Reproduction Modes and Conservation Implications in Three Polyploid Sorbus Stenoendemics in Eastern Slovakia (Central Europe)
Abstract
:1. Introduction
2. Results
2.1. Flow Cytometric Determination of Ploidy Level of Mature Trees
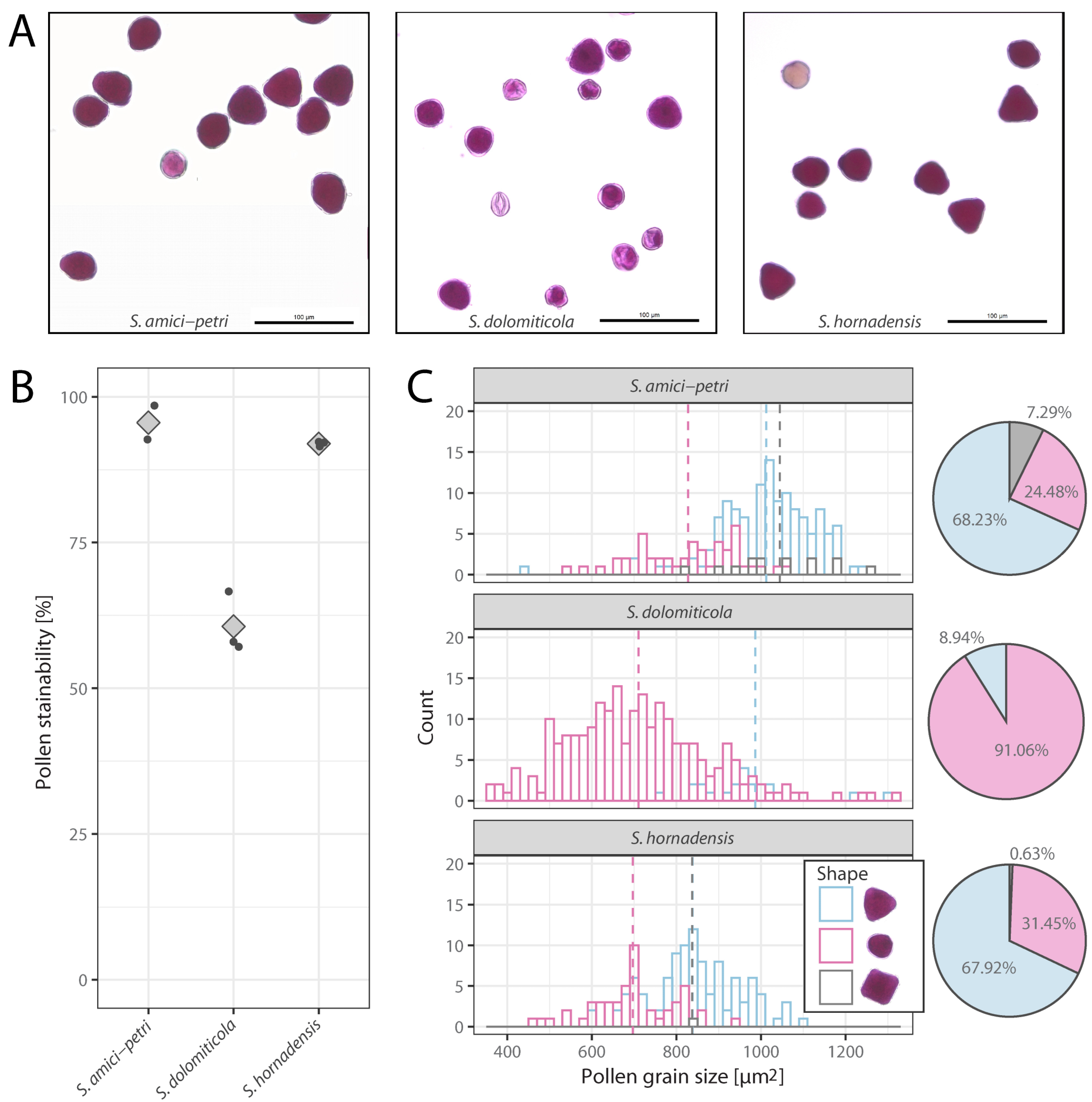
2.2. Pollen Stainability and Pollen Size
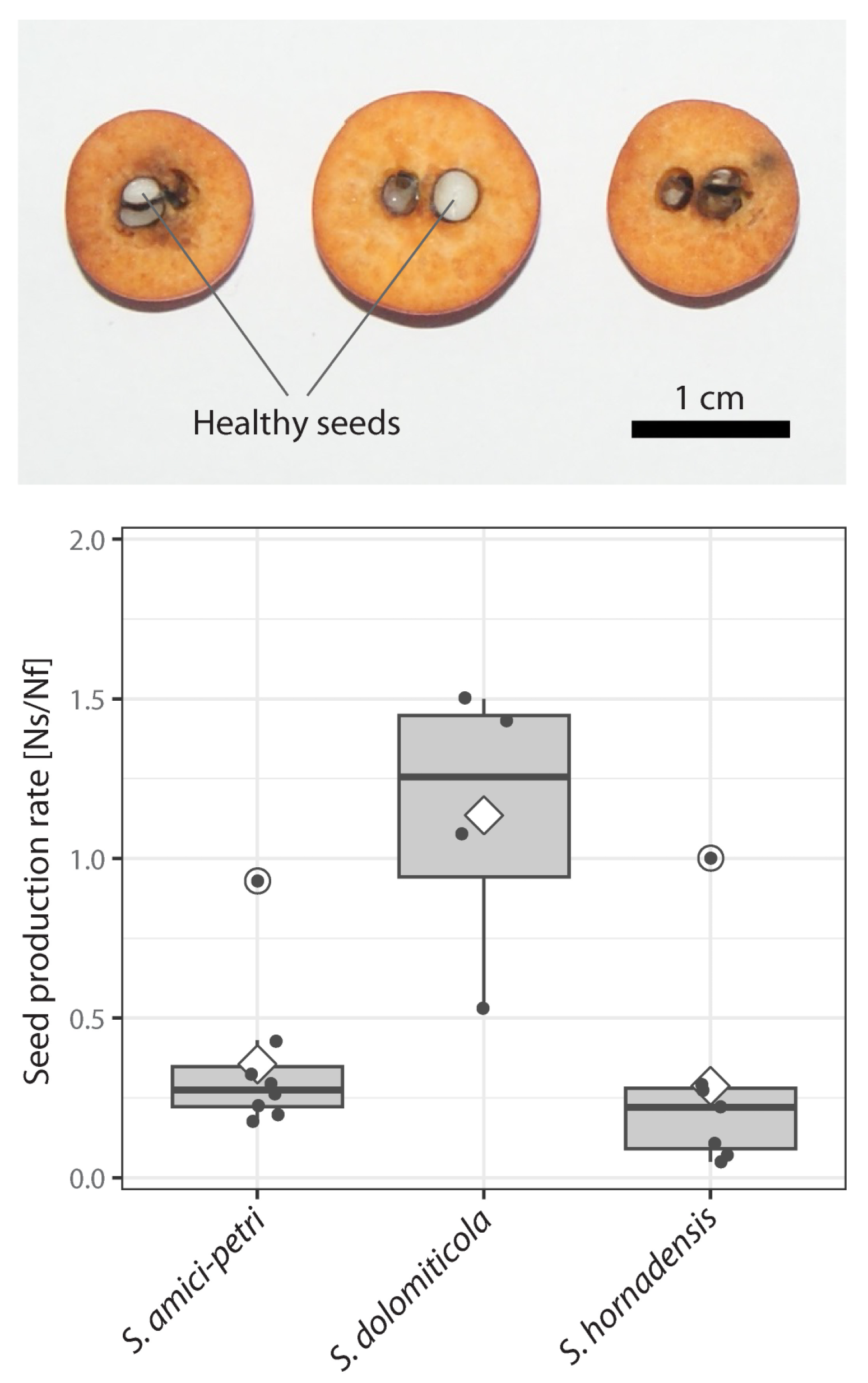
2.3. Seed Production Rate
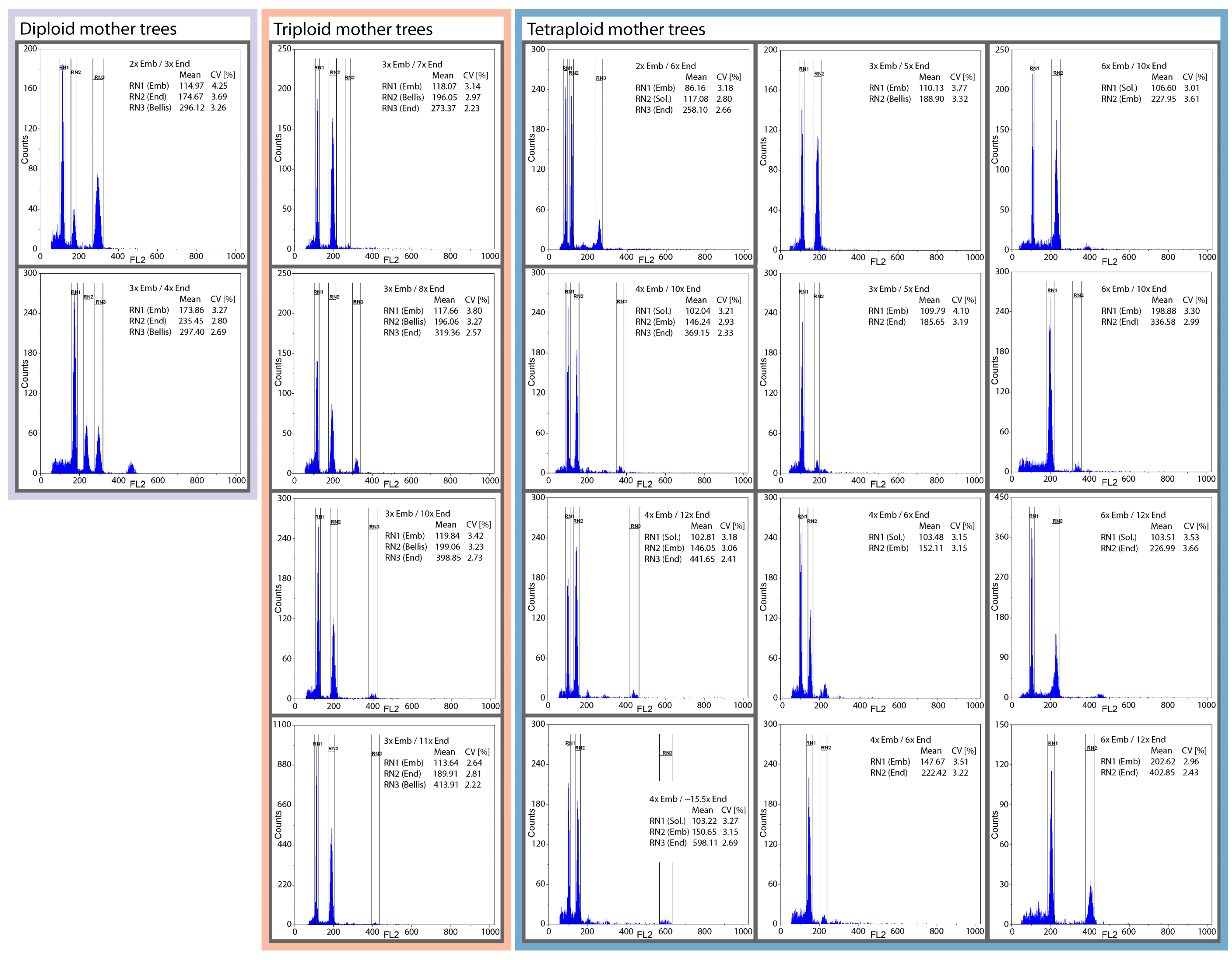
2.4. Flow Cytometric Seed Screen
2.5. Reproduction Modes in S. amici-petri, S. dolomiticola, and S. hornadensis
3. Discussion
3.1. Reproduction Characteristics and Variation of Tetraploid S. amici-petri and S. hornadensis
3.2. Dependence of Seed Production of Apomict S. dolomiticola on Heterospecific Pollen and Conservation Implications
3.3. Microevolutionary Dynamics in Mixed-Cytotype Populations in Stredné Pohornádie Valley
4. Materials and Methods
4.1. Plant Material
4.2. Pollen Stainability and Pollen Grain Size Determination
4.3. Seed Production Rate
4.4. Flow Cytometric Determination of the Ploidy Level of Mother Plant, Embryo, and Endosperm
4.5. Examination of Reproduction Modes
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faure, J.-E. Double Fertilization in Flowering Plants: Discovery, Study Methods and Mechanisms. Comptes Rendus De L’académie Des Sci. -Ser. III-Sci. De La Vie 2001, 324, 551–558. [Google Scholar] [CrossRef]
- Raghavan, V. Some Reflections on Double Fertilization, from Its Discovery to the Present. New Phytol. 2003, 159, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H. Major Evolutionary Transitions in Flowering Plant Reproduction: An Overview. Int. J. Plant Sci. 2008, 169, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Maheswari, P. An Introduction to the Embryology of Angiosperms; McGraw-Hill: New York, NY, USA, 1950. [Google Scholar]
- Kolarčik, V.; Kocová, V.; Vašková, D. Flow Cytometric Seed Screen Data Are Consistent with Models of Chromosome Inheritance in Asymmetrically Compensating Allopolyploids. Cytom. Part A 2018, 93, 737–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savidan, Y. Gametophytic Apomixis. In Current Trends in the Embryology of Angiosperms; Bhojwani, S.S., Soh, W.-Y., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2001; pp. 419–433. [Google Scholar]
- Normark, B.B. 13-Unusual Gametic and Genetic Systems. In Sperm Biology an Evolutionary Perspective; Birkhead, T.R., Hosken, D.J., Pitnick, S., Eds.; Academic Press: London, UK, 2009; pp. 507–538. ISBN 978-0-12-372568-4. [Google Scholar]
- Vallejo-Marín, M.; Dorken, M.; Barrett, S.C.H. The Ecological and Evolutionary Consequences of Clonality for Plant Mating. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Barrett, S.C.H. Influences of Clonality on Plant Sexual Reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 8859–8866. [Google Scholar] [CrossRef] [Green Version]
- De Meeûs, T.; Prugnolle, F.; Agnew, P. Asexual Reproduction: Genetics and Evolutionary Aspects. Cell. Mol. Life Sci. 2007, 64, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Ozias-Akins, P. Apomixis: Developmental Characteristics and Genetics. Crit. Rev. Plant Sci. 2006, 25, 199–214. [Google Scholar] [CrossRef]
- Vozárová, R.; Herklotz, V.; Kovařík, A.; Tynkevich, Y.; Volkov, R.; Ritz, C.; Lunerová, J. Ancient Origin of Two 5S RDNA Families Dominating in the Genus Rosa and Their Behavior in the Canina-Type Meiosis. Front. Plant Sci. 2021, 12, 643548. [Google Scholar] [CrossRef] [PubMed]
- Pellino, M.; Hojsgaard, D.; Schmutzer, T.; Scholz, U.; Hörandl, E.; Vogel, H.; Sharbel, T. Asexual Genome Evolution in the Apomictic Ranunculus auricomus Complex: Examining the Effects of Hybridization and Mutation Accumulation. Mol. Ecol. 2013, 22, 5908–5921. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard, D.; Hörandl, E. A Little Bit of Sex Matters for Genome Evolution in Asexual Plants. Front. Plant Sci. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojsgaard, D.; Klatt, S.; Baier, R.; Carman, J.; Hörandl, E. Taxonomy and Biogeography of Apomixis in Angiosperms and Associated Biodiversity Characteristics. Crit. Rev. Plant Sci. 2014, 33, 414–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sennikov, A.; Kurtto, A. A Phylogenetic Checklist of Sorbus s.l. (Rosaceae) in Europe. Memo. Soc. Pro Fauna Flora Fenn. 2017, 93, 1–78. [Google Scholar]
- Ludwig, S.; Robertson, A.; Rich, T.; Đorđević, M.; Cerović, R.; Houston, L.; Harris, S.; Hiscock, S. Breeding Systems, Hybridization and Continuing Evolution in Avon Gorge Sorbus. Ann. Bot. 2013, 111, 563–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajrudinović, A.; Siljak-Yakovlev, S.; Brown, S.C.; Pustahija, F.; Bourge, M.; Ballian, D.; Bogunić, F. When Sexual Meets Apomict: Genome Size, Ploidy Level and Reproductive Mode Variation of Sorbus aria s.l. and S. austriaca (Rosaceae) in Bosnia and Herzegovina. Ann. Bot. 2015, 116, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepší, M.; Lepší, P.; Koutecký, P.; Bílá, J.; Vít, P. Taxonomic Revision of Sorbus Subgenus Aria Occurring in the Czech Republic. Preslia 2015, 87, 109–162. [Google Scholar]
- Lepší, M.; Koutecký, P.; Nosková, J.; Lepší, P.; Urfus, T.; Rich, T.C.G. Versatility of Reproductive Modes and Ploidy Level Interactions in Sorbus s.l. (Malinae, Rosaceae). Bot. J. Linn. Soc. 2019, 191, 502–522. [Google Scholar] [CrossRef]
- Robertson, A.; Rich, T.C.G.; Allen, A.M.; Houston, L.; Roberts, C.A.T.; Bridle, J.O.N.R.; Harris, S.A.; Hiscock, S.J. Hybridization and Polyploidy as Drivers of Continuing Evolution and Speciation in Sorbus. Mol. Ecol. 2010, 19, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Uhrinová, V.; Zozomová-Lihová, J.; Bernátová, D.; Paule, J.; Paule, L.; Gömöry, D. Origin and Genetic Differentiation of Pink-Flowered Sorbus Hybrids in the Western Carpathians. Ann. Bot. 2017, 120, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Chester, M.; Cowan, R.S.; Fay, M.F.; Rich, T.C.G. Parentage of Endemic Sorbus L. (Rosaceae) Species in the British Isles: Evidence from Plastid DNA. Bot. J. Linn. Soc. 2007, 154, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Liljefors, A. Studies on Propagation, Embryology, and Pollination in Sorbus. Acta Horti Bergiani 1953, 16, 277–329. [Google Scholar]
- Jankun, A.; Kovanda, M. Apomixis in Sorbus sudetica (Embryological Studies in Sorbus 1). Preslia 1986, 58, 7–19. [Google Scholar]
- Jankun, A.; Kovanda, M. Apomixis and Origin of Sorbus bohemica (Embryological Studies in Sorbus 2). Preslia 1987, 59, 97–116. [Google Scholar]
- Hojsgaard, D.; Greilhuber, J.; Pellino, M.; Paun, O.; Sharbel, T.F.; Hörandl, E. Emergence of Apospory and Bypass of Meiosis via Apomixis after Sexual Hybridisation and Polyploidisation. New Phytol. 2014, 204, 1000–1012. [Google Scholar] [CrossRef] [Green Version]
- Vít, P.; Lepší, M.; Lepší, P. There Is No Diploid Apomict among Czech Sorbus Species: A Biosystematic Revision of S. eximia and Discovery of S. barrandienica. Preslia 2013, 84, 71–96. [Google Scholar]
- Somlyay, L.; Sennikov, A. Atlas Florae Europaeae Notes 23. The Typification and Revised Taxonomic Circumscription of Sorbus bakonyensis (Rosaceae), with a Description of Sorbus udvardyana, a New Apomictic Species Endemic to Hungary. Phytotaxa 2014, 164, 265–275. [Google Scholar] [CrossRef]
- Hajrudinović, A.; Frajman, B.; Schönswetter, P.; Silajdžić, E.; Siljak-Yakovlev, S.; Bogunić, F. Towards a Better Understanding of Polyploid Sorbus (Rosaceae) from Bosnia and Herzegovina (Balkan Peninsula), Including Description of a Novel, Tetraploid Apomictic Species. Bot. J. Linn. Soc. 2015, 178, 670–685. [Google Scholar] [CrossRef] [Green Version]
- Somlyay, L.; Lisztes-Szabó, Z.; Sennikov, A.N. Atlas Florae Europaeae Notes 29. Two New Species of Sorbus (Rosaceae) Endemic to Hungary, Previously Confused with S. subdanubialis. Ann. Bot. Fenn. 2016, 53, 361–372. [Google Scholar] [CrossRef]
- Levin, J.; Fay, M.F.; Pellicer, J.; Hedrén, M. Multiple Independent Origins of Intermediate Species between Sorbus aucuparia and S. hybrida (Rosaceae) in the Baltic Region. Nord. J. Bot. 2018, 36, e02035. [Google Scholar] [CrossRef]
- Velebil, J.; Lepší, M.; Nosková, J.; Lepší, P. Taxonomic Assessment of Sorbus Subgenus Aria in the Malé Karpaty Mountains. Preslia 2022, 94, 305–334. [Google Scholar] [CrossRef]
- Mikoláš, V. Sorbus dolomiticola Mikoláš, a New Hybridogenous Species of the Genus Sorbus s.l. from Eastern Slovakia. Thaiszia J. Bot. 1996, 6, 1–12. [Google Scholar]
- Mikoláš, V. Sorbus amici-petri Mikoláš, a New Hybridogenous Species of the Genus Sorbus s.l. from Eastern Slovakia. Thaiszia J. Bot. 2003, 13, 127–133. [Google Scholar]
- Mikoláš, V. Sorbus hornadensis Mikoláš (Rosaceae, Pyreae), a New Hybridogenous Species from Eastern Slovakia. Thaiszia J. Bot. 2015, 25, 21–27. [Google Scholar]
- Rivers, M.; Beech, E.; Bazos, I.; Bogunić, F.; Buira, A.; Caković, D.; Carapeto, A.; Carta, A.; Cornier, B.; Fenu, G.; et al. European Red List of Trees; IUCN: Cambridge, UK; Brussels, Belgium, 2019. [Google Scholar]
- Hamston, T.J.; Wilson, R.J.; de Vere, N.; Rich, T.C.G.; Stevens, J.R.; Cresswell, J.E. Breeding System and Spatial Isolation from Congeners Strongly Constrain Seed Set in an Insect-Pollinated Apomictic Tree: Sorbus subcuneata (Rosaceae). Sci. Rep. 2017, 7, 45122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talent, N.; Dickinson, T. Endosperm Formation in Aposporous Crataegus (Rosaceae, Spiraeoideae, Tribe Pyreae): Parallels to Ranunculaceae and Poaceae. New Phytol. 2007, 173, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Matzk, F.; Meister, A.; Schubert, I. An Efficient Screen for Reproductive Pathways Using Mature Seeds of Monocots and Dicots. Plant J. 2000, 21, 97–108. [Google Scholar] [CrossRef]
- Matzk, F.; Meister, A.; Brutovská, R.; Schubert, I. Reconstruction of Reproductive Diversity in Hypericum perforatum L. Opens Novel Strategies to Manage Apomixis. Plant J. 2001, 26, 275–282. [Google Scholar] [CrossRef]
- Dobeš, C.; Lückl, A.; Hülber, K.; Paule, J. Prospects and Limits of the Flow Cytometric Seed Screen—Insights from Potentilla Sensu Lato (Potentilleae, Rosaceae). New Phytol. 2013, 198, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, O.; Mazzucato, A. Screening Procedures to Identify and Quantify Apomixis. In The Flowering of Apomixis: From Mechanisms to Genetic Engineering; Savidan, Y., Carman, J.G., Dresselhaus, T., Eds.; CIMMYT, IRD, European Commission DG VI (FAIR): Mexico City, Mexico, 2001; pp. 121–136. [Google Scholar]
- Matzk, F. Reproduction Mode Screening. In Flow Cytometry with Plant Cells; Doležel, J., Greilhuber, J., Suda, J., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2007; pp. 131–152. ISBN 9783527610921. [Google Scholar]
- Hojsgaard, D.; Hörandl, E. The Rise of Apomixis in Natural Plant Populations. Front. Plant Sci. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Talent, N.; Dickinson, T.A. The Potential for Ploidy Level Increases and Decreases in Crataegus (Rosaceae, Spiraeoideae, Tribe Pyreae). Can. J. Bot. 2007, 85, 570–584. [Google Scholar] [CrossRef] [Green Version]
- Burgess, M.B.; Cushman, K.R.; Doucette, E.T.; Talent, N.; Frye, C.T.; Campbell, C.S. Effects of Apomixis and Polyploidy on Diversification and Geographic Distribution in Amelanchier (Rosaceae). Am. J. Bot. 2014, 101, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Macková, L.; Nosková, J.; Ďurišová, L.; Urfus, T. Insights into the Cytotype and Reproductive Puzzle of Cotoneaster integerrimus in the Western Carpathians. Plant Syst. Evol. 2020, 306, 58. [Google Scholar] [CrossRef]
- Bogunić, F.; Siljak-Yakovlev, S.; Mahmutović-Dizdarević, I.; Hajrudinović-Bogunić, A.; Bourge, M.; Brown, S.C.; Muratović, E. Genome Size, Cytotype Diversity and Reproductive Mode Variation of Cotoneaster integerrimus (Rosaceae) from the Balkans. Plants 2021, 10, 2798. [Google Scholar] [CrossRef]
- Kolarčik, V.; Kocová, V.; Mikoláš, V.; Mártonfiová, L.; Hajdučeková, N.; Mártonfi, P. Variability of Reproduction Pathways in the Central-European Populations of Hawthorns with Emphasis on Triploids. Plants 2022, 11, 3497. [Google Scholar] [CrossRef]
- Aliyu, O.M.; Schranz, M.E.; Sharbel, T.F. Quantitative Variation for Apomictic Reproduction in the Genus Boechera (Brassicaceae). Am. J. Bot. 2010, 97, 1719–1731. [Google Scholar] [CrossRef]
- Vašková, D.; Kolarčik, V. Breeding Systems in Diploid and Polyploid Hawthorns (Crataegus): Evidence from Experimental Pollinations of C. monogyna, C. subsphaerica, and Natural Hybrids. Forests 2019, 10, 1059. [Google Scholar] [CrossRef] [Green Version]
- Comai, L. The Advantages and Disadvantages of Being Polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Hamston, T.; de Vere, N.; King, A.; Pellicer, J.; Fay, M.; Cresswell, J.; Stevens, J. Apomixis and Hybridization Drives Reticulate Evolution and Phyletic Differentiation in Sorbus L.: Implications for Conservation. Front. Plant Sci. 2018, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Marvier, M.A.; Smith, D.L. Conservation Implications of Host Use for Rare Parasitic Plants. Conserv. Biol. 1997, 11, 839–848. [Google Scholar] [CrossRef]
- Moir, M.; Coates, D.; Kennington, W.; Barrett, S.R.; Taylor, G. Concordance in Evolutionary History of Threatened Plant and Insect Populations Warrant Unified Conservation Management Approaches. Biol. Conserv. 2016, 198, 135–144. [Google Scholar] [CrossRef]
- Kolarčik, V.; (Pavol Jozef Šafárik University, Košice, Slovakia); Mikoláš, V.; (independent researcher, Košice, Slovakia). Microevolutionary Processes in Mixed-Cytotype Population of Sexuals and Pseudogamous Apomicts: A Case Study of the Genus Sorbus. 2022; unpublished work. [Google Scholar]
- Futák, J. Fytogeografické Členenie Slovenska. In Flóra Slovenska IV/1; Bertová, L., Ed.; Veda: Bratislava, Slovakia, 1984; pp. 418–420. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA, 2022. [Google Scholar]
- Kearns, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University Press of Colorado: Niwot, CO, USA, 1993. [Google Scholar]
- Ross, P.; Slovin, J.P.; Chen, C. A Simplifed Method for Differential Staining of Aborted and Non-Aborted Pollen Grains. Int. J. Plant Biol. 2010, 1, e13. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, F.J. The Tps Series of Software. Hystrix Ital. J. Mammal. 2015, 26, 9–12. [Google Scholar] [CrossRef]
- Suda, J.; Kron, P.; Husband, B.C.; Trávníček, P. Flow Cytometry and Ploidy: Applications in Plant Systematics, Ecology and Evolutionary Biology. In Flow Cytometry with Plant Cells; Doležel, J., Greilhuber, J., Suda, J., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2007; pp. 103–130. [Google Scholar]
- Doležel, J.; Göhde, W. Sex Determination in Dioecious Plants Melandrium album and M. rubrum Using High-Resolution Flow Cytometry. Cytometry 1995, 19, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Doležel, J. Flow Cytometric Analysis of Nuclear DNA Content in Higher Plants. Phytochem. Anal. 1991, 2, 143–154. [Google Scholar] [CrossRef]
- Greilhuber, J.; Temsch, E.M.; Loureiro, J.C.M. Nuclear DNA Content Measurement. In Flow Cytometry with Plant Cells; Doležel, J., Greilhuber, J., Suda, J., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2007; pp. 67–101. [Google Scholar]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of Three DNA Fluorochromes for Flow Cytometric Estimation of Nuclear DNA Content in Plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Schönswetter, P.; Suda, J.; Popp, M.; Weiss-Schneeweiss, H.; Brochmann, C. Circumpolar Phylogeography of Juncus biglumis (Juncaceae) Inferred from AFLP Fingerprints, CpDNA Sequences, Nuclear DNA Content and Chromosome Numbers. Mol. Phylogenet. Evol. 2007, 42, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santos, C. Two New Nuclear Isolation Buffers for Plant DNA Flow Cytometry: A Test with 37 Species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Pellicer, J.; Clermont, S.; Houston, L.; Rich, T.C.G.; Fay, M.F. Cytotype Diversity in the Sorbus Complex (Rosaceae) in Britain: Sorting out the Puzzle. Ann. Bot. 2012, 110, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

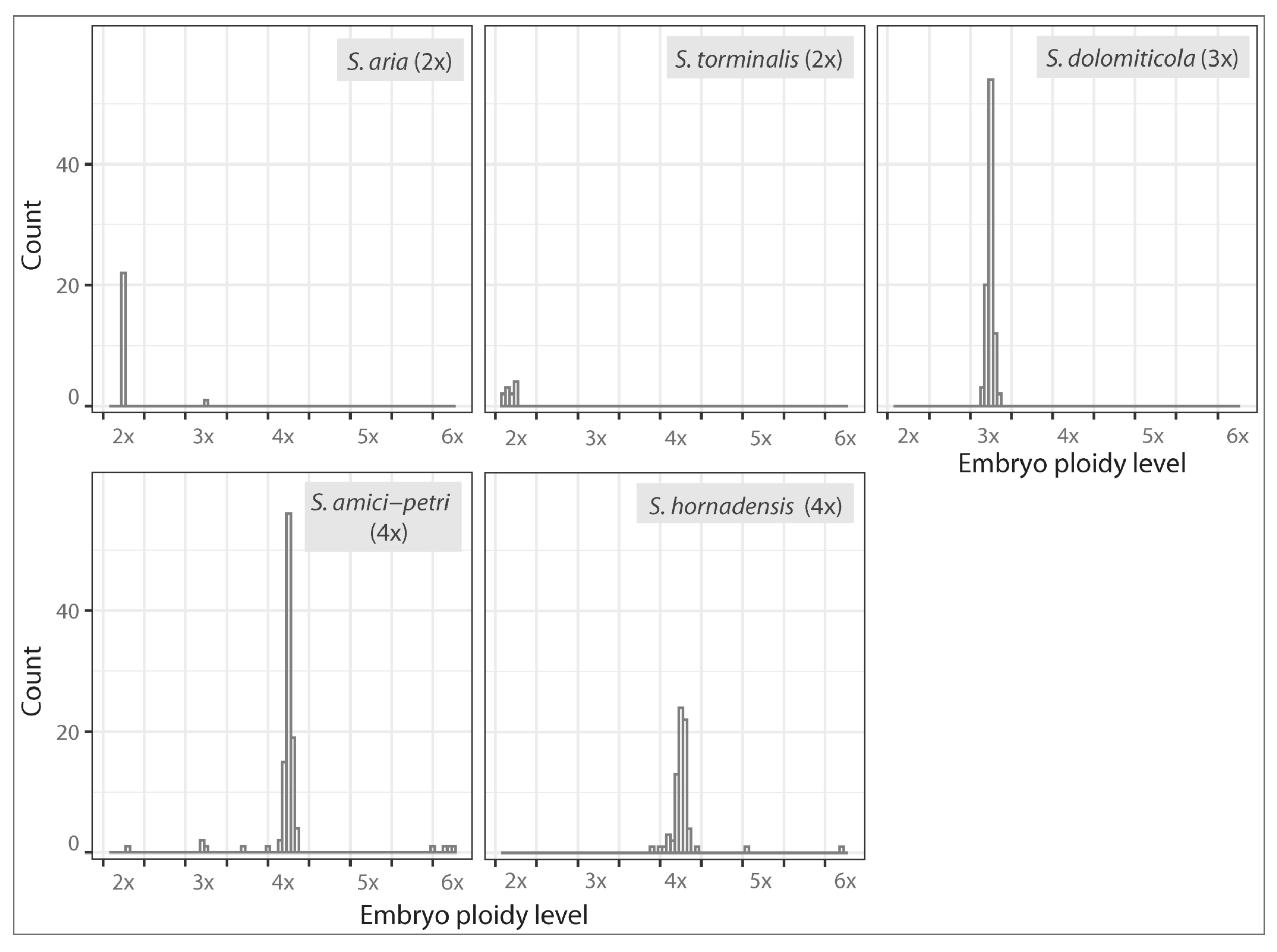
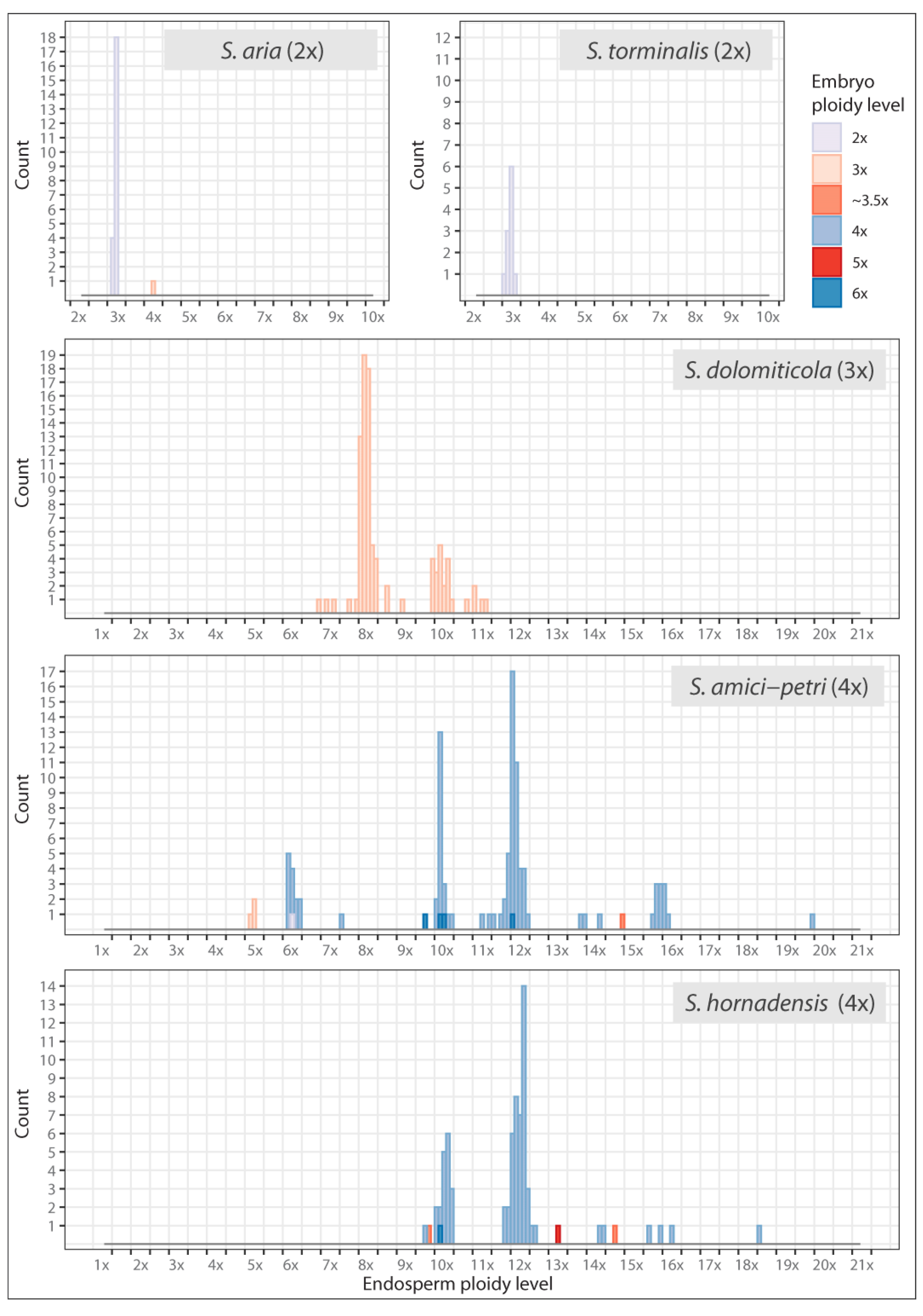
| Endosperm Ploidy Level | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. amici-petri | ||||||||||||||||||||||||||
| 3x | 4x | 5x | 6x | ~6.5x | 7x | ~7.5x | 8x | ~8.5x | 9x | ~9.5x | 10x | ~10.5x | 11x | ~11.5x | 12x | ~12.5x | 13x | ~13.5x | 14x | ~14.5x | ~15.5x | 16x | ~18.5x | ~19.5x | ||
| Embryo ploidy level | 2x | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 3x | . | . | 3 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |
| ~3.5x | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | |
| 4x | . | . | . | 13 | . | . | 1 | . | . | . | . | 20 | . | 2 | 9 | 37 | . | . | 2 | 1 | . | 7 | 4 | . | 1 | |
| 6x | . | . | . | . | . | . | . | . | . | . | 1 | 2 | . | . | . | 1 | . | . | . | . | . | . | . | . | . | |
| S. dolomiticola | ||||||||||||||||||||||||||
| 3x | 4x | 5x | 6x | ~6.5x | 7x | ~7.5x | 8x | ~8.5x | 9x | ~9.5x | 10x | ~10.5x | 11x | ~11.5x | 12x | ~12.5x | 13x | ~13.5x | 14x | ~14.5x | ~15.5x | 16x | ~18.5x | ~19.5x | ||
| 3x | . | . | . | . | 1 | 2 | 1 | 60 | 2 | 1 | 3 | 16 | 1 | 4 | . | . | . | . | . | . | . | . | . | . | . | |
| S. hornadensis | ||||||||||||||||||||||||||
| 3x | 4x | 5x | 6x | ~6.5x | 7x | ~7.5x | 8x | ~8.5x | 9x | ~9.5x | 10x | ~10.5x | 11x | ~11.5x | 12x | ~12.5x | 13x | ~13.5x | 14x | ~14.5x | ~15.5x | 16x | ~18.5x | ~19.5x | ||
| ~3.5x | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | |
| 4x | . | . | . | . | . | . | . | . | . | . | 1 | 18 | . | . | 4 | 38 | 2 | 1 | . | 2 | . | 2 | 1 | 1 | . | |
| 5x | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | |
| 6x | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | |
| Species | Pollen Stainability | Pollen Grain Size | Fruits | FCP | FCSS |
|---|---|---|---|---|---|
| Sorbus amici-petri | 2 (401) | 2 (192) | 8 (327) | 15 | 11 (107/106) |
| S. dolomiticola | 3 (550) | 3 (246) | 4 (46) | 15 | 9 (93/91) |
| S. hornadensis | 3 (409) | 3 (159) | 7 (176) | 12 | 10 (82/74) |
| SUM | 8 (1360) | 8 (597) | 19 (549) | 42 | 30 (282/271) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolarčik, V.; Mirková, M.; Mikoláš, V. Reproduction Modes and Conservation Implications in Three Polyploid Sorbus Stenoendemics in Eastern Slovakia (Central Europe). Plants 2023, 12, 373. https://doi.org/10.3390/plants12020373
Kolarčik V, Mirková M, Mikoláš V. Reproduction Modes and Conservation Implications in Three Polyploid Sorbus Stenoendemics in Eastern Slovakia (Central Europe). Plants. 2023; 12(2):373. https://doi.org/10.3390/plants12020373
Chicago/Turabian StyleKolarčik, Vladislav, Mária Mirková, and Vlastimil Mikoláš. 2023. "Reproduction Modes and Conservation Implications in Three Polyploid Sorbus Stenoendemics in Eastern Slovakia (Central Europe)" Plants 12, no. 2: 373. https://doi.org/10.3390/plants12020373
APA StyleKolarčik, V., Mirková, M., & Mikoláš, V. (2023). Reproduction Modes and Conservation Implications in Three Polyploid Sorbus Stenoendemics in Eastern Slovakia (Central Europe). Plants, 12(2), 373. https://doi.org/10.3390/plants12020373





