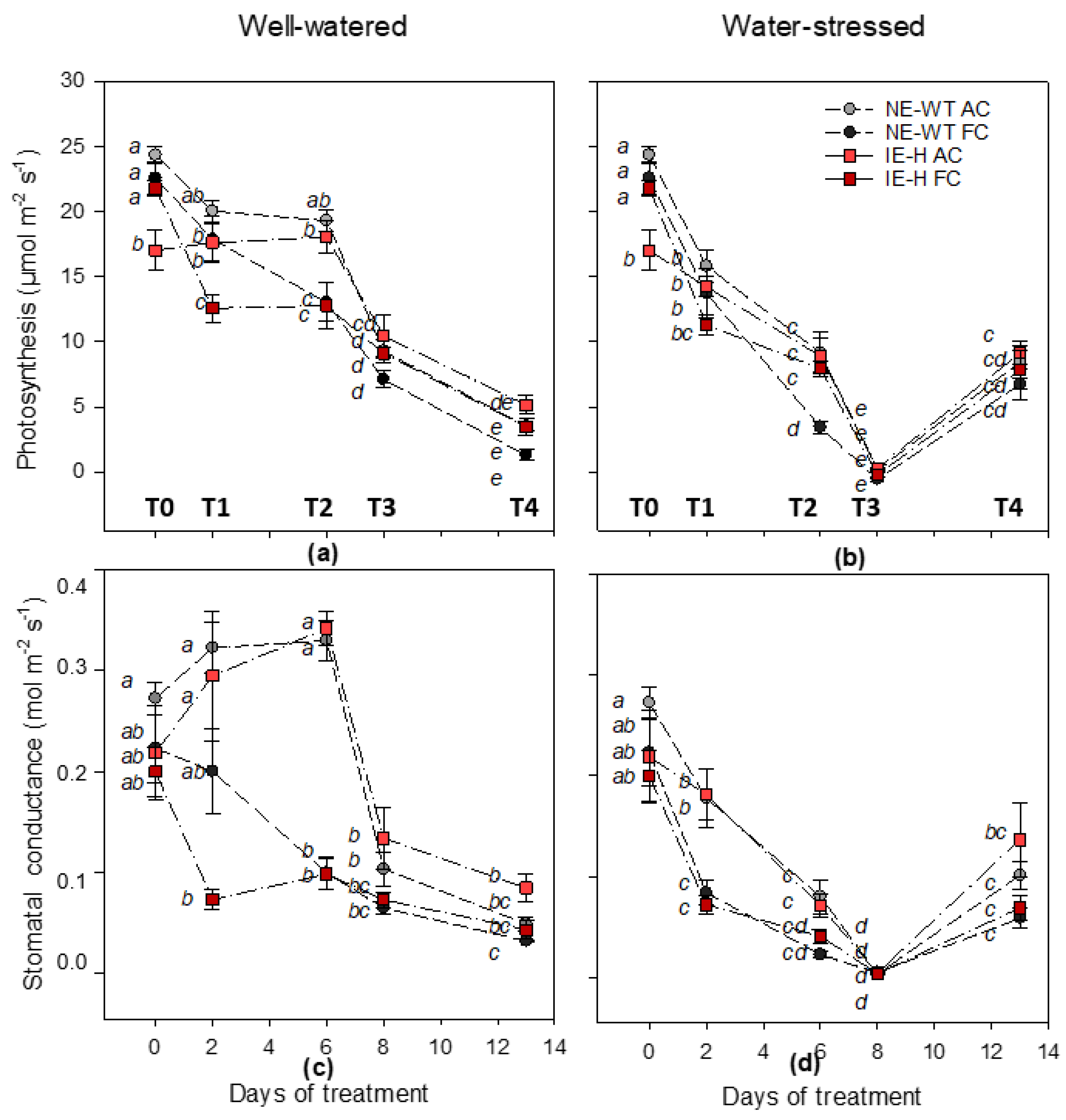
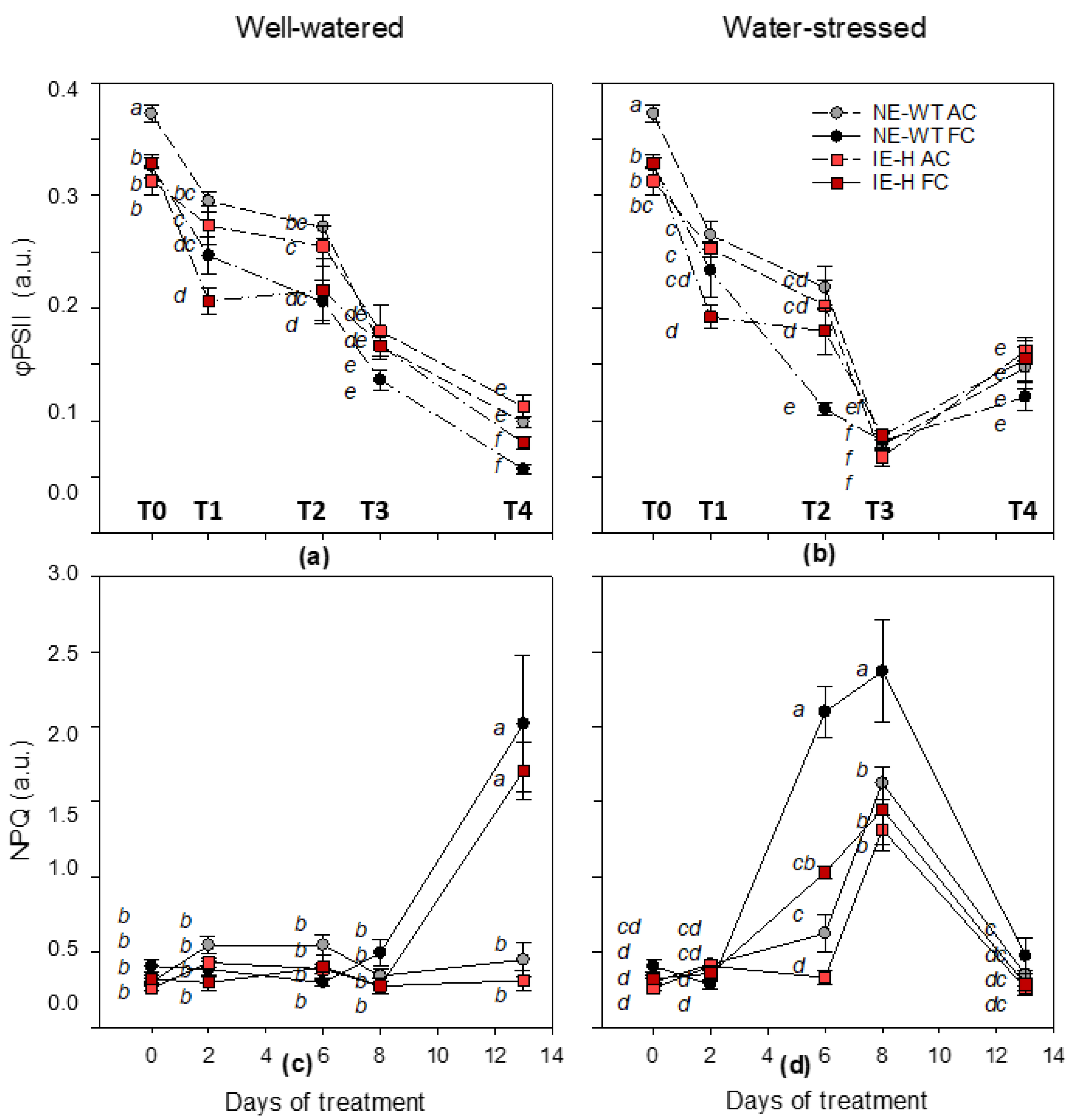
2.1. Physiological Parameters
2.2. Genes Expression
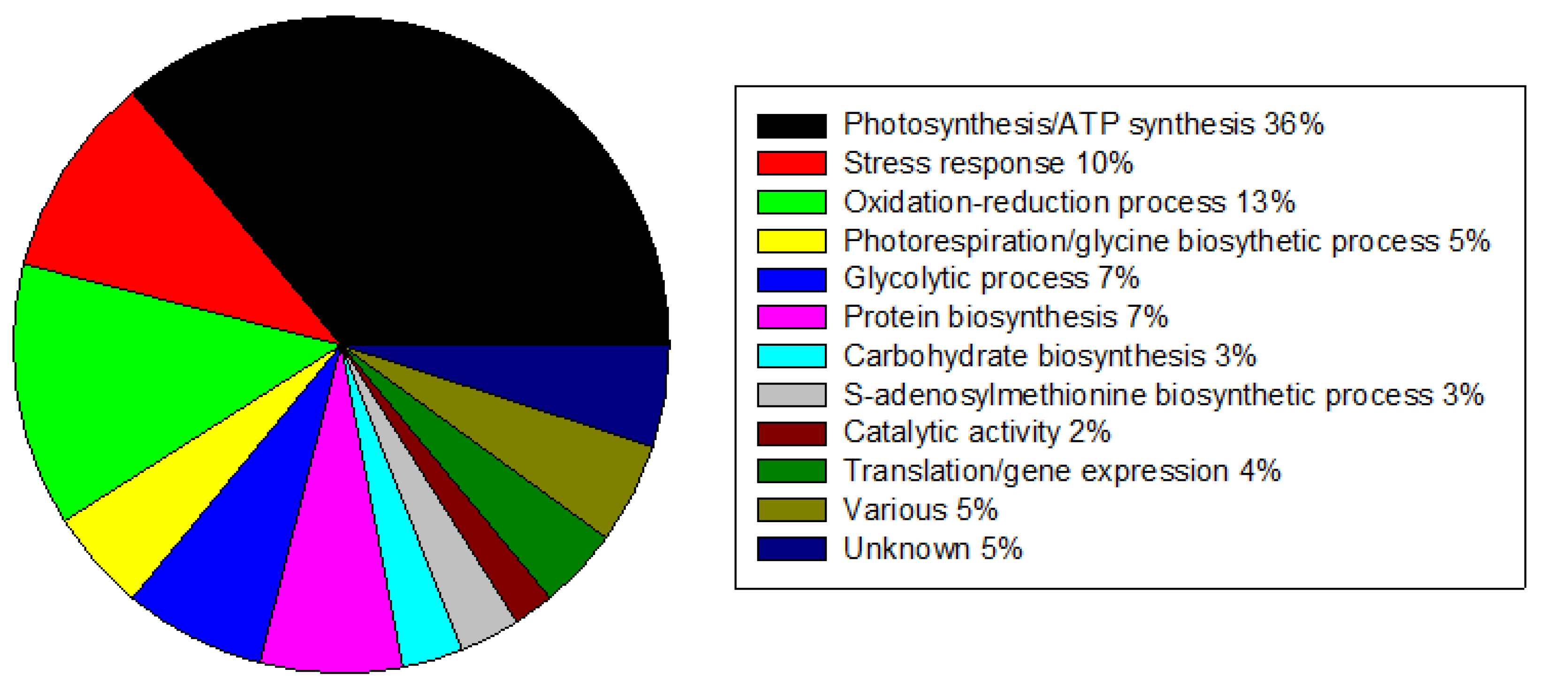
2.3. Proteins
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth and Sampling Conditions
4.1.1. First Experiment
4.1.2. Second Experiment
4.2. Gas Exchange Measurements and Chlorophyll Fluorescence
4.3. Relative Water Content, Fresh and Dry Weight Measurements
4.4. Gene Expression Analysis
4.5. Proteins Extraction and Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Singsaas, E.L.; Lerdau, M.; Winter, K.; Sharkey, T.D. Isoprene Increases Thermotolerance of Isoprene-Emitting Species. Plant Physiol. 1997, 115, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Várkonyi, Z.; Szabó, M.; Maslenkova, L.; Nogues, I.; Kovács, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 2011, 157, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Müller, C.; Ghirardo, A.; Rock, T.M.; Aichler, M.; Walch, A.; Schmitt-Kopplin, P.; Schnitzler, J.P. Knocking Down of Isoprene Emission Modifies the Lipid Matrix of Thylakoid Membranes and Influences the Chloroplast Ultrastructure in Poplar. Plant Physiol. 2015, 168, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Velikova, V.; Pinelli, P.; Pasqualini, S.; Reale, L.; Ferranti, F.; Loreto, F. Isoprene decreases the concentration of nitric oxide in leaves exposed to elevated ozone. New Phytol. 2005, 166, 419–426. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Behnke, K.; Kaiser, A.; Zimmer, I.; Brüggemann, N.; Janz, D.; Polle, A.; Hampp, R.; Hänsch, R.; Popko, J.; Schmitt-Kopplin, P.; et al. RNAi-mediated suppression of isoprene emission in poplar impacts phenolic metabolism: A transcriptomic and metabolomic analysis. Plant Mol. Biol. 2010, 74, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velikova, V.; Ghirardo, A.; Vanzo, E.; Merl, J.; Hauck, S.M.; Schnitzler, J.P. Genetic manipulation of isoprene emissions in poplar plants remodels the chloroplast proteome. J. Proteome Res. 2014, 13, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Behnke, K.; Ehlting, B.; Teuber, M.; Bauerfeind, M.; Louis, S.; HaЁnsch, R.; Polle, A.; Bohlmann, J.; Schnitzler, J.P. Transgenic, non-isoprene-emitting poplars don’t like it hot. Plant J. 2007, 51, 485–499. [Google Scholar] [CrossRef]
- Pollastri, S.; Tsonev, T.; Loreto, F. Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J. Exp. Bot. 2014, 65, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Pollastri, S.; Jorba, I.; Hawkins, T.J.; Llusià, J.; Michelozzi, M.; Navajas, D.; Peñuelas, J.; Hussey, P.J.; Knight, M.R.; Loreto, F. Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol. 2019, 223, 1307–1318. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, A.; Wright, L.P.; Bi, Z.; Rosenkranz, M.; Pulido, P.; Rodríguez-Concepción, M.; Niinemets, Ü.; Brüggemann, N.; Gershenzon, J.; Schnitzler, J.P. Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol. 2014, 165, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef]
- Beckett, M.; Loreto, F.; Velikova, V.; Brunetti, C.; Di Ferdinando, M.; Tattini, M.; Calfapietra, C.; Farrant, J.M. Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilis during dehydration and rehydration. Plant Cell Environ. 2012, 35, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, A.; Gutknecht, J.; Zimmer, I.; Brüggemann, N.; Schnitzler, J.P. Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: Online tracing of C translocation dynamics in poplar plants. PLoS ONE 2011, 6, e17393. [Google Scholar] [CrossRef] [Green Version]
- Trowbridge, A.M.; Asensio, D.; Eller, A.S.D.; Way, D.A.; Wilkinson, M.J.; Schnitzler, J.P.; Jackson, R.B.; Monson, R.K. Contribution of various carbon sources toward isoprene biosynthesis in poplar leaves mediated by altered atmospheric CO2 concentrations. PLoS ONE 2012, 7, e32387. [Google Scholar]
- Funk, J.L.; Mak, J.E.; Lerdau, M.T. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 2004, 27, 747–755. [Google Scholar] [CrossRef]
- Behnke, K.; Ghirardo, A.; Janz, D.; Kanawati, B.; Esperschütz, J.; Zimmer, I.; Schmitt-Kopplin, P.; Niinemets, Ü.; Polle, A.; Schnitzler, J.P.; et al. Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiol. 2013, 33, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Brüggemann, N.; Schnitzler, J.P. Relationship of isopentenyl diphosphate (IDP) isomerase activity to isoprene emission of oak leaves. Tree Physiol. 2002, 22, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Pegoraro, E.; Rey, A.; Greenberg, J.; Harley, P.; Grace, J.; Malhi, Y.; Guenther, A. Effect of drought on isoprene emission rates from leaves of Quercus virginiana Mill. Atmos. Environ. 2004, 38, 6149–6156. [Google Scholar] [CrossRef] [Green Version]
- Tattini, M.; Velikova, V.; Vickers, C.; Brunetti, C.; Di Ferdinando, M.; Trivellini, A.; Fineschi, S.; Agati, G.; Ferrini, F.; Loreto, F. Isoprene production in transgenic tobacco alters isoprenoid, non-structural carbohydrate and phenylpropanoid metabolism, and protects photosynthesis from drought stress. Plant Cell Environ. 2014, 37, 1950–1964. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.C.; Hewitt, C.N.; Possell, M.; Vickers, C.E.; Purnell, A.; Mullineaux, P.M.; Davies, W.J.; Dodd, I.C. Isoprene emission protects photosynthesis but reduces plant productivity during drought in transgenic tobacco (Nicotiana tabacum) plants. New Phytol. 2014, 201, 205–216. [Google Scholar] [CrossRef]
- Behnke, K.; Grote, R.; Brüggemann, N.; Zimmer, I.; Zhou, G.; Elobeid, M.; Janz, D.; Polle, A.; Schnitzler, J.P. Isoprene emission-free poplars—A chance to reduce the impact from poplar plantations on the atmosphere. New Phytol. 2012, 194, 70–82. [Google Scholar] [CrossRef]
- Vanzo, E.; Jud, W.; Li, Z.; Albert, A.; Domagalska, M.A.; Ghirardo, A.; Niederbacher, B.; Frenzel, J.; Beemster, G.T.S.; Asard, H.; et al. Facing the Future: Effects of Short-Term Climate Extremes on Isoprene-Emitting and Nonemitting Poplar. Plant Physiol. 2015, 169, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.M.; Sharkey, T.D. Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell Environ. 2016, 39, 1251–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene Acts as a Signaling Molecule in Gene Networks Important for Stress Responses and Plant Growth. Plant Physiol. 2019, 180, 124–152. [Google Scholar] [CrossRef] [Green Version]
- Monson, R.K.; Winkler, B.; Rosenstiel, T.N.; Block, K.; Merl-Pham, J.; Strauss, S.H.; Ault, K.; Maxfield, J.; Moore, D.J.P.; Trahan, N.A.; et al. High productivity in hybrid-poplar plantations without isoprene emission to the atmosphere. Proc. Natl. Acad. Sci. USA 2020, 117, 1596–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. In IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018; Available online: https://www.ipcc.ch/sr15/ (accessed on 20 June 2022).
- Way, D.; Ghirardo, A.; Kanawati, B.; Esperschütz, J.; Monson, R.K.; Jackson, R.B.; Schmitt-Kopplin, P.; Schnitzler, J.P. Increasing atmospheric CO2 reduces metabolic and physiological differences between isoprene- and non-isoprene-emitting poplars. New Phytol. 2013, 200, 534–546. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Tissue, D.T. To what extent can rising [CO2] ameliorate plant drought stress? New Phytol. 2021, 231, 2118–2124. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia plants during Mediterranean summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef]
- Centritto, M.; Nascetti, P.; Petrilli, L.; Raschi, A.; Loreto, F. Profiles of isoprene emission and photosynthetic parameters in hybrid poplars exposed to free-air CO2 enrichment. Plant Cell Environ. 2004, 27, 403–412. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Loreto, F.; Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Jamie, I.M.; Prentice, I.C.; Atwell, B.J. Evolution of isoprene emission capacity in plants. Trends Plant Sci. 2014, 19, 439–446. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Chen, X.; Yeh, S. Isoprene Increases Thermotolerance of Fosmidomycin-Fed Leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Pollastri, S.; Pinosio, S.; Reichelt, M.; Sharkey, T.D.; Schnitzler, J.P.; Loreto, F. Isoprene enhances leaf cytokinin metabolism and induces early senescence. New Phytol. 2022, 234, 961–974. [Google Scholar] [CrossRef]
- Vanzo, E.; Merl-Pham, J.; Velikova, V.; Ghirardo, A.; Lindermayr, C.; Hauck, S.M.; Bernhardt, J.; Riedel, K.; Durner, J.; Schnitzler, J.P. Modulation of Protein S-Nitrosylation by Isoprene Emission in Poplar. Plant Physiol. 2016, 170, 1945–1961. [Google Scholar] [CrossRef] [Green Version]
- Dani, K.G.S.; Loreto, F. Plant volatiles as regulators of hormone homeostasis. New Phytol. 2022, 234, 804–812. [Google Scholar] [CrossRef]
- Rott, M.; Martins, N.F.; Thiele, W.; Lein, W.; Bock, R.; Kramer, D.M.; Schöttler, M.A. ATP Synthase Repression in Tobacco Restricts Photosynthetic Electron Transport, CO2 Assimilation, and Plant Growth by Overacidification of the Thylakoid Lumen. Plant Cell 2011, 23, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone quenching properties of isoprene and its antioxidant role in plants. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Dreher, K.; Callis, J. Ubiquitin, Hormones and Biotic Stress in Plants. Ann. Bot. 2007, 99, 787–822. [Google Scholar] [CrossRef] [PubMed]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Sadanandom, A.; Bailey, M.; Ewan, R.; Lee, J.; Nelis, S. The ubiquitin-proteasome system: Central modifier of plant signalling. New Phytol. 2012, 196, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Behnke, K.; Kleist, E.; Uerlings, R.; Wildt, J.; Rennenberg, H.; Schnitzler, J.P. RNAi-mediated suppression of isoprene biosynthesis in hybrid poplar impacts ozone tolerance. Tree Physiol. 2009, 29, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Trainotti, L.; Li, M.; Varotto, C. Overexpression of isoprene synthase affects ABA-and drought-related gene expression and enhances tolerance to abiotic stress. Int. J. Mol. Sci. 2020, 21, 4276. [Google Scholar] [CrossRef]
- Pollastri, S.; Baccelli, I.; Loreto, F. Isoprene: An Antioxidant Itself or a Molecule with Multiple Regulatory Functions in Plants? Antioxidants 2021, 10, 684. [Google Scholar] [CrossRef]
- Kurepa, J.; Toh-E, A.; Smalle, J. 26S proteasome regulatory particle mutants have increased oxidative stress tolerance. Plant J. 2008, 53, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Y.S.; Xing, X.J.; Xu, Z.S.; Zhu, B.; Fu, X.Y.; Xu, Z.S.; Zhu, B.; Fu, X.Y.; Peng, R.H.; et al. Enhancement of phenol stress tolerance in transgenic Arabidopsis plants overexpressing glutathione S-transferase. Plant Growth Regul. 2017, 82, 37–45. [Google Scholar] [CrossRef]
- Sharma, R.; Sahoo, A.; Devendran, R.; Jain, M. Over-expression of a rice tau class glutathione S-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PLoS ONE 2014, 9, e92900. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Li, Y.S.; Chen, X.F.; Hu, J.; Chang, X.; Zhu, Y.G. Transgenic tobacco plants overexpressing cotton glutathione S-transferase (GST) show enhanced resistance to methyl viologen. J. Plant Physiol. 2003, 160, 1305–1311. [Google Scholar] [CrossRef]
- Roxas, V.P.; Lodhi, S.A.; Garrett, D.K.; Mahan, J.R.; Allen, R.D. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 2000, 41, 1229–1234. [Google Scholar] [CrossRef]
- Monson, R.K.; Weraduwage, S.M.; Rosenkranz, M.; Schnitzler, J.P.; Sharkey, T.D. Leaf isoprene emission as a trait that mediates the growth-defense tradeoff in the face of climate stress. Oecologia 2021, 197, 885–902. [Google Scholar] [CrossRef]
- Zhang, N.; Kallis, R.P.; Ewy, R.G.; Portis, A.R., Jr. Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc. Natl. Acad. Sci. USA 2002, 99, 3330–3334. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.-M.; Zhou, L.; He, Y.; Wang, D.; Qi, Y.-H.; He, Y.; Wang, D.; Qi, Y.H.; Jiang, D.A. Rubisco Activase Is Also a Multiple Responder to Abiotic Stresses in Rice. PLoS ONE 2015, 10, e0140934. [Google Scholar] [CrossRef] [Green Version]
- Crafts-Brandner, S.J.; Salvucci, M.E. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. USA 2000, 97, 13430–13435. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [Green Version]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [Green Version]
- Salekdeh, G.H.; Siopongco, J.; Wade, L.J.; Ghareyazie, B.; Bennett, J. Proteomic analysis of rice leaves during drought stress and recovery. Proteomics 2002, 2, 1131–1145. [Google Scholar] [CrossRef]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoya, S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 2008, 27, 329–334. [Google Scholar] [CrossRef]
- Kirschner, M.; Winkelhaus, S.; Thierfelder, J.; Nover, L. Transient expression and heat stress induced aggregation of endogenous and heterologous small heat stress proteins in tobacco protoplasts. Plant J. 2000, 24, 397–412. [Google Scholar] [CrossRef]
- Eyles, S.J.; Gierasch, L.M. Nature’s molecular sponges: Small heat shock proteins grow into their chaperone roles. Proc. Natl. Acad. Sci. USA 2010, 107, 2727–2728. [Google Scholar] [CrossRef] [Green Version]
- Ghirardo, A.; Nosenko, T.; Kreuzwieser, J.; Winkler, J.B.; Kruse, J.; Albert, A.; Merl-Pham, J.; Lux, T.; Ache, P.; Zimmer, I.; et al. Protein expression plasticity contributes to heat and drought tolerance of date palm. Oecologia 2021, 197, 903–919. [Google Scholar] [CrossRef]
- Chowdary, T.K.; Raman, B.; Ramakrishna, T.; Rao, C.M. Interaction of mammalian Hsp22 with lipid membranes. Biochem. J. 2007, 401, 437–445. [Google Scholar] [CrossRef]
- Nakamoto, H.; Vígh, L. The small heat shock proteins and their clients. Cell. Mol. Life Sci. 2007, 64, 294–306. [Google Scholar] [CrossRef]
- Balogi, Z.; Cheregi, O.; Giese, K.C.; Vierling, E.; Vass, I.; Vígh, L.; Horváth, I. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in Synechocystis 6803. J. Biol. Chem. 2008, 283, 22983–22991. [Google Scholar] [CrossRef] [Green Version]
- Siwko, M.E.; Marrink, S.J.; de Vries, A.H.; Kozubek, A.; Uiterkamp, A.J.S.; Mark, A.E. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochim. Biophys. Acta Biomembr. 2007, 1768, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Nelson, C.J.; Trosch, J.; Castleden, I.; Huang, S.; Millar, A.H. Protein degradation rate in Arabidopsis thaliana leaf growth and development. Plant Cell 2017, 29, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Xu, Z.Y.; Na, Y.J.; Yoo, Y.J.; Lee, J.; Sohn, E.J.; Hwang, I. Small heat shock protein Hsp17.8 functions as an AKR2A cofactor in the targeting of chloroplast outer membrane proteins in Arabidopsis. Plant Physiol. 2011, 157, 132–146. [Google Scholar] [CrossRef] [Green Version]
- Treger, J.M.; McEntee, K. Structure of the DNA damage-inducible gene DDR48 and evidence for its role in mutagenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1990, 10, 3174–3184. [Google Scholar] [CrossRef]
- Konrad, Z.; Bar-Zvi, D. Synergism between the chaperone-like activity of the stress regulated ASR1 protein and the osmolyte glycine-betaine. Planta 2008, 227, 1213–1219. [Google Scholar] [CrossRef]
- Carrari, F.; Fernie, A.R.; Iusem, N.D. Heard it through the grapevine? ABA and sugar cross-talk: The ASR story. Trends Plant Sci. 2004, 9, 57–59. [Google Scholar] [CrossRef]
- Dai, J.R.; Liu, B.; Feng, D.R.; Liu, H.; Liu, H.Y.; He, Y.M.; Qi, K.B.; Wang, H.B.; Wang, J.F. MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep. 2011, 30, 1219–1230. [Google Scholar] [CrossRef]
- Hu, W.; Huang, C.; Deng, X.; Zhou, S.; Chen, L.; Li, Y.I.N.; Wang, C.; Ma, Z.; Yuan, Q.; Wang, Y.A.N.; et al. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 2013, 36, 1449–1464. [Google Scholar] [CrossRef]
- Barta, C.; Loreto, F. The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiol. 2006, 141, 1676–1683. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: Control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef]
- Roy, J.; Rineau, F.; De Boeck, H.J.; Nijs, I.; Pütz, T.; Abiven, S.; Arnone, J.A.; Barton, C.V.; Beenaerts, N.; Brüggemann, N.; et al. Ecotrons: Powerful and versatile ecosystem analysers for ecology, agronomy and environmental science. Glob. Chang. Biol. 2021, 27, 1387–1407. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Et Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Techniques for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Szopinska, A.; Christ, E.; Planchon, S.; König, H.; Evers, D.; Renaut, J. Stuck at work? Quantitative proteomics of environmental wine yeast strains reveals the natural mechanism of overcoming stuck fermentation. Proteomics 2016, 16, 593–608. [Google Scholar] [CrossRef]
- Printz, B.; Guerriero, G.; Sergeant, K.; Audinot, J.N.; Guignard, C.; Renaut, J.; Lutts, S.; Hausman, J.F. Combining -Omics to Unravel the Impact of Copper Nutrition on Alfalfa (Medicago sativa) Stem Metabolism. Plant Cell Physiol. 2016, 57, 407–422. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollastri, S.; Velikova, V.; Castaldini, M.; Fineschi, S.; Ghirardo, A.; Renaut, J.; Schnitzler, J.-P.; Sergeant, K.; Winkler, J.B.; Zorzan, S.; et al. Isoprene-Emitting Tobacco Plants Are Less Affected by Moderate Water Deficit under Future Climate Change Scenario and Show Adjustments of Stress-Related Proteins in Actual Climate. Plants 2023, 12, 333. https://doi.org/10.3390/plants12020333
Pollastri S, Velikova V, Castaldini M, Fineschi S, Ghirardo A, Renaut J, Schnitzler J-P, Sergeant K, Winkler JB, Zorzan S, et al. Isoprene-Emitting Tobacco Plants Are Less Affected by Moderate Water Deficit under Future Climate Change Scenario and Show Adjustments of Stress-Related Proteins in Actual Climate. Plants. 2023; 12(2):333. https://doi.org/10.3390/plants12020333
Chicago/Turabian StylePollastri, Susanna, Violeta Velikova, Maurizio Castaldini, Silvia Fineschi, Andrea Ghirardo, Jenny Renaut, Jörg-Peter Schnitzler, Kjell Sergeant, Jana Barbro Winkler, Simone Zorzan, and et al. 2023. "Isoprene-Emitting Tobacco Plants Are Less Affected by Moderate Water Deficit under Future Climate Change Scenario and Show Adjustments of Stress-Related Proteins in Actual Climate" Plants 12, no. 2: 333. https://doi.org/10.3390/plants12020333
APA StylePollastri, S., Velikova, V., Castaldini, M., Fineschi, S., Ghirardo, A., Renaut, J., Schnitzler, J. -P., Sergeant, K., Winkler, J. B., Zorzan, S., & Loreto, F. (2023). Isoprene-Emitting Tobacco Plants Are Less Affected by Moderate Water Deficit under Future Climate Change Scenario and Show Adjustments of Stress-Related Proteins in Actual Climate. Plants, 12(2), 333. https://doi.org/10.3390/plants12020333









