Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain
Abstract
1. Introduction
2. Results and Discusion
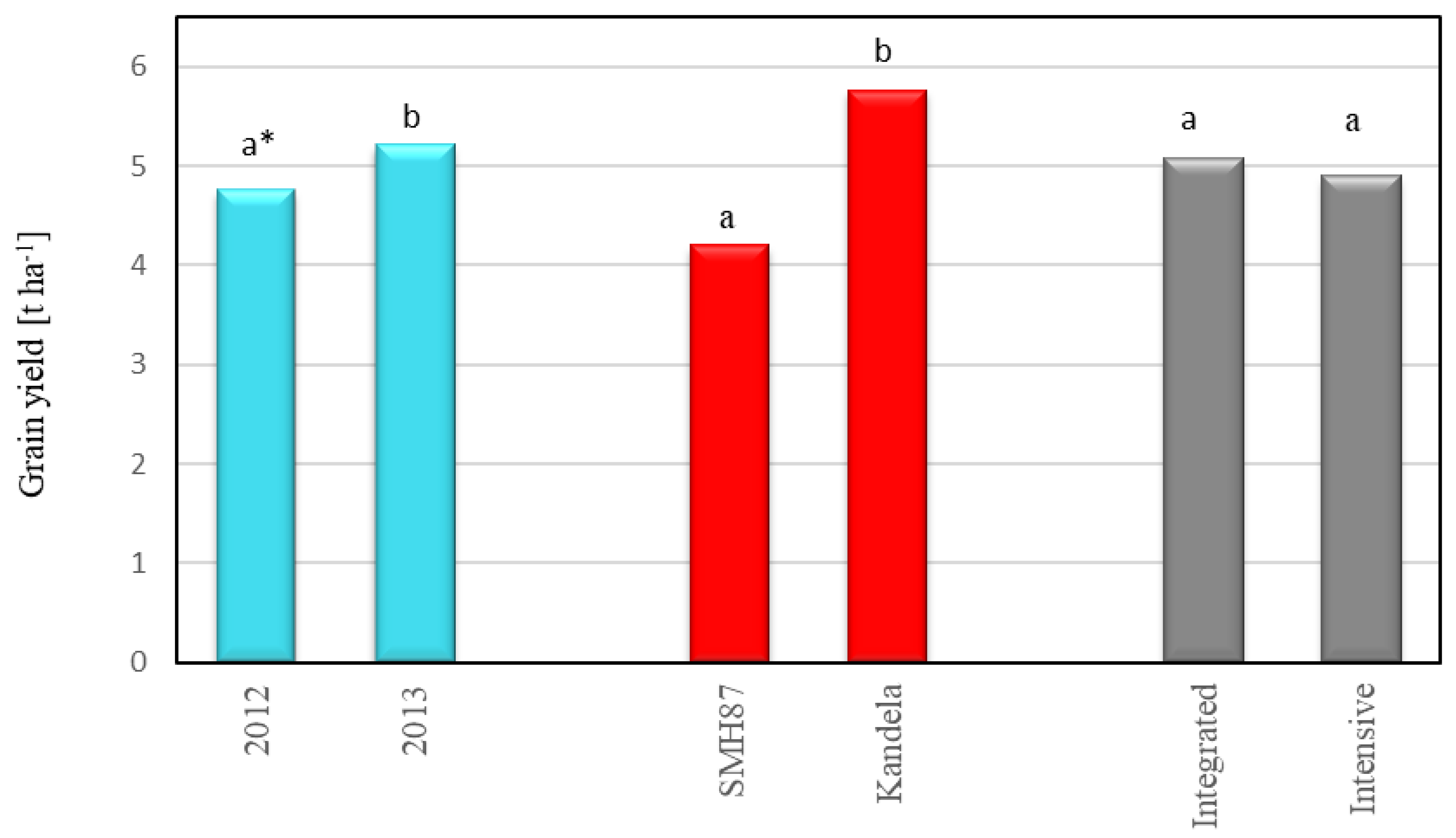
2.1. Grain Yield
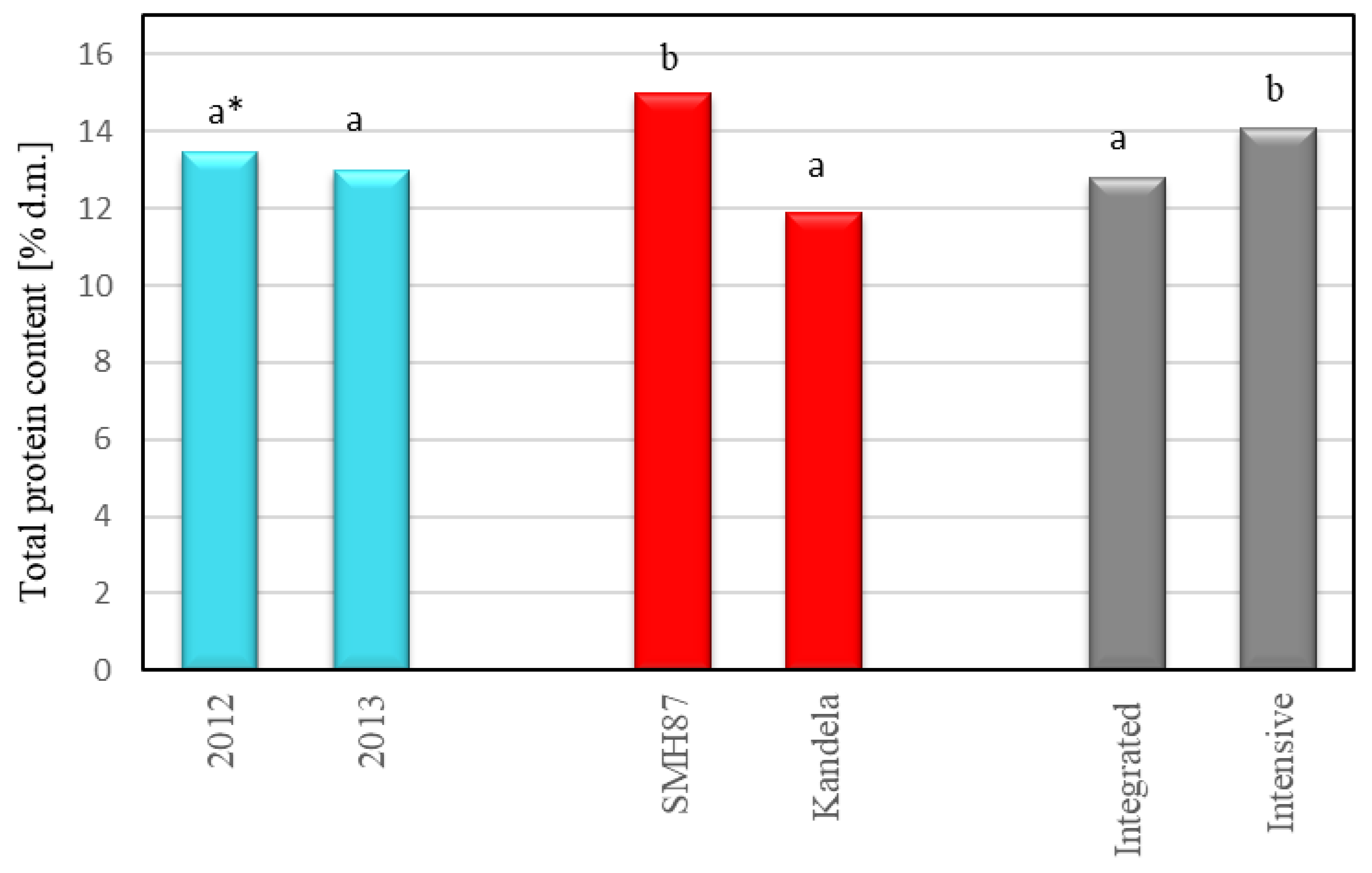
2.2. Total Protein Content
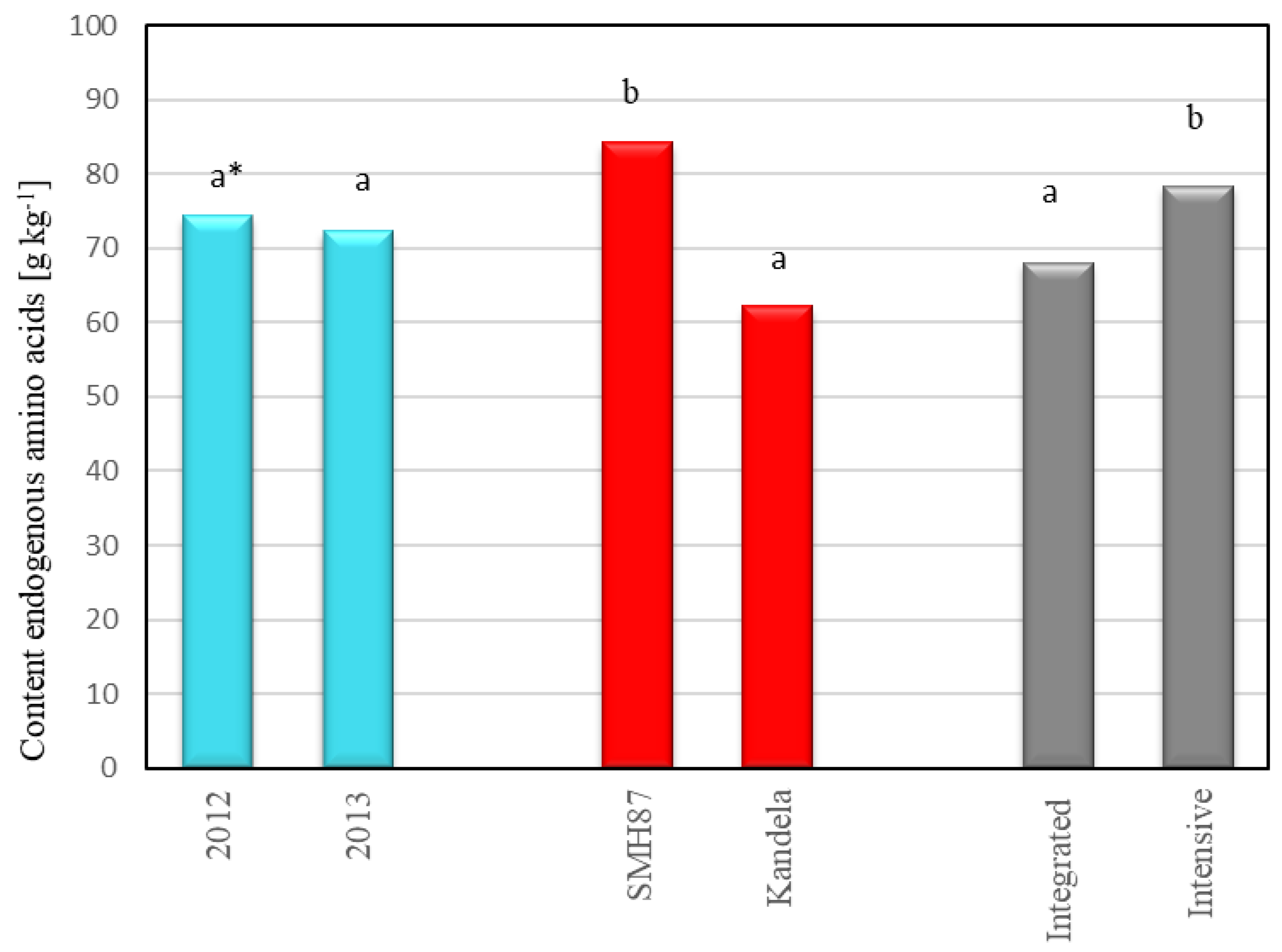
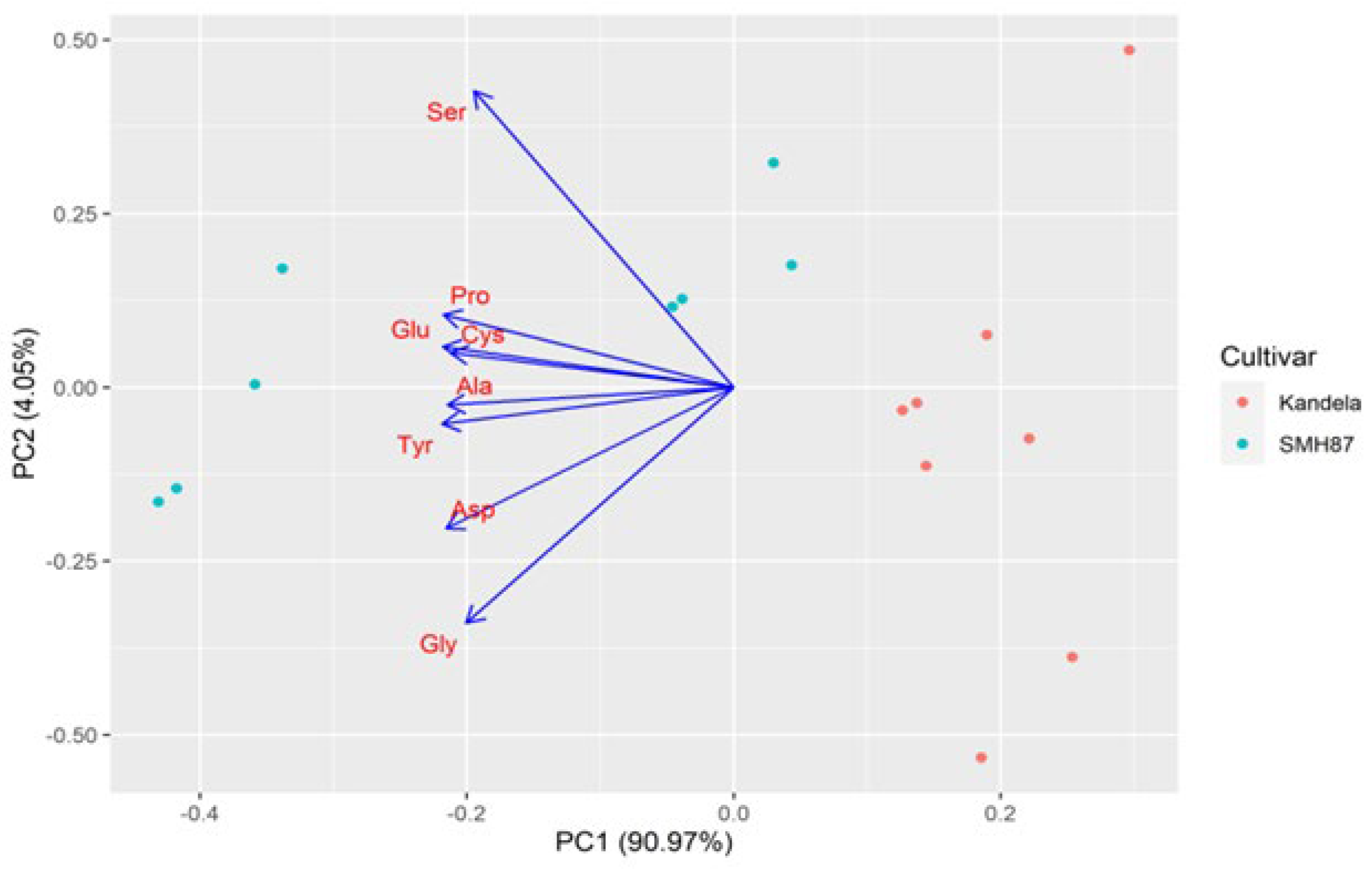
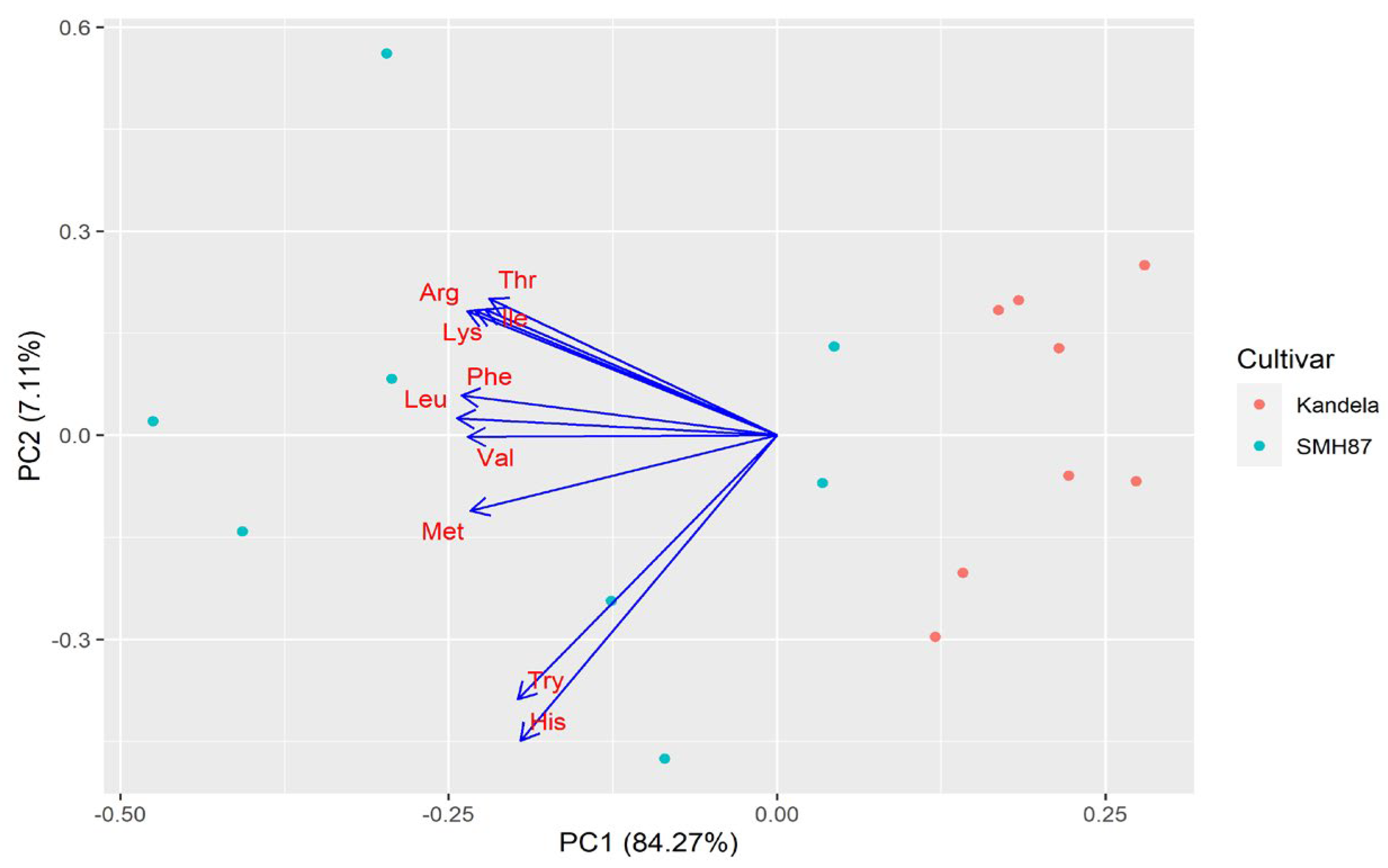
2.3. Protein Amino Acid Profile
3. Materials and Methods
3.1. Site Characteristics, Experimental Design, and Agronomic Practices
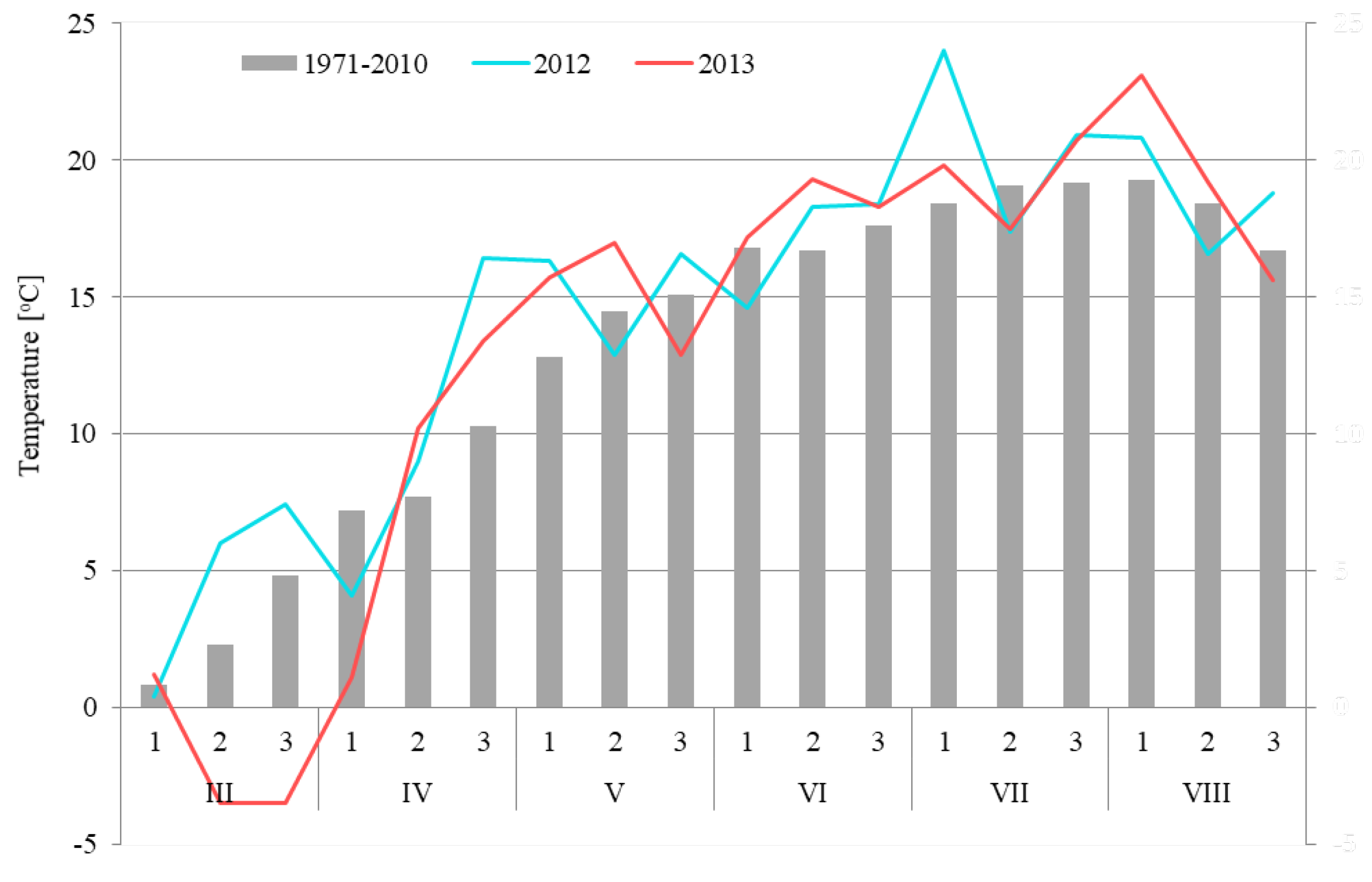
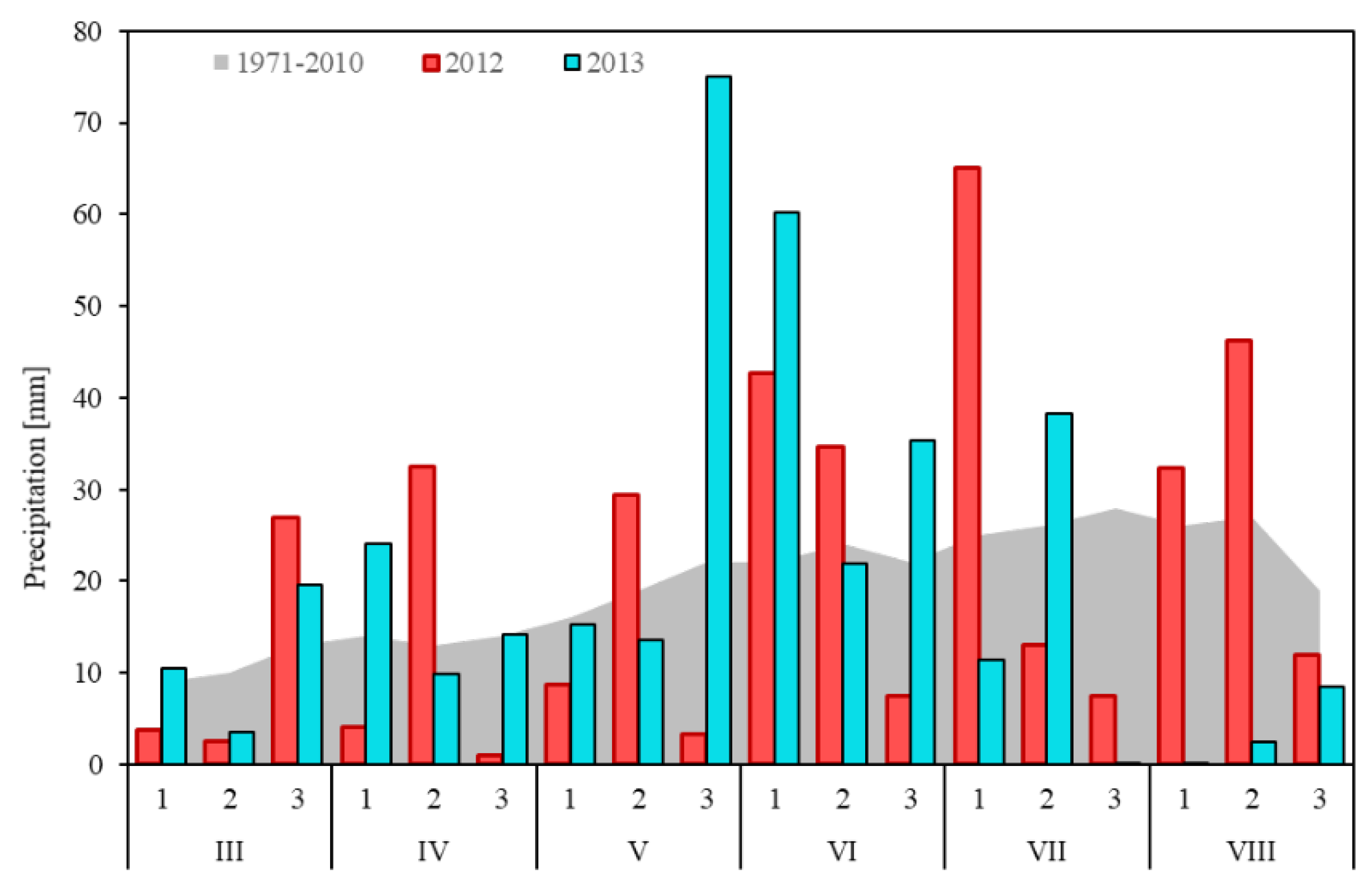
3.2. Meteorological Conditions
3.3. Grain Yield Assessment
3.4. Chemical Analyses
3.4.1. Determination of Total Protein Content
3.4.2. Identification and Determination of Amino Acids by High-Performance Liquid Chromatography (HPLC)
Apparatus
Analytical Procedure for the Determination of Amino Acids
Analytical Procedure for the Determination of Tryptophan Content
- —free or total tryptophan content, in g kg−1,
- —peak area of tryptophan in extract or hydrolysate of test sample,
- —peak area of α-methyl-tryptophan in an extract or hydrolysate of the test sample,
- —concentration of the internal standard (α-methyl-tryptophan) in the extract or hydrolysate of the test sample, in µg mL−1,
- —average calibration factor,
- —final volume of extract or hydrolysate of sample, w mL,
- —dilution factor,
- —sample weight, in mg,
- —sum of the calibration factors for all titrations of tryptophan and α-methyl-tryptophan calibration solution during the analysis,
- —calibration factor for a single calibration injection of the standard solution of tryptophan and α-methyl-tryptophan during the analysis,
- —number of rates of tryptophan and α-methyl-tryptophan calibration solution during the analysis,
- —peak area of standard tryptophan, calibration standard solution of tryptophan and α-methyl-tryptophan,
- —peak area of internal standard, calibration standard solution of tryptophan and α-methyl-tryptophan,
- —concentration of the internal standard in the calibration standard solution, in µg mL−1,
- —concentration of the tryptophan standard in the calibration standard solution, in µg mL−1.
3.4.3. Determination of Biological Value of Protein
- ai—The exogenous amino acid content of the tested protein,
- as—Exogenous amino acid content of the reference protein.
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Peña, R.J. Current and Future Trends of Wheat Quality Needs. In Wheat Production in Stressed Environments. Developments in Plant Breeding; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 12. [Google Scholar] [CrossRef]
- Litwinek, D.; Gambuś, H.; Mickowska, B.; Zięć, G.; Berski, W. Aminoacids composition of proteins in wheat and oat flours used in breads production. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 1725–1733. [Google Scholar]
- Kuchciak, J.; Czubaszek, A. Quality and milling properties of wheat grain obtained from different cereal producers–Assessent under laboratory and industrial conditions. Zesz. Probl. Post. Nauk Roln. 2015, 581, 29–39. (In Polish) [Google Scholar]
- Łaba, S.; Cacak-Pietrzak, G.; Łaba, R.; Sułek, A.; Szczepański, K. Food Losses in Consumer Cereal Production in Poland in the Context of Food Security and Environmental Impact. Agriculture 2022, 12, 665. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 17 October 2022).
- Cacak-Pietrzak, G. Use of wheat in various branches of the food industry-technological requirements. Przegl. Zboż. Młyn. 2008, 11, 11–13. (In Polish) [Google Scholar]
- Manzano, J.R. Prolaminas y Marcadores Moleculares Relacionadas Con la Calidad en Trigo Duro (Triticum turgidum L.). Ph.D. Thesis, Escuela Técnica Superio de Ingenieros Agró nomos, Universidad Politécnica de Madrid, Madrid, Spain, 2007. [Google Scholar]
- Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops#Cereals (accessed on 18 October 2022).
- Rozbicki, J.; Ceglińska, A.; Gozdowski, D.; Jakubczyk, M.; Cacak-Pietrzak, G.; Mądry, W.; Golba, J.; Piechociński, M.; Sobczyński, G.; Stadnicki, M.; et al. Influence of the cultivar, environment and managment on the grain yield and bread-making quality in winter wheat. J. Cer. Sci. 2015, 61, 126–132. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G. The influence of production technology on yield selected quality parameters of spring wheat cultivars. Agric. Sci. Crop Sci. Animal Sci. Res. Rural Develop. 2018, 2, 42–48. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G.; Wyzinska, M.; Nieróbca, A. Influence of Nitroden Fertilization on the Yields and Grain Quality of Winter Wheat under Different Environmental Conditions. Int. J. Agric. Biol. Eng. 2019, 13, 127–133. [Google Scholar]
- Kołodziejczyk, M.; Szmigiel, A. Influence of intensity cultivation technology of some spring wheat cultivars. Fragm. Agron. 2014, 31, 75–84. (In Polish) [Google Scholar]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Kuś, J.; Jończyk, K.; Kawalec, A. Factors limiting the yields of winter wheat in different crop production systems. Acta Agroph. 2007, 10, 407–417. (In Polish) [Google Scholar]
- Korbas, M.; Mrówczyński, M. Integrated Production of Winter and Spring Wheat; IOR-PIB: Poznań, Poland, 2009; pp. 1–166. (In Polish) [Google Scholar]
- Mariem, S.B.; Gonzalez-Torralba, J.; Collar, C.; Aranjuelo, I.; Morales, F. Durum Wheat Grain Yield and Quality under Lowand High Nitrogen Conditions: Insights into Natural Variation in Low-and High-Yielding Genotypes. Plants 2020, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Marzec, A.; Cacak-Pietrzak, G.; Gondek, E. Mechanical and acoustic properties of spring wheat versus its technological quality factors. J. Texture Stud. 2011, 42, 319–329. [Google Scholar] [CrossRef]
- Dziki, D.; Cacak-Pietrzak, G.; Gawlik-Dziki, U.; Miś, A.; Różyło, R.; Jończyk, K. Physicochemical Properties and Milling Characteristic of Spring Wheat from Different Farming Systems. J. Agr. Sci. Tech. 2017, 19, 1253–1266. [Google Scholar]
- Wu, W.; Ma, B.L.; Fan, J.J.; Sun, M.; Yi, Y.; Guo, W.S.; Voldeng, H.D. Management of nitrogen fertilization to balance reducing lodging risk and increasing yield and protein content in spring wheat. Field Crops Res. 2019, 241, 107584. [Google Scholar] [CrossRef]
- Awika, J.M. Major cereal grains production and use around the world. In: Advances in cereal science: Implications to food processing and health promotion. J. Am. Chem. Soc. 2011, 1089, 1–13. [Google Scholar]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Del Moral, G.L.F.; Rharrabti, Y.; Martos, V.; Royo, C. Environmentally induced changes in amino acid composition in the grain of durum wheat grown under different water and temperature regimes in a Mediterranean environment. J. Agric. Food Chem. 2007, 3, 8144–8151. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, P.; Tian, J. Genetic analysis of amino acid content in wheat grain. J. Fenet. 2014, 93, 451–458. [Google Scholar] [CrossRef]
- Thorwarth, P.; Piepho, H.P.; Zhao, Y.; Ebmeyer, E.; Schacht, J.; Schachschneider, R.; Kazman, E.; Rief, J.C.; Würschum, T.; Longin, C.F.H. Higher grain yield and higher grain protein deviation underline the potential of hybrid wheat for a sustainable agriculture. Plant Breed 2018, 137, 326–337. [Google Scholar] [CrossRef]
- Yu, Z.; Islam, S.; She, M.; Diepeveen, D.; Zhang, Y.; Tang, G.; Zhang, J.; Juhasz, A.; Yang, R.; Ma, W. Wheat grain protein accumulation and polymerization mechanisms driven by nitrogen fertilization. Plant J. 2018, 96, 1160–1177. [Google Scholar] [CrossRef] [PubMed]
- Araya-Flores, J.; Guzmán, C.; Matus, I.; Parada, R.; Jarpa, G.; de Camargo, A.C.; Shahidi, F.; Schwember, A.R. New Findings in the Amino Acid Profile and Gene Expression in Contrasting Durum Wheat Gluten Strength Genotypes during Grain Filling. J. Agric. Food Chem. 2020, 68, 5521–5528. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sadras, V.O.; Lu, G.; Zhang, P.; Han, Y.; Liu, L.; Xie, J.; Yang, X.; Zhang, S. A global meta-analysis of split nitrogen application for improved wheat yield and grain protein content. Soil Tillage Res. 2021, 213, 105111. [Google Scholar] [CrossRef]
- Hamany Djande, C.Y.; Pretorius, C.; Tugizimana, F.; Piater, L.A.; Dubery, I.A. Metabolomics: A tool for cultivar phenotyping and investigation of grain crops. Agronomy 2020, 10, 831. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Protein content prediction in single wheat kernels using hyperspectral imaging. Food Chem. 2018, 240, 32–42. [Google Scholar] [CrossRef]
- Joye, I. Protein digestibility of cereal products. Foods 2019, 8, 199. [Google Scholar] [CrossRef]
- Reeds, P.J. Dispensable and indispensable amino acids for humans. J. Nutri. 2000, 130, 1835–1840. [Google Scholar] [CrossRef]
- FAO/WHO. Protein Quality Evaluation: Report of a Joint FAO-WHO Expert Consultation; FAO: Rome, Italy, 1991; Volume 51. [Google Scholar]
- Chernova, E.; Bazhenova, I.; Bazhenova, T. Development of the composition of cereal dishes of higher biological value. BIO Web Conf. EDP Sci. 2021, 29, 01022. [Google Scholar] [CrossRef]
- Radzka, E.; Jankowska, J. Effect of hydrothermal conditions on spring wheat yield in central-eastern Poland (1975–2005). Acta Agroph. 2015, 22, 269–277. (In Polish) [Google Scholar]
- Jolánkai, M.; Kassai, M.K.; Tarnawa, Á.; Pósa, B.; Birkás, M. Impact of precipitation and temperature on the grain and protein yield of wheat (Triticum aestivum L.) varieties. J. Hung. Meteorol. Serv. 2018, 122, 31–40. [Google Scholar] [CrossRef]
- Rachoń, L.; Woźniak, A. Variability of spring durum and common wheat yields in the decade 2009-2018 in the Lublin region. Agron. Sci. 2020, 75, 67–74. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Gromadzka, K.; Dziki, D. Milling and Baking Quality of Spring Wheat (Triticum aestivum L.) from Organic Farming. Agriculture 2021, 11, 765. [Google Scholar] [CrossRef]
- Spychaj, R.; Gil, Z.; Chrzanowska-Dróżdż, B. Technological value of winter durum wheat cv. Komnata as dependent on chemical plant protection. Biul. IHAR 2011, 262, 25–38. (In Polish) [Google Scholar]
- Kulyk, M.I.; Rozhkov, A.O.; Kalinichenko, O.V.; Taranenko, A.O.; Onopriienko, O.V. Effect of winter wheat variety, hydrothermal coefficient (HTC) and thousand kernel weight (TKW) on protein content, grain and protein yield. Agron. Res. 2020, 18, 2103–2116. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Y.; Shi, Z.; Rentsch, D.; Ward, J.L.; Hassall, K.; Hawkesford, M.J. Wheat amino acid transporters highly expressed in grain cells regulate amino acid accumulation in grain. PLoS ONE 2021, 16, e0246763. [Google Scholar] [CrossRef]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef]
- Buczek, J.; Borrecka-Jarmo, D.; Jarecki, W. Yield and quality of grain of selected spring wheat cultivars depending on the dose and the of nitrogen application. Fragm. Agron. 2011, 28, 7–15. (In Polish) [Google Scholar]
- Lollato, R.P.; Jaenisch, B.R.; Silva, S.R. Genotype-specific nitrogen uptake dynamics and fertilizer management explain contrasting wheat protein concentration. Crop Sci. 2021, 61, 2048–2066. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative study on gluten protein composition of ancient (einkorn, emmer and spelt) and modern wheat species (durum and common wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef]
- Giancaspro, A.; Giove, S.L.; Zacheo, S.A.; Blanco, A.; Gadaleta, A. Genetic variation for protein content and yield-related traits in a durum population derived from an inter-specific cross between hexaploid and tetraploid wheat cultivars. Front. Plant Sci. 2019, 10, 1509. [Google Scholar] [CrossRef]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.B.; Pozniak, C.; Fahima, T.; Krugman, T. Grain protein content and thousand kernel weight QTLs identified in a durum× wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2020, 133, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Besaliev, I.N.; Panfilov, A.L.; Karavaytsev, Y.A.; Reger, N.S.; Kholodilina, T.N. Content of prolin and essential amino acids in spring wheat grain in dry conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012116. [Google Scholar] [CrossRef]
- Hospodarenko, H.; Karpenko, V.; Liubych, V.; Novikov, V. Characterization of amino acid content of grain of new wheat cultivars and lines. Agric. Sci. Pract. 2018, 5, 12–18. [Google Scholar] [CrossRef]
- Johansson, E.; Branlard, G.; Cuniberti, M.; Flagella, Z.; Hüsken, A.; Nurit, E.; Peña, R.J.; Sissons, M.; Vazquez, D. Genotypic and Environmental Effects on Wheat Technological and Nutritional Quality. In Wheat Quality for Improving Processing and Human Health; Springer: Cham, Switzerland, 2020; pp. 171–204. [Google Scholar]
- Spychaj-Fabisiak, E.; Barczak, B.; Nowak, K.; Jagielski, J. Amino acid composition of winter wheat grain protein depending on the seed certification class and on the cultivar. Rom. Agric. Res. 2014, 31, 89–94. [Google Scholar]
- Jaśkiewicz, B.; Szczepanek, M. Amino acids content in triticale grain depending on meteorological, agro-technical and genetic factors. Agric. Sci. Crop Sci. Animal Sci. Res. Rural Develop. 2018, 2, 28–34. [Google Scholar] [CrossRef]
- Dvořáćek, V.; Čurn, V.; Moudrý, J. Evaluation of Amino Acid Content and composition in Spelt Wheat Varieties. Cereale Res. Commun. 2002, 30, 187–193. [Google Scholar] [CrossRef]
- Knezević, D.S.; Djukic, N.; Paunovic, A.; Madic, M. Amino acid contents in grain of different winter wheat (Triticum aestivum L.) varieties. Cereal Res. Commun. 2009, 37, 647–650. [Google Scholar] [CrossRef]
- Biel, W.; Maciorowski, R. Assessing nutritional value of grains of selected wheat cultivars. Żywn Nauka Technol. Jakość 2012, 2, 45–55. (In Polish) [Google Scholar]
- Majcherczak, E.; Cwojdziński, W.; Nowak, K. Effect of increasing nitrogen fertilisation on the amino acid composition in winter barley grain protein. Acta Sci. Pol. Agric. 2003, 2, 114–118. [Google Scholar]
- Barczak, B.; Nowak, K. Content of amino acids of winter barley (Hordeum vulgare L.) biomas depending on the plant growth stage and nitrogen fertilization. Acta. Sci. Pol. Agric. 2008, 7, 3–15. [Google Scholar]
- Crista, F.; Radulov, I.; Crista, L.; Berbecea, A.; Lato, A. Influence of mineral fertilization on the amino acid content and raw protein of wheat grain. J. Food Agric. Environ. 2012, 10, 47–50. [Google Scholar]
- Crista, F.; Radulov, I.; Sala, F.; Crista, L.; Berbecea, A. Influence of NPK fertilizers upon winter wheat grain quality. Res. J. Agric. Sci. 2012, 44, 30–35. [Google Scholar]
- Gondek, K.; Kopec, M.; Glab, T. The contents of microelements and exogenous amino acids in spring wheat (Triticum aestivum L.) grain after municipal sewage sludge fertilization. J. Agric. Sci. 2012, 4, 294–303. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, D.; Wang, C.; Zhao, H.; Zhu, Y.; Guo, T. Responses of Amino Acid Composition to Nitrogen Application in High-and Low-Protein Wheat Cultivars at Two Planting Environments. Crop Sci. 2016, 56, 1277–1287. [Google Scholar] [CrossRef]
- Isaychev, V.; Andreev, N.; Mudarisov, F. Influence of macro and microelements on the biological value of wheat grain. IOP Conf. Ser. Environ. Earth Sci. 2021, 937, 022130. [Google Scholar] [CrossRef]
- Nowak, K.; Spychaj-Fabisiak, E.; Barczak, B.; Knapowski, T.; Kozera, W. Preliminary studies of the amino acid composition of spelled grain protein, fertilization with nitrogen and microelements. Inż. Ap. Chem. 2015, 54, 265–266. [Google Scholar]
- Biel, W.; Hury, G.; Maciorowski, R.; Kotlarz, A.; Jaśkowska, I. Effect of nitrogen fertilization on chemical composition of spelt wheat (Triticum aestivum ssp. spelta L.) two varieties. Acta Sci. Pol. Zootech. 2010, 9, 5–14. [Google Scholar]
- Ralcewicz, M.; Knapowski, T. The effect of some agrotechnical factors on grain yield and amino acid composition of protein of oat. Biul. IHAR 2006, 239, 193–204. [Google Scholar]
- Wiater, J.; Kozera, P. Effect of organic and mineral fertilization on the content and amino acid composition of winter wheat protein. Zesz. Probl. Post. Nauk Roln. 2010, 111, 85–97. [Google Scholar]
- Kowieska, A.; Jaskowska, A.; Lipiński, P. Carbohydrate fraction and amino acids content in wheat grain in two consequent years. Acta Sci. Pol. Zootechnica 2010, 4, 135–145. [Google Scholar]
- Biel, W.; Bobko, K.; Maciorowski, R. Chemical composition and nutritive value of husked and naked oats grain. J. Cer. Sci. 2009, 49, 413–418. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, G.; Wang, C.; Lu, H.; Li, S.; Xie, Y.; Guo, T. Effect of irrigation and nitrogen application on grain amino acid composition and protein quality in winter wheat. PLoS ONE 2017, 12, e0178494. [Google Scholar] [CrossRef] [PubMed]
- Barczak, B.; Cwojdzinski, W.; Nowak, K. Influence of increasing of nitrogen on the yield of winter barley grain and protein. Zesz. Probl. Post. Nauk Roln. 1994, 414, 235–244. (In Polish) [Google Scholar]
- AACC. AACC. AACC Method 46-11.02: Crude Protein–Improved Kjeldahl Method, Copper Catalyst Modyfication. In Official Methods of the American Association of Cereal Chemists, 11th ed.; AACC: St. Paul, MN, USA, 2010; p. 39. [Google Scholar]
- Szkudzińska, K.; Smutniak, I.; Rubaj, J.; Korol, W.; Bielecka, G. Method validation for determination of amino acids in feed by UPLC. Accred. Qual. Assur. 2017, 22, 247–252. [Google Scholar] [CrossRef]
- Tome, D. Criteria and markers for protein quality assessment—A review. Br. J. Nutr. 2012, 108, S222–S229. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Energy and Protein Requirements. Report of a Joint FAO-WHO Nutritional Meeting; Technical Report; FAO/WHO/UNU: Geneva, Switzerland, 1985. [Google Scholar]

| Production Technology | S * | K ** | S | K | S | K | S | K | S | K | S | K | S | K | S | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endogenous Amino Acids | ||||||||||||||||
| Ser *** | Asp | Glu | Pro | Gly | Ala | Tyr | Cys | |||||||||
| Integrated | 5.56 b **** | 4.88 a | 5.36 b | 4.79 a | 38.77 b | 31,87 a | 12.20 b | 9.39 a | 3.80 b | 3.63 a | 3.56 b | 3.35 a | 2.10 a | 1.80 a | 2.74 a | 2.29 a |
| Intensive | 6.82 a | 4.07 b | 6.69 a | 4.88 a | 50.50 a | 32.49 a | 15.11 a | 9.63 a | 4.50 a | 3.76 a | 4.65 a | 3.24 a | 2.71 a | 1.84 a | 3.16 a | 2.45 a |
| Production Technology | S * | K ** | S | K | S | K | S | K | S | K | S | K | S | K | S | K | S | K | S | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exogenous Amino Acids | ||||||||||||||||||||
| Thr *** | Val | Ile | Leu | Phe | His | Lys | Arg | Met | Trp | |||||||||||
| Integrated | 3.28 b **** | 3.05 a | 4.31 b | 3.40 a | 4.03 b | 3.48 a | 7.34 b | 5.48 b | 5.19 a | 4.81 a | 2.34 a | 2.19 a | 2.45 b | 2.26 a | 4.41 a | 3.73 a | 2.99 a | 2.36 b | 1.4 a | 1.3 a |
| Intensive | 3.65 a | 2.99 a | 5.30 a | 3.63 a | 5.46 a | 3.58 a | 8.96 a | 6.30 a | 6.03 a | 4.37 a | 2.57 a | 2.51 a | 3.15 a | 2.16 a | 5.24 a | 4.05 a | 3.28 a | 2.83 a | 1.5 a | 1.4 a |
| Amino Acid | FAO/WHO * Amino Acid Composition of the Egg White (mg g−1) | CS (%) | |||
|---|---|---|---|---|---|
| SMH87 (Durum Wheat) | Kandela (Common Wheat) | ||||
| Integrated | Intensive | Integrated | Intensive | ||
| Isoleucine | 3.01 * | 135 | 180 | 115 | 119 |
| Leucine | 5.30 | 140 | 169 | 118 | 119 |
| Lysine | 4.50 | 54 | 70 | 53 | 48 |
| Methionine + cysteine | 2.21 | 259 | 291 | 210 | 217 |
| Tyrosine + phenylalanate | 3.81 | 191 | 236 | 160 | 163 |
| Threonine | 2.30 | 143 | 159 | 152 | 130 |
| Tryptophan | 0.61 | 238 | 248 | 220 | 230 |
| Valina | 3.9 | 111 | 136 | 88 | 93 |
| Cultivar | SMH87 (Durum Wheat) | Kandela (Common Wheat) | ||
|---|---|---|---|---|
| Production technology | integrated | intensive | integrated | intensive |
| EAAI MH | 93.9 | 96.8 | 88.7 | 91.6 |
| EAAI WE | 62.6 | 64.5 | 59.1 | 61.1 |
| Production Technology | Fertilization (kg ha−1) | Herbicides | Fungicides | Insecticides | Retardants | ||
|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | |||||
| Integrated | 110 * | 70 ** | 105 ** | Mustang 306 SE (florasulan) 0.6 L ha−1, Axial 100 EC (pinoxaden) 0.4 L ha−1 | Input 460 EC (prothioconazole, spiroxamine) 1.0 L ha−1 | Fury 100 EW (zeta-cypermethrin) 0.1 L ha−1 | - |
| Intensive | 140 * | 80 ** | 100 ** | Mustang 306 SE (florasulan) 0.6 L ha−1, Axial 100 EC (pinoxaden) 0.4 L ha−1 | Amistar 250 SC (azoxystrobin) 0.6 L ha−1 Artea 330 EC (propiconazole + cyproconazole) 0.5 L ha−1 | Fury 100 EW (zeta-cypermethrin) 0.1 L ha−1 | Modus 250 EW (ethyl trinexapac) 0.4 L ha−1 |
| Column Temperature | 25 °C |
|---|---|
| Moving phase | A mixture of 3.00 g acetic acid, 900 mL distilled water and 50.0 mL of 1,1,1-trichloro-2-methyl-2-propanol solution. The mixture was brought to pH 5 using sodium hydroxide solution. The pH value was controlled with a pH meter. The mixture was then made up to 1 L with distilled water. |
| Flow rate | 1 mL min−1 |
| Detection wavelength | Excitation: = 280 nm, emission: = 356 nm, |
| Volume to be dosed | 20 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułek, A.; Cacak-Pietrzak, G.; Różewicz, M.; Nieróbca, A.; Grabiński, J.; Studnicki, M.; Sujka, K.; Dziki, D. Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain. Plants 2023, 12, 364. https://doi.org/10.3390/plants12020364
Sułek A, Cacak-Pietrzak G, Różewicz M, Nieróbca A, Grabiński J, Studnicki M, Sujka K, Dziki D. Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain. Plants. 2023; 12(2):364. https://doi.org/10.3390/plants12020364
Chicago/Turabian StyleSułek, Alicja, Grażyna Cacak-Pietrzak, Marcin Różewicz, Anna Nieróbca, Jerzy Grabiński, Marcin Studnicki, Katarzyna Sujka, and Dariusz Dziki. 2023. "Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain" Plants 12, no. 2: 364. https://doi.org/10.3390/plants12020364
APA StyleSułek, A., Cacak-Pietrzak, G., Różewicz, M., Nieróbca, A., Grabiński, J., Studnicki, M., Sujka, K., & Dziki, D. (2023). Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain. Plants, 12(2), 364. https://doi.org/10.3390/plants12020364







