Increasing the Salt Stress Tolerance of Some Tomato Cultivars under the Influence of Growth Regulators
Abstract
1. Introduction
2. Results
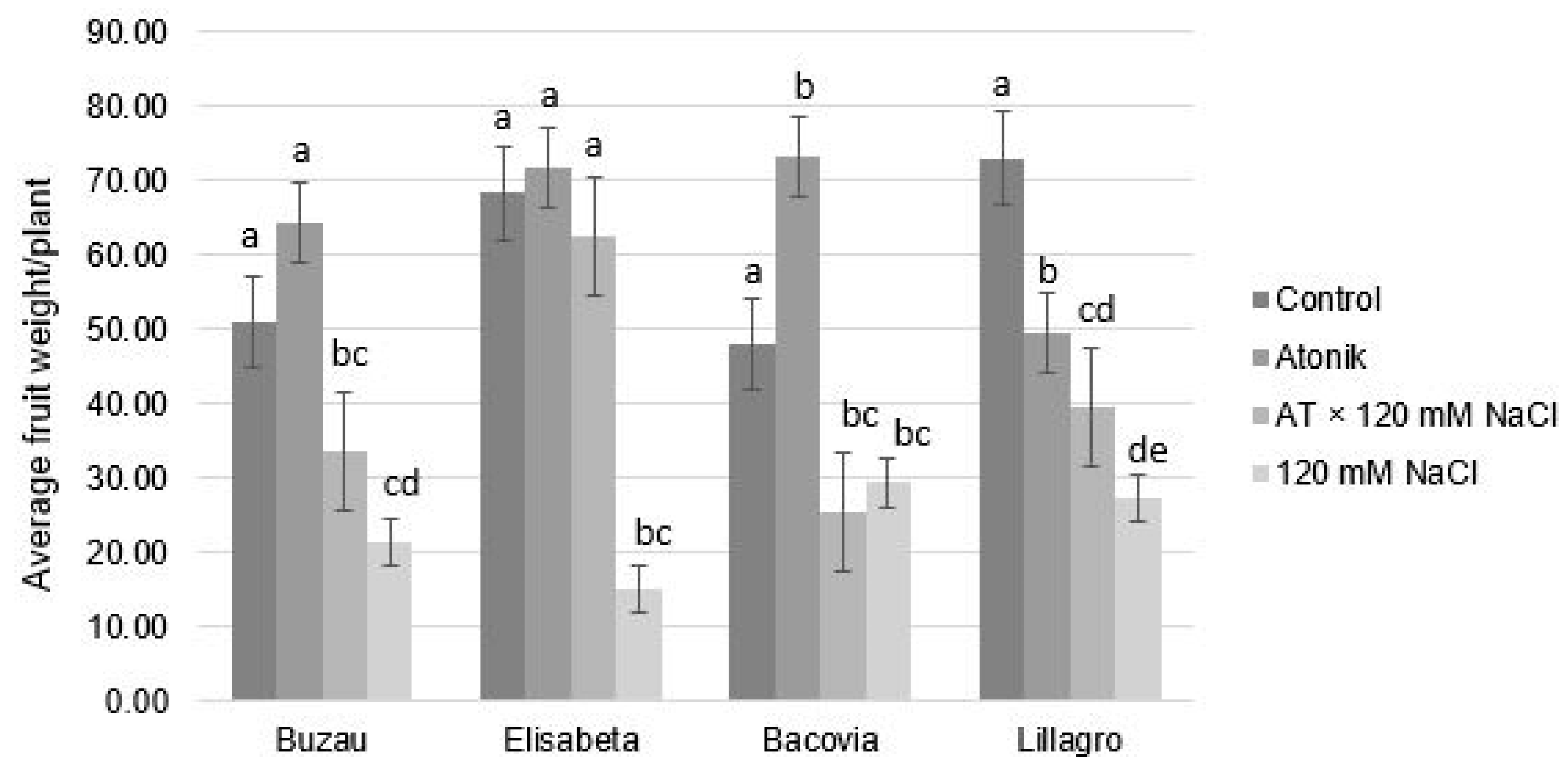
2.1. Effect of Atonik Biostimulant on the Growth and Fruiting Characters of Tomato Plants under the Action of Saline Stress
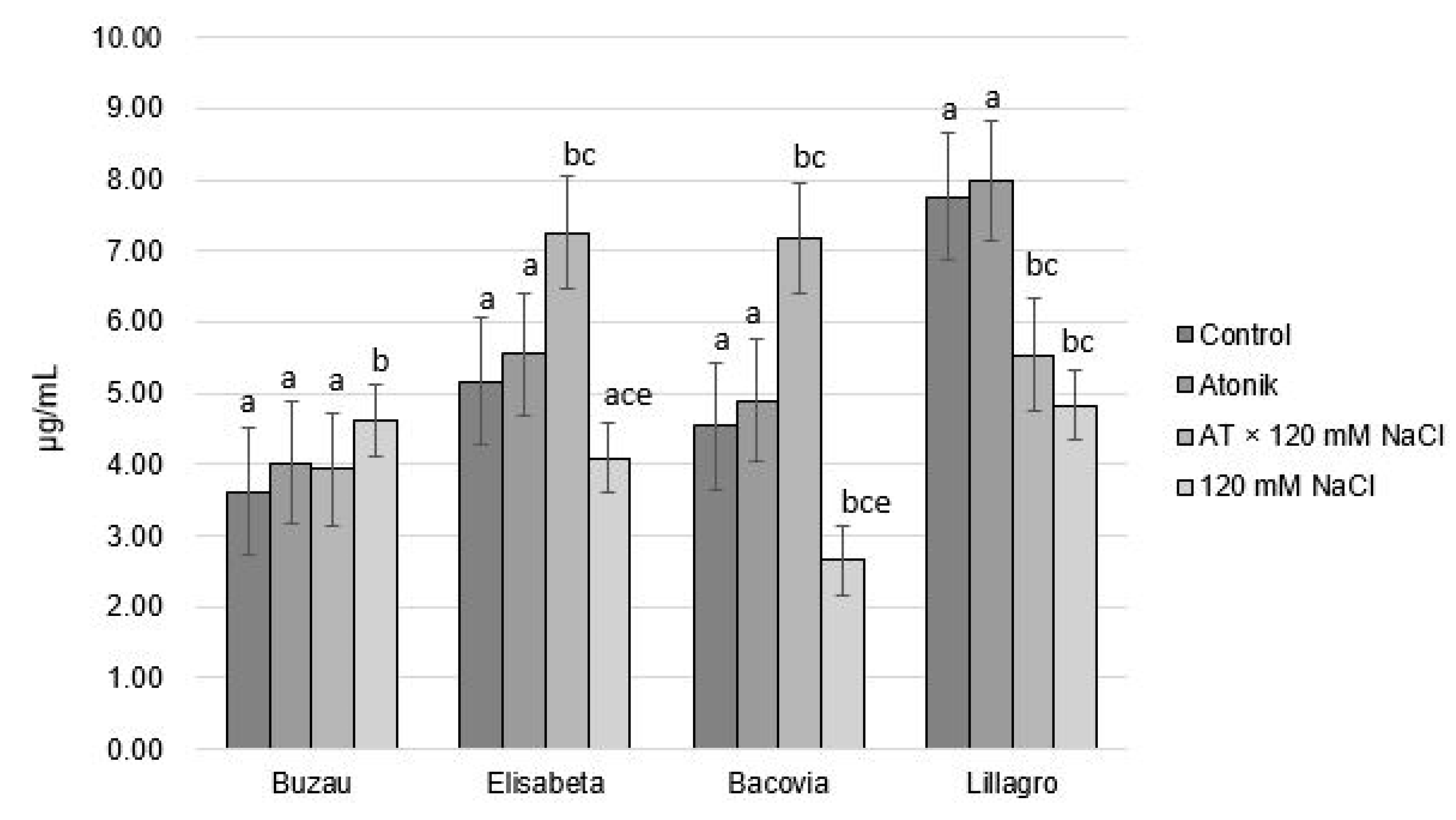
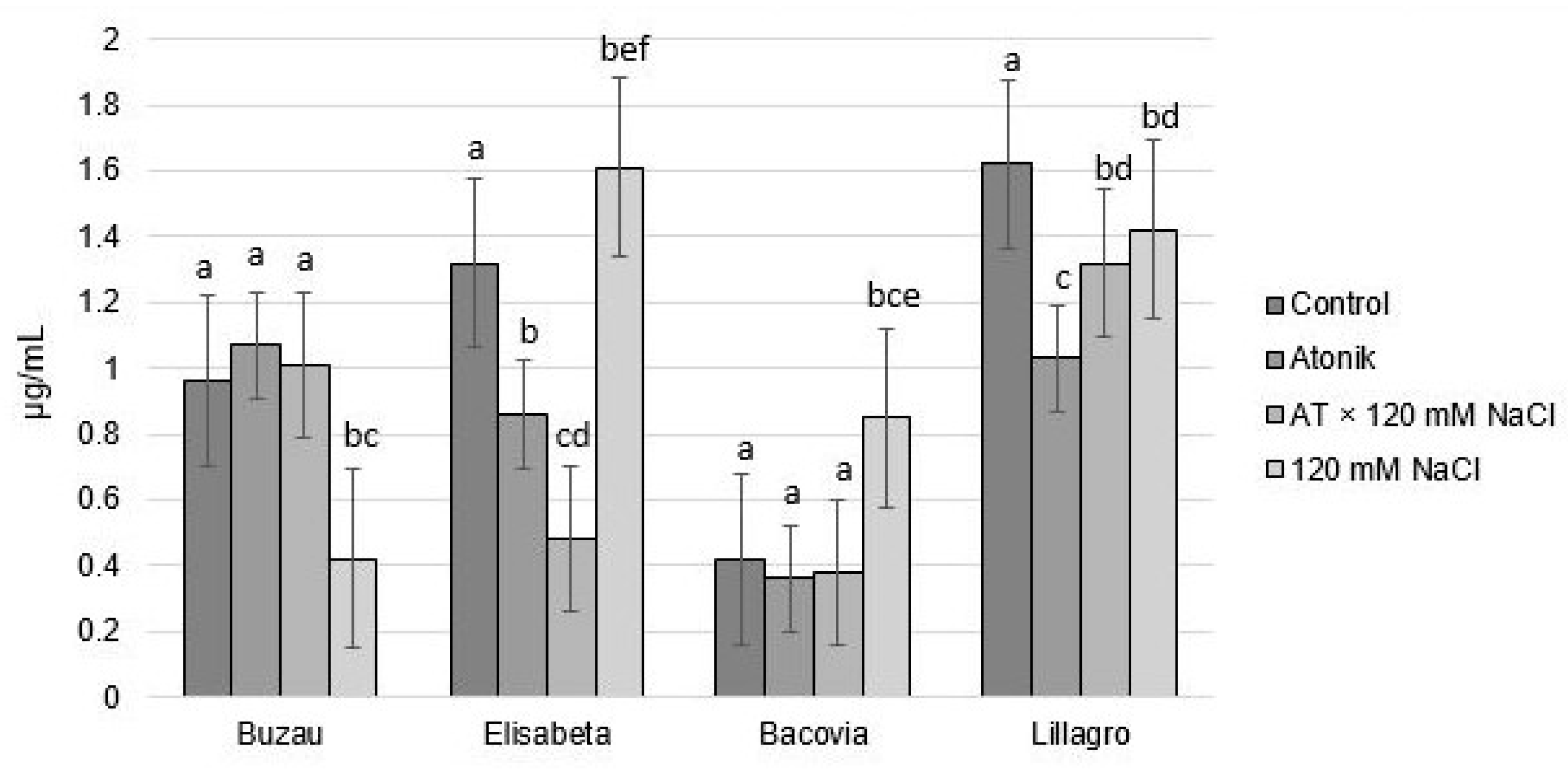
2.2. Effect of Atonik Biostimulant on Some Biochemical Indicators of Tomato Plants under the Action of Saline Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material, Treatments Used and the Growth Conditions
4.2. Biometric and Gravimetric Measurements
4.3. Biochemical Analyses
4.3.1. Quantitative Determination of Photosynthetic Pigments
4.3.2. Proline Determination
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity Stress in Arid and Semi-Arid Climates: Effects and Management in Field Crops, Climate Change and Agriculture; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Tofei, A.M.; Boscaiu, M.; Moreno, H.R.; Ibáñez-Asensio, S.; Vicente, O. Effect of a biostimulant based on polyphenols and glycine betaine on tomato plants’ responses to salt stress. Agronomy 2022, 12, 2142. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.; Li, Z.; Sheng, J.; Shen, L. SIMAPK 3 enhances tolerance to salt stress in tomato plants by scavenging ROS accumulation and up-regulating the expression of ethylene signaling related genes. Environ. Exp. Bot. 2022, 193, 104698. [Google Scholar] [CrossRef]
- Koyro, H.W.; Geissler, N.; Hussin, S.; Debez, A.; Huchzermeyer, B. Strategies of halophytes to survive in a salty environment. In Abiotic Stress and Plant Responses; Khan, N.A., Singh, S., Eds.; IK International Publishing House: New Delhi, India, 2008; pp. 83–104. [Google Scholar]
- Kawanabe, S.; Zhu, T.C. Degeneration and conservation of Aneurolepidium chinense grassland in Northern China. J. JPN Grassl. Sci. 1991, 37, 91–99. [Google Scholar]
- Dajic, Z.; Rao, K.; Raghavendra, A.; Eddy, K. Salt stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 41–99. [Google Scholar]
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef]
- Drobek, M.; Frac, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A Review. Agron. J. 2019, 9, 335. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef]
- Kaur, P.; Yadav, P.; Bali, S.; Guatum, V.; Kaur Kohli, S.; Kapoor, D.; Arora, S.; Pal Vig, A.; Kaur, R.; Bhardwaj, R. Chapter 6, Pytohormones is Improving Abiotic Stress Tolerance. In Plant Tolerance to Environmental Stress, Role of Phytoprotectants; Hasanuzzaman, M., Fujita, M., Oku, M., Tofazzal, I., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 81–103. [Google Scholar]
- Amini, F.; Ehsanpour, A.A. Response of tomato (Lycopersicon esculentum Mill.) cultivars to MS, water agar and salt stress in in vitro culture. Pak. J. Biol. Sci. 2006, 9, 170–175. [Google Scholar]
- Wang, X.; Geng, S.; Ri, Y.J.; Cao, D.; Liu, J.; Shi, D.; Yang, C. Physiological responses and adaptive strategies of tomato plants to salt and alkali stresses. Sci. Hortic. 2011, 130, 248–255. [Google Scholar] [CrossRef]
- Raza, M.A.; Saeed, A.; Munir, H.; Ziaf, K.; Shakeel, A.; Saeed, N.; Munawar, A.; Rehman, F. Screening of tomato genotypes for salinity tolerance based on early growth attributes and leaf inorganic osmolytes. Arch. Agron. Soil Sci. 2017, 63, 501–512. [Google Scholar] [CrossRef]
- Al-Daej, M.I. Research Article—Salt tolerance of some tomato (Solanum lycoversicum L.) Cultivars for salinity under controlled conditions. Am. J. Plant Physiol. 2018, 13, 58–64. [Google Scholar]
- Hnilickova, L.H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Ehret, D.L.; Usher, K.; Helmer, T.; Block, G.; Steinke, D.; Frey, B.; Kuang, T.; Diarra, M. Tomato fruit antioxidants in relation to salinity and greenhouse climate. J. Agric. Food Chem. 2013, 61, 1138–1145. [Google Scholar] [CrossRef]
- Covaşă, M.; Jităreanu, C.D.; Slabu, C.; Marta, A.E. The influence of salt stress on ascorbic acid (vitamin c) in the fruits of some tomato cultivars from N-E Romania. Lucr. Ştiinţifice Ser. Hortic. Usamv Iaşi 2017, 60, 17–22. [Google Scholar]
- Karima, F.; Abdelgawad, M.; El-Mogy, M.; Garchery, C.; Stevens, R.G. Increasing ascorbic acid content and salinity tolerance of cherry tomato plants by suppressed expression of the ascorbate oxidase gene. Agronomy 2019, 9, 51. [Google Scholar]
- Muuns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 6, 15–24. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Eggink, L.; Park, H.; Hoober, J.K. The role of chlorophyll b in photosynthesis: Hypothesis. BMC Plant Biol. 2001, 1, 2. [Google Scholar] [CrossRef]
- Swapnil, P.; Mukesh, M.; Kumar Singh, S.; Dhuldhaj, U.P.; Marwal, H.A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Lee, J.J.; Crosby, K.M.; Pike, L.M.; Yoo, K.S.; Leskovar, D.I. Impact of genetic and environmental variation on development of flavonoids and carotenoids in pepper (Capsicum spp.). Sci. Hortic. 2005, 106, 341–352. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Yldiz, M.; Terzi, H. Effect of NaCl stress on chlorophyll biosynthesis, proline, lipid peroxidation and antioxidative enzymes in leaves of salt tolerant and salt sensitive barley cultivars. J. Agric. Sci. 2013, 19, 79–88. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, P.V.; Prasadecum, S.M. Proline and salinity tolerance in plants. Biochem. Pharmacol. 2014, 3, 6. [Google Scholar] [CrossRef]
- Szabos, L.; Savoure, A. Proline: A multifunctional aminoacid. Trends Plant Sci. 2009, 15, 89–97. [Google Scholar] [CrossRef]
- Molazem, D.; Qurbanov, E.M.; Dunyamaliyev, S.A. Role of proline, Na and chlorophyll content in salt tolerance of corn (Zea mays L.). Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 319–324. [Google Scholar]
- Available online: https://www.aectra.ro/produs/atonik/ (accessed on 12 December 2022).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Carillo, P.; Gibbon, Y. PROTOCOL: Extraction and Determination of Proline. PrometheusWiki. 2011. Available online: http://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 12 November 2022).
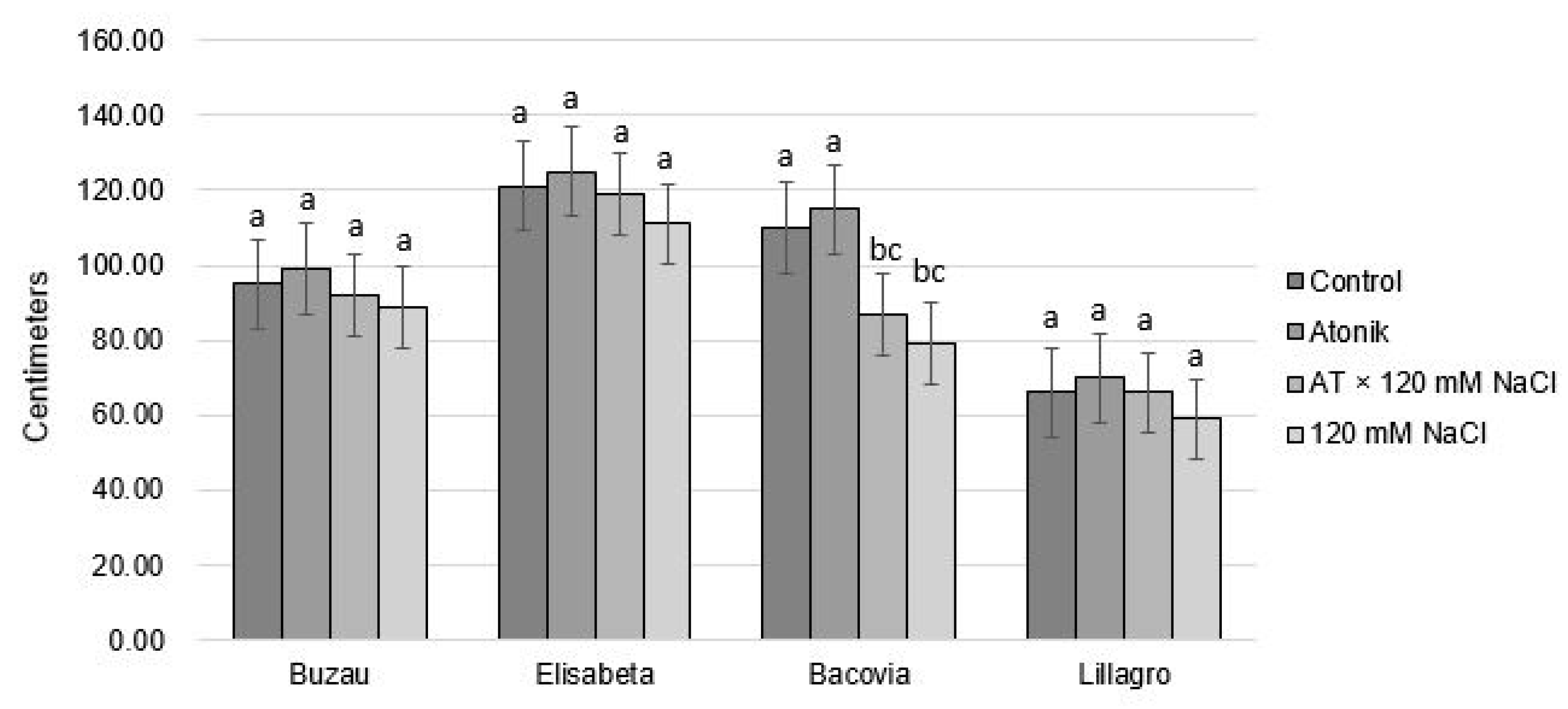
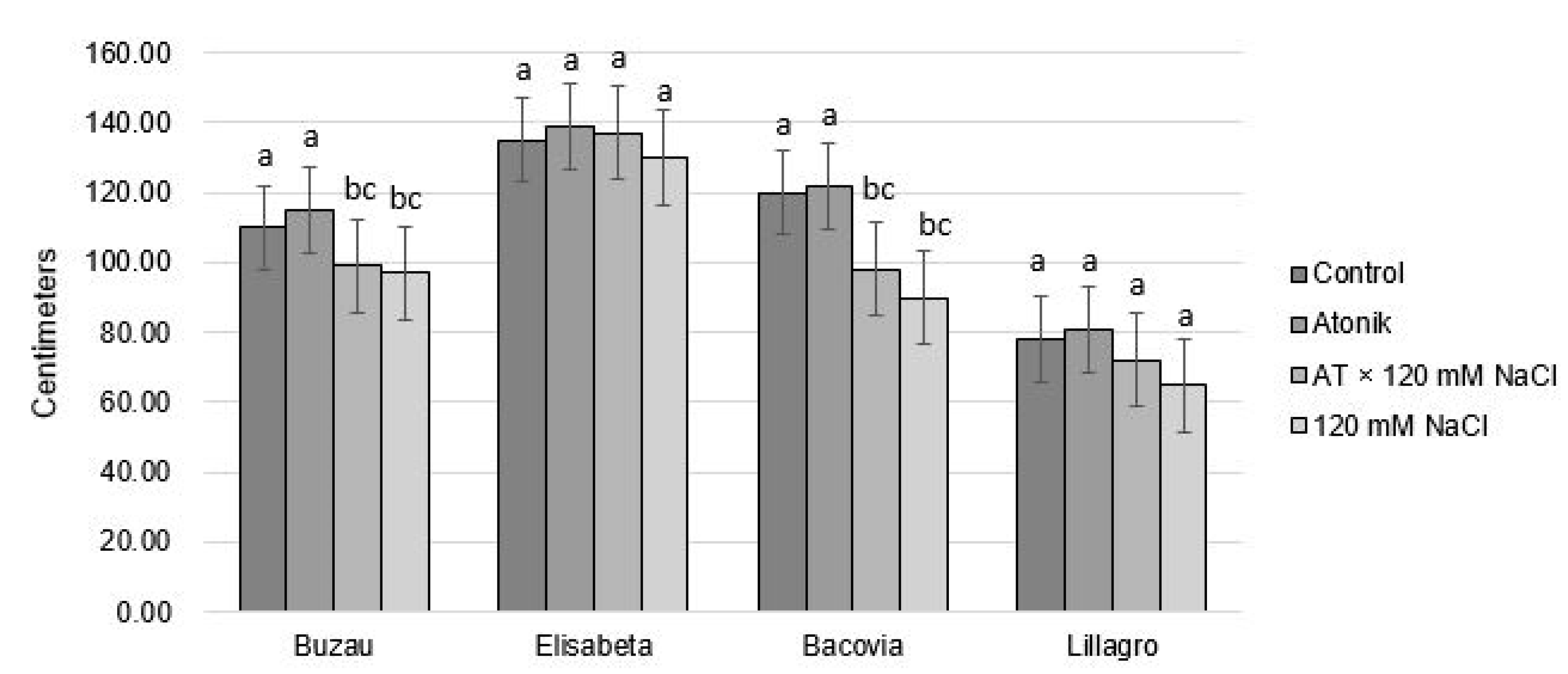
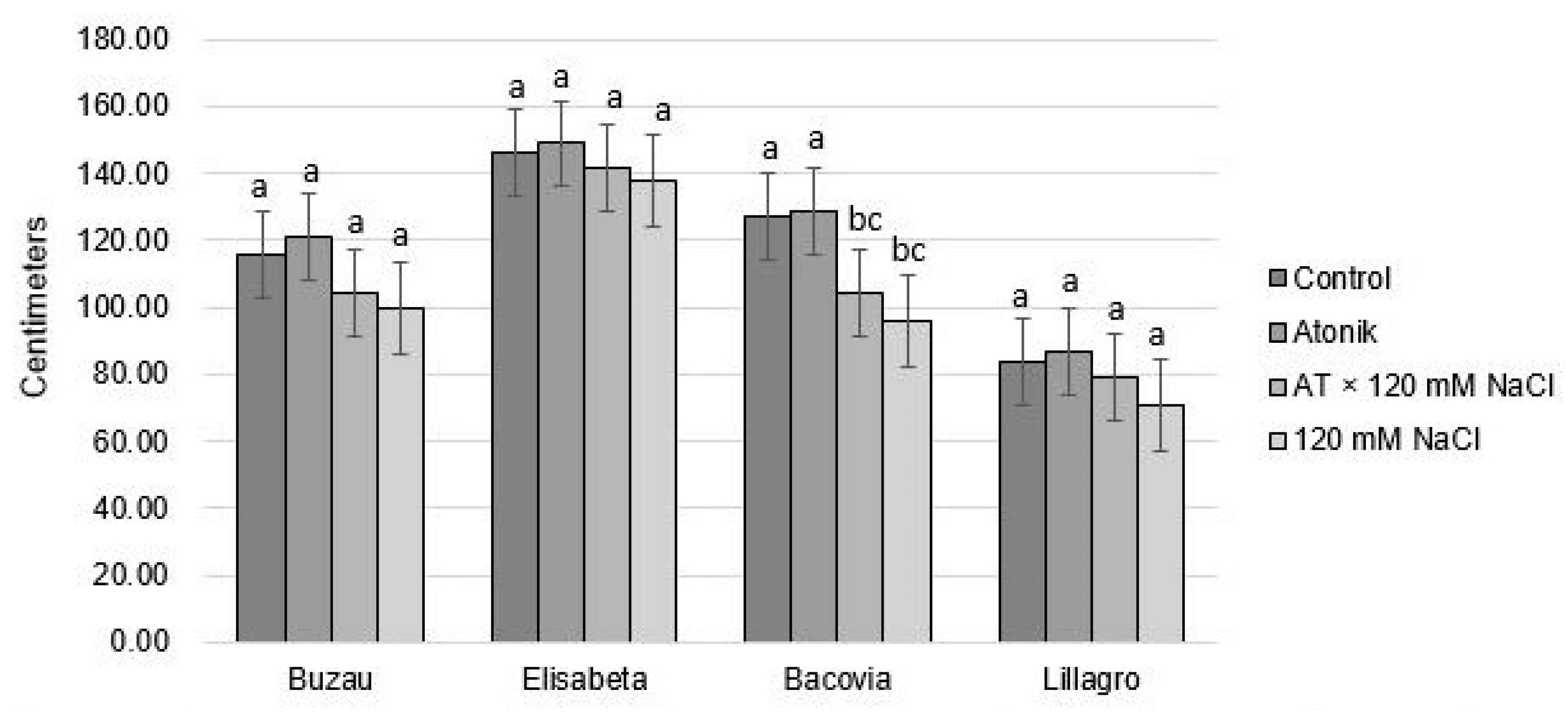

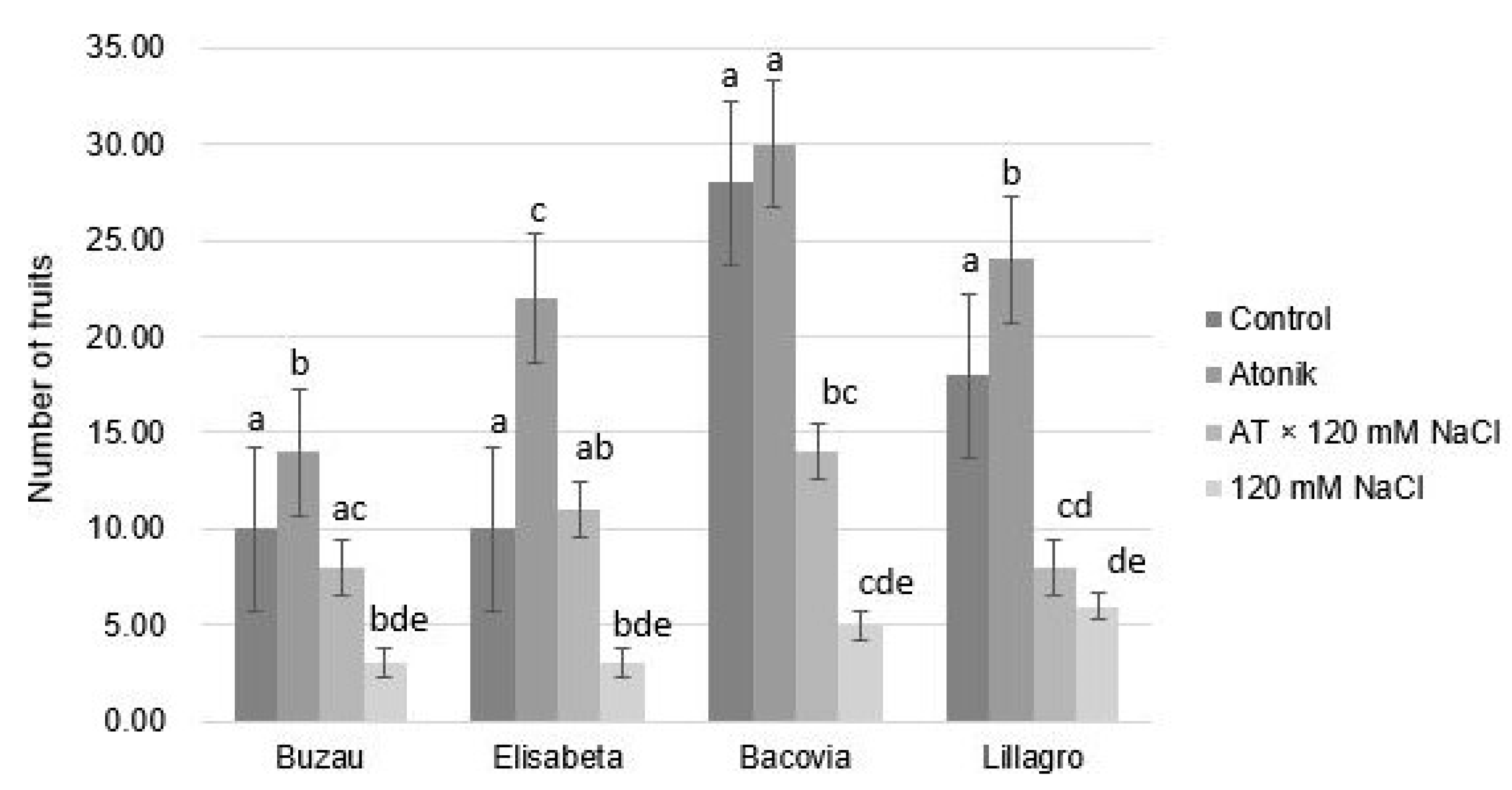

| Proline Content (nmol mg−1 FW) after the Third Treatments | ||||
|---|---|---|---|---|
| Treatments | Buzau | Elisabeta | Bacovia | Lillagro |
| Control | 0.084 a | 0.098 a | 0.055 a | 0.094 a |
| Atonik | 0.084 a | 0.081 a | 0.084 a | 0.114 a |
| AT×120 mM NaCl | 0.186 a | 0.121 ab | 0.038 ab | 0.030 bc |
| 120 mM NaCl | 0.084 a | 0.431 bc | 0.070 a | 0.133 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covașă, M.; Slabu, C.; Marta, A.E.; Jităreanu, C.D. Increasing the Salt Stress Tolerance of Some Tomato Cultivars under the Influence of Growth Regulators. Plants 2023, 12, 363. https://doi.org/10.3390/plants12020363
Covașă M, Slabu C, Marta AE, Jităreanu CD. Increasing the Salt Stress Tolerance of Some Tomato Cultivars under the Influence of Growth Regulators. Plants. 2023; 12(2):363. https://doi.org/10.3390/plants12020363
Chicago/Turabian StyleCovașă, Mihaela, Cristina Slabu, Alina Elena Marta, and Carmenica Doina Jităreanu. 2023. "Increasing the Salt Stress Tolerance of Some Tomato Cultivars under the Influence of Growth Regulators" Plants 12, no. 2: 363. https://doi.org/10.3390/plants12020363
APA StyleCovașă, M., Slabu, C., Marta, A. E., & Jităreanu, C. D. (2023). Increasing the Salt Stress Tolerance of Some Tomato Cultivars under the Influence of Growth Regulators. Plants, 12(2), 363. https://doi.org/10.3390/plants12020363









