Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems
Abstract
:1. Introduction
2. Plant-Associated Bacteria
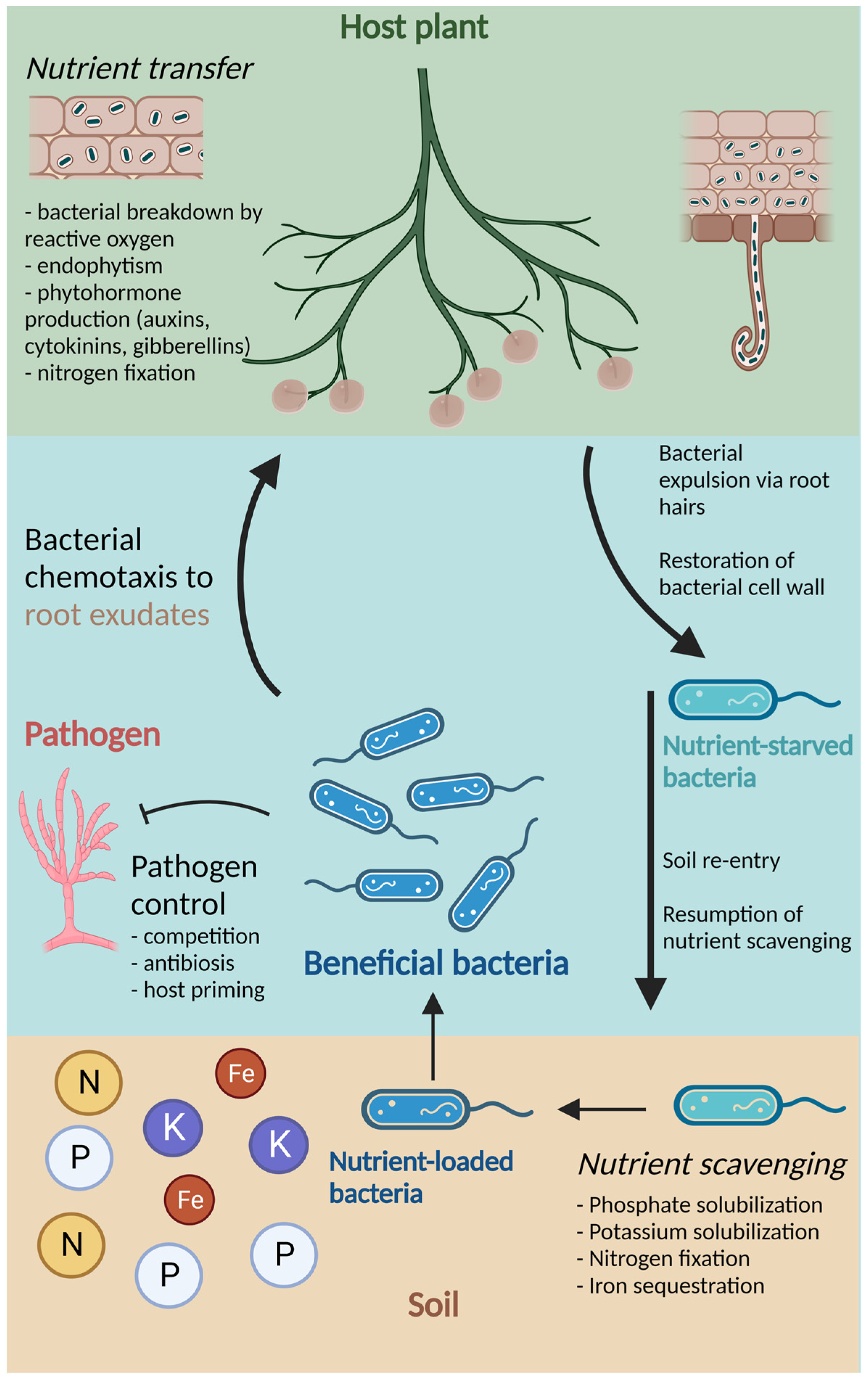
2.1. Mechanisms of Bacterial Association with Plants
2.2. Functions of Beneficial Bacteria
2.2.1. Biological Nitrogen Fixation
2.2.2. Other nutrient Benefits
2.2.3. Phytohormone Production
2.2.4. Abiotic Stress Relief
2.2.5. Pathogen Control
2.3. Field Inconsistencies of PGPB
3. Vertical Farming
3.1. Vertical Farming Systems and Setups
3.2. Advantages of Vertical Farming
3.3. Challenges to Vertical Farming
4. Intersection of PGPB and CEA
4.1. Microflora in CEA Systems
4.2. Factors That Can Influence PGPB Success in CEA
4.2.1. Substrate
4.2.2. Oxygenation and Flow Rate
4.2.3. Temperature
4.2.4. Light Quality
4.2.5. Root Exudates and Implications for CO2 Supplementation
4.2.6. Plant Age
5. Conclusions
6. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Projections to 2050|Global Perspectives Studies|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/global-perspectives-studies/food-agriculture-projections-to-2050/en/ (accessed on 5 December 2022).
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, e963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Connor, E.W. Translating Phytobiomes from Theory to Practice: Ecological and Evolutionary Considerations. Phytobiomes J. 2017, 1, 57–69. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [Green Version]
- Despommier, D.D. The Vertical Farm: Feeding the World in the 21st Century; Macmillan: New York, NY, USA, 2010; ISBN 978-1-4299-4604-9. [Google Scholar]
- Elkazzaz, A. Soilless Agriculture a New and Advanced Method for Agriculture Development: An Introduction. Agric. Res. Technol. Open Access J. 2017, 3, 63–72. [Google Scholar] [CrossRef]
- Kalantari, F.; Mohd Tahir, O.; Mahmoudi Lahijani, A.; Kalantari, S. A Review of Vertical Farming Technology: A Guide for Implementation of Building Integrated Agriculture in Cities. Adv. Eng. Forum 2017, 24, 76–91. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Boon, N.; Geelen, D. Vertical Farming: The Only Way Is Up? Agronomy 2022, 12, 2. [Google Scholar] [CrossRef]
- Jones, J. Hydroponics: A Practical Guide for the Soilless Grower; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4200-3770-8. [Google Scholar]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern Plant Cultivation Technologies in Agriculture under Controlled Environment: A Review on Aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef] [Green Version]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless Culture. FAO Plant Production and Protection. Paper 2013, 217, 303–354. [Google Scholar]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca Sativa L. Var. Sucrine) Growth Performance in Complemented Aquaponic Solution Outperforms Hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Lykas, C.; Katsoulas, N.; Giaglaras, P.; Kittas, C. Electrical Conductivity and PH Prediction in a Recirculated Nutrient Solution of a Greenhouse Soilless Rose Crop. J. Plant Nutr. 2006, 29, 1585–1599. [Google Scholar] [CrossRef]
- Badri, D.V.; Weir, T.L.; van der Lelie, D.; Vivanco, J.M. Rhizosphere Chemical Dialogues: Plant–Microbe Interactions. Curr. Opin. Biotechnol. 2009, 20, 642–650. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy Cycle: An Oxidative Process in Plants for Nutrient Extraction from Symbiotic Microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Rentsch, D.; Robatzek, S.; Webb, R.I.; Sagulenko, E.; Näsholm, T.; Schmidt, S.; Lonhienne, T.G.A. Turning the Table: Plants Consume Microbes as a Source of Nutrients. PLoS ONE 2010, 5, e11915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Kingsley, K.L.; White, J.F. Chemical Interactions at the Interface of Plant Root Hair Cells and Intracellular Bacteria. Microorganisms 2021, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic Microbes and Their Potential Applications in Crop Management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H. How Do Lettuce Seedlings Adapt to Low-PH Stress Conditions? A Mechanism for Low-PH-Induced Root Hair Formation in Lettuce Seedlings. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, N., Anjum, N.A., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 125–155. ISBN 978-3-642-25828-2. [Google Scholar]
- Silber, A. Chemical Characteristics of Soilless Media. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–148. ISBN 978-0-444-63696-6. [Google Scholar]
- Paungfoo-Lonhienne, C.; Schmidt, S.; Lonhienne, T.G.A. Uptake of Non-Pathogenic E. Coli by Arabidopsis Induces down-Regulation of Heat Shock Proteins. Plant Signal. Behav. 2010, 5, 1626–1628. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.K.; Kingsley, K.; Bergen, M.; English, C.; Elmore, M.; Kharwar, R.N.; White, J.F. Bacterial Endophytes from Rice Cut Grass (Leersia Oryzoides L.) Increase Growth, Promote Root Gravitropic Response, Stimulate Root Hair Formation, and Protect Rice Seedlings from Disease. Plant Soil 2018, 422, 223–238. [Google Scholar] [CrossRef]
- Roley, S.S.; Duncan, D.S.; Liang, D.; Garoutte, A.; Jackson, R.D.; Tiedje, J.M.; Robertson, G.P. Associative Nitrogen Fixation (ANF) in Switchgrass (Panicum virgatum) across a Nitrogen Input Gradient. PLoS ONE 2018, 13, e0197320. [Google Scholar] [CrossRef] [Green Version]
- Irizarry, I.; White, J.f. Application of Bacteria from Non-Cultivated Plants to Promote Growth, Alter Root Architecture and Alleviate Salt Stress of Cotton. J. Appl. Microbiol. 2017, 122, 1110–1120. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.f. Bacillus Amyloliquefaciens Alters Gene Expression, ROS Production and Lignin Synthesis in Cotton Seedling Roots. J. Appl. Microbiol. 2018, 124, 1589–1603. [Google Scholar] [CrossRef]
- White, J.F., Jr.; Torres, M.S.; Sullivan, R.F.; Jabbour, R.E.; Chen, Q.; Tadych, M.; Irizarry, I.; Bergen, M.S.; Havkin-Frenkel, D.; Belanger, F.C. Occurrence of Bacillus Amyloliquefaciens as a Systemic Endophyte of Vanilla Orchids. Microsc. Res. Tech. 2014, 77, 874–885. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Yeoh, Y.K.; Webb, R.I.; Lakshmanan, P.; Chan, C.X.; Lim, P.-E.; Ragan, M.A.; Schmidt, S.; Hugenholtz, P. A New Species of Burkholderia Isolated from Sugarcane Roots Promotes Plant Growth. Microb. Biotechnol. 2014, 7, 142–154. [Google Scholar] [CrossRef]
- Soares, M.A.; Li, H.-Y.; Bergen, M.; da Silva, J.M.; Kowalski, K.P.; White, J.F. Functional Role of an Endophytic Bacillus Amyloliquefaciens in Enhancing Growth and Disease Protection of Invasive English Ivy (Hedera helix L.). Plant Soil 2016, 405, 107–123. [Google Scholar] [CrossRef]
- Elmore, M.T.; White, J.F.; Kingsley, K.L.; Diehl, K.H.; Verma, S.K. Pantoea Spp. Associated with Smooth Crabgrass (Digitaria ischaemum) Seed Inhibit Competitor Plant Species. Microorganisms 2019, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- White, J.F.; Chang, X.; Kingsley, K.L.; Zhang, Q.; Chiaranunt, P.; Micci, A.; Velazquez, F.; Elmore, M.; Crane, S.; Li, S.; et al. Endophytic Bacteria in Grass Crop Growth Promotion and Biostimulation. Grass Res. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Cocking, E.; Dent, D. The Prospect of N2-Fixing Crops Galore! Biochem. 2019, 41, 14–17. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- de Bruijn, F.J. Biological Nitrogen Fixation. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 215–224. ISBN 978-3-319-08575-3. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Dos Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.C.; Dixon, R. Distribution of Nitrogen Fixation and Nitrogenase-like Sequences amongst Microbial Genomes. BMC Genom. 2012, 13, 162. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef]
- McGlynn, S.E.; Boyd, E.S.; Peters, J.W.; Orphan, V.J. Classifying the Metal Dependence of Uncharacterized Nitrogenases. Front. Microbiol. 2012, 3, 419. [Google Scholar] [CrossRef] [PubMed]
- Berman-Frank, I.; Chen, Y.-B.; Gerchman, Y.; Dismukes, G.C.; Falkowski, P.G. Inhibition of Nitrogenase by Oxygen in Marine Cyanobacteria Controls the Global Nitrogen and Oxygen Cycles. Biogeosciences Discuss. 2005, 2, 261–273. [Google Scholar]
- Padda, K.P.; Puri, A.; Chanway, C. Endophytic Nitrogen Fixation—A Possible ‘Hidden’ Source of Nitrogen for Lodgepole Pine Trees Growing at Unreclaimed Gravel Mining Sites. FEMS Microbiol. Ecol. 2019, 95, fiz172. [Google Scholar] [CrossRef] [PubMed]
- Knoth, J.L.; Kim, S.-H.; Ettl, G.J.; Doty, S.L. Biological Nitrogen Fixation and Biomass Accumulation within Poplar Clones as a Result of Inoculations with Diazotrophic Endophyte Consortia. New Phytol. 2014, 201, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Burris, R.H.; Gunapala, N.; Kennedy, C. Comparison of Benefit to Sugarcane Plant Growth and 15N2 Incorporation Following Inoculation of Sterile Plants with Acetobacter Diazotrophicus Wild-Type and Nif¯ Mutant Strains. Mol. Plant-Microbe Interact. 2001, 14, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Iniguez, A.L.; Dong, Y.; Triplett, E.W. Nitrogen Fixation in Wheat Provided by Klebsiella Pneumoniae 342. Mol. Plant-Microbe Interact. 2004, 17, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Boddey, R.M.; de Oliveira, O.C.; Urquiaga, S.; Reis, V.M.; de Olivares, F.L.; Baldani, V.L.D.; Döbereiner, J. Biological Nitrogen Fixation Associated with Sugar Cane and Rice: Contributions and Prospects for Improvement. In Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems: Extended Versions of Papers Presented at the Symposium on Biological Nitrogen Fixation for Sustainable Agriculture at the 15th Congress of Soil Science, Acapulco, Mexico, 1994; Ladha, J.K., Peoples, M.B., Eds.; Developments in Plant and Soil Sciences; Springer Netherlands: Dordrecht, The Netherlands, 1995; pp. 195–209. ISBN 978-94-011-0055-7. [Google Scholar]
- Gyaneshwar, P.; James, E.K.; Reddy, P.M.; Ladha, J.K. Herbaspirillum Colonization Increases Growth and Nitrogen Accumulation in Aluminium-Tolerant Rice Varieties. New Phytol. 2002, 154, 131–145. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; do Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust Biological Nitrogen Fixation in a Model Grass–Bacterial Association. Plant J. 2015, 81, 907–919. [Google Scholar] [CrossRef]
- Landeta, C.; Marchant, F. Biostimulants: Emerging Trend and Opportunities. In Biostimulants: Exploring Sources and Applications; Ramawat, N., Bhardwaj, V., Eds.; Plant Life and Environment Dynamics; Springer Nature: Singapore, 2022; pp. 263–290. ISBN 9789811670800. [Google Scholar]
- Son, J.E.; Kim, H.J.; Ahn, T.I. Chapter 20—Hydroponic Systems. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 273–283. ISBN 978-0-12-816691-8. [Google Scholar]
- Arcas-Pilz, V.; Parada, F.; Villalba, G.; Rufí-Salis, M.; Rosell-Melé, A.; Gabarrell Durany, X. Improving the Fertigation of Soilless Urban Vertical Agriculture through the Combination of Struvite and Rhizobia Inoculation in Phaseolus Vulgaris. Front. Plant Sci. 2021, 12, 649304. [Google Scholar] [CrossRef]
- Kontopoulou, C.-K.; Liasis, E.; Iannetta, P.P.; Tampakaki, A.; Savvas, D. Impact of Rhizobial Inoculation and Reduced N Supply on Biomass Production and Biological N2 Fixation in Common Bean Grown Hydroponically. J. Sci. Food Agric. 2017, 97, 4353–4361. [Google Scholar] [CrossRef]
- Razmjooei, Z.; Etemadi, M.; Eshghi, S.; Ramezanian, A.; Mirazimi Abarghuei, F.; Alizargar, J. Potential Role of Foliar Application of Azotobacter on Growth, Nutritional Value and Quality of Lettuce under Different Nitrogen Levels. Plants 2022, 11, 406. [Google Scholar] [CrossRef]
- Franchini, M. Investigations on the Interactions between the Endophyte Nitrogen Fixing Bacterium Gluconacetobacter Diazotrophicus and Tomato Plants. Available online: https://eprints.nottingham.ac.uk/66671/ (accessed on 3 January 2023).
- Sebring, R.L.; Duiker, S.W.; Berghage, R.D.; Regan, J.M.; Lambert, J.D.; Bryant, R.B. Gluconacetobacter Diazotrophicus Inoculation of Two Lettuce Cultivars Affects Leaf and Root Growth under Hydroponic Conditions. Appl. Sci. 2022, 12, 1585. [Google Scholar] [CrossRef]
- Aziz, T.; Sabir, M.; Farooq, M.; Maqsood, M.A.; Ahmad, H.R.; Warraich, E.A. Phosphorus Deficiency in Plants: Responses, Adaptive Mechanisms, and Signaling. In Plant Signaling: Understanding the Molecular Crosstalk; Hakeem, K.R., Rehman, R.U., Tahir, I., Eds.; Springer India: New Delhi, India, 2014; pp. 133–148. ISBN 978-81-322-1542-4. [Google Scholar]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects—A Review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium Deficiency in Plants: Effects and Signaling Cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, e4917256. [Google Scholar] [CrossRef]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Saqlan, S.M.; Rasheed, M. Phosphorus Solubilizing Bacteria: Occurrence, Mechanisms and Their Role in Crop Production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Selvi, K.B.; Paul, J.J.A.; Vijaya, V.; Saraswathi, K. Analyzing the Efficacy of Phosphate Solubilizing Microorganisms by Enrichment Culture Techniques. Biochem. Mol. Biol. J. 2017, 3, 100029. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.A.; Khavazi, K.; Moezi, A.A.; Rejali, F.; Nadian, H.A. Phosphate Solubilizing Bacteria and Arbuscular Mycorrhizal Fungi Impacts on Inorganic Phosphorus Fractions and Wheat Growth. World Appl. Sci. J. 2011, 15, 1310–1318. [Google Scholar]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and Their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Joyce, A.; Goddek, S.; Kotzen, B.; Wuertz, S. Aquaponics: Closing the Cycle on Limited Water, Land and Nutrient Resources. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. ISBN 978-3-030-15943-6. [Google Scholar]
- Goddek, S.; Delaide, B.P.L.; Joyce, A.; Wuertz, S.; Jijakli, M.H.; Gross, A.; Eding, E.H.; Bläser, I.; Reuter, M.; Keizer, L.C.P.; et al. Nutrient Mineralization and Organic Matter Reduction Performance of RAS-Based Sludge in Sequential UASB-EGSB Reactors. Aquac. Eng. 2018, 83, 10–19. [Google Scholar] [CrossRef]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The Effect of Anaerobic and Aerobic Fish Sludge Supernatant on Hydroponic Lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Da Cerozi, B.S.; Fitzsimmons, K. Use of Bacillus spp. to Enhance Phosphorus Availability and Serve as a Plant Growth Promoter in Aquaponics Systems. Sci. Hortic. 2016, 211, 277–282. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial Siderophores Efficiently Provide Iron to Iron-Starved Tomato Plants in Hydroponics Culture. Antonie Van Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, J.; Guerinot, M.L. Iron Uptake and Transport in Plants: The Good, the Bad, and the Ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef] [Green Version]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial Siderophores in Community and Host Interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Delaporte-Quintana, P.; Lovaisa, N.C.; Rapisarda, V.A.; Pedraza, R.O. The Plant Growth Promoting Bacteria Gluconacetobacter Diazotrophicus and Azospirillum Brasilense Contribute to the Iron Nutrition of Strawberry Plants through Siderophores Production. Plant Growth Regul. 2020, 91, 185–199. [Google Scholar] [CrossRef]
- Abiraami, T.V.; Suman, A.; Singh, B.; Aswini, K.; Annapurna, K. Radiochemical Evidence for the Contribution of Chemotyped Siderophore Producing Bacteria Towards Plant Iron Nutrition. Curr. Microbiol. 2021, 78, 4072–4083. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions between Plants and Non-Symbiotic Growth Promoting Bacteria under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. Phytohormonal Roles in Plant Responses to Heavy Metal Stress: Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef]
- Skalický, V.; Kubeš, M.; Napier, R.; Novák, O. Auxins and Cytokinins—The Role of Subcellular Organization on Homeostasis. Int. J. Mol. Sci. 2018, 19, 3115. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Endophytic Phytohormones and Their Role in Plant Growth Promotion. In Functional Importance of the Plant Microbiome: Implications for Agriculture, Forestry and Bioenergy; Doty, S.L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 89–105. ISBN 978-3-319-65897-1. [Google Scholar]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin Signaling Networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of Plant Growth by Cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Schmülling, T. Cytokinin Action in Plant Development. Curr. Opin. Plant Biol. 2009, 12, 527–538. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin Producing Bacteria Enhance Plant Growth in Drying Soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved Drought Stress Response in Alfalfa Plants Nodulated by an IAA Over-Producing Rhizobium Strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef] [Green Version]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voß, U.; Postaire, O.; Luu, D.-T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin Regulates Aquaporin Function to Facilitate Lateral Root Emergence. Nat. Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A. The Role of Auxin in Shaping Shoot Architecture. J. Exp. Bot. 2013, 64, 2593–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernoux, T.; Besnard, F.; Traas, J. Auxin at the Shoot Apical Meristem. Cold Spring Harb. Perspect. Biol. 2010, 2, a001487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin Oxidase Regulates Rice Grain Production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Takei, K.; Hirose, N. Interactions between Nitrogen and Cytokinin in the Regulation of Metabolism and Development. Trends Plant Sci. 2006, 11, 440–448. [Google Scholar] [CrossRef]
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin Controls Local Cytokinin Biosynthesis in the Nodal Stem in Apical Dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-Producing, Plant Growth-Promoting Rhizobacteria That Confer Resistance to Drought Stress in Platycladus Orientalis Container Seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting Plant–Microbe Partnerships to Improve Biomass Production and Remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine Kinase Homologs That Act as Cytokinin Receptors Possess Overlapping Functions in the Regulation of Shoot and Root Growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [Green Version]
- Hooley, R. Gibberellins: Perception, Transduction and Responses. Plant Mol. Biol. 1994, 26, 1529–1555. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant Growth-Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis Sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin Production by Newly Isolated Strain Leifsonia Soli SE134 and Its Potential to Promote Plant Growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin Secreting Rhizobacterium, Pseudomonas Putida H-2-3 Modulates the Hormonal and Stress Physiology of Soybean to Improve the Plant Growth under Saline and Drought Conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Gomaa, E.Z. Effect of Plant Growth Promoting Bacillus Subtilis and Pseudomonas Fluorescens on Growth and Pigment Composition of Radish Plants (Raphanus sativus) under NaCl Stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Gibberellins Producing Bacillus Methylotrophicus KE2 Supports Plant Growth and Enhances Nutritional Metabolites and Food Values of Lettuce. Plant Physiol. Biochem. 2016, 109, 181–189. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, A.; Yarnia, M.; Hassanpanah, D.; Farahvash, F.; Khalilvand, E. The Response of Different Potato Cultivars to Plant Growth-Promoting Rhizobacteria (PGPRs) and Chemical Fertilizers in Aeroponic Culture Conditions. J. Plant Nutr. 2022, 45, 2975–2985. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.-Y.; Wang, K.-X.; Hu, J.-J.; Tang, M.-X.; He, W.; Vander Zaag, P. Manipulating Aeroponically Grown Potatoes with Gibberellins and Calcium Nitrate. Am. J. Potato Res. 2018, 95, 351–361. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and Pathogenic Plant-Microbe Interactions during Flooding Stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant Bacteria Mitigate the Effects of Salinity Stress on Soybean Growth by Regulating Secondary Metabolites and Molecular Responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Roy Choudhury, A.; Walitang, D.I.; Anandham, R.; Senthilkumar, M.; Sa, T. Salt Stress Tolerance-Promoting Proteins and Metabolites under Plant-Bacteria-Salt Stress Tripartite Interactions. Appl. Sci. 2022, 12, 3126. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Kasotia, A.; Jain, S.; Vaishnav, A.; Kumari, S.; Sharma, K.P.; Varma, A. Bacterial-Mediated Tolerance and Resistance to Plants Under Abiotic and Biotic Stresses. J. Plant Growth Regul. 2016, 35, 276–300. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Rhizobacteria (PGPR): Their Potential as Antagonists and Biocontrol Agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [Green Version]
- Velivelli, S.; Sessitsch, A.; Doyle, B. The Role of Microbial Inoculants in Integrated Crop Management Systems. Potato Res. 2014, 57, 291–309. [Google Scholar] [CrossRef]
- Montalbán-López, M.; Scott, T.A.; Ramesh, S.; Rahman, I.R.; van Heel, A.; Viel, J.H.; Bandarian, V.; Dittmann, E.; Genilloud, O.; Goto, Y.; et al. New Developments in RiPP Discovery, Enzymology and Engineering. Nat. Prod. Rep. 2021, 38, 130–239. [Google Scholar] [CrossRef]
- Rebuffat, S. The Manifold Roles of Microbial Ribosomal Peptide-Based Natural Products in Physiology and Ecology. J. Biol. Chem. 2020, 295, 34–54. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Núñez, M.A.; López, V.E.L.y. Nonribosomal Peptides Synthetases and Their Applications in Industry. Sustain. Chem. Process. 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of Linear Non-Ribosomal Peptide in Biocontrol Fungi. J. Fungi 2020, 6, 61. [Google Scholar] [CrossRef]
- Xu, X.; Qu, R.; Wu, W.; Jiang, C.; Shao, D.; Shi, J. Applications of Microbial Co-Cultures in Polyketides Production. J. Appl. Microbiol. 2021, 130, 1023–1034. [Google Scholar] [CrossRef]
- Michelsen, C.F.; Watrous, J.; Glaring, M.A.; Kersten, R.; Koyama, N.; Dorrestein, P.C.; Stougaard, P. Nonribosomal Peptides, Key Biocontrol Components for Pseudomonas Fluorescens In5, Isolated from a Greenlandic Suppressive Soil. MBio 2015, 6, e00079-15. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.; Ullah, N.; Janjua, H.A. In Vitro Evaluation and Genome Mining of Bacillus Subtilis Strain RS10 Reveals Its Biocontrol and Plant Growth-Promoting Potential. Agriculture 2021, 11, 1273. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S.; Sheikh, I.H.; Khan, A. Role of Cyanide Production by Pseudomonas Fluorescens CHA0 in the Suppression of Root-Knot Nematode, Meloidogyne Javanica in Tomato. World J. Microbiol. Biotechnol. 2006, 22, 641–650. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S.S. Influence of Hydrogen Cyanide-Producing Rhizobacteria in Controlling the Crown Gall and Root-Knot Nematode, Meloidogyne Incognita. Egypt. J. Biol. Pest Control 2019, 29, 41. [Google Scholar] [CrossRef]
- Flury, P.; Vesga, P.; Péchy-Tarr, M.; Aellen, N.; Dennert, F.; Hofer, N.; Kupferschmied, K.P.; Kupferschmied, P.; Metla, Z.; Ma, Z.; et al. Antimicrobial and Insecticidal: Cyclic Lipopeptides and Hydrogen Cyanide Produced by Plant-Beneficial Pseudomonas Strains CHA0, CMR12a, and PCL1391 Contribute to Insect Killing. Front. Microbiol. 2017, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Olivera, M.; Delgado, N.; Cádiz, F.; Riquelme, N.; Montenegro, I.; Seeger, M.; Bravo, G.; Barros-Parada, W.; Pedreschi, R.; Besoain, X. Diffusible Compounds Produced by Hanseniaspora Osmophila and Gluconobacter Cerinus Help to Control the Causal Agents of Gray Rot and Summer Bunch Rot of Table Grapes. Antibiotics 2021, 10, 664. [Google Scholar] [CrossRef]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and Their Role in Biocontrol by Pseudomonas Bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva, M.O.; Vicedo, B.; Finiti, I.; Flors, V.; Del Amo, G.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Preventive and Post-Infection Control of Botrytis Cinerea in Tomato Plants by Hexanoic Acid. Plant Pathol. 2008, 57, 1038–1046. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Dunne, C.; Crowley, J.J.; Moënne-Loccoz, Y.; Dowling, D.N.; Bruijn, S.; O’Gara, F. Biological Control of Pythium Ultimum by Stenotrophomonas Maltophilia W81 Is Mediated by an Extracellular Proteolytic Activity. Microbiology 1997, 143, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.Y.; Reedy, R.M.; Bick, J.; Oudemans, P.V. Characterization of a Chitinase Gene from Stenotrophomonas Maltophilia Strain 34S1 and Its Involvement in Biological Control. Appl. Environ. Microbiol. 2002, 68, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [Green Version]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Mulders, I.H.M.; Dekkers, L.C.; Lugtenberg, B.J.J. Root Colonization by Phenazine-1-Carboxamide-Producing Bacterium Pseudomonas Chlororaphis PCL1391 Is Essential for Biocontrol of Tomato Foot and Root Rot. Mol. Plant-Microbe Interact. 2000, 13, 1340–1345. [Google Scholar] [CrossRef] [Green Version]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; van Loon, L.C. Induced Systemic Resistance by Fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; van Wees, S.C.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A Novel Signaling Pathway Controlling Induced Systemic Resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Dessaux, Y.; Grandclément, C.; Faure, D. Engineering the Rhizosphere. Trends Plant Sci. 2016, 21, 266–278. [Google Scholar] [CrossRef]
- Ji, S.-H.; Kim, J.-S.; Lee, C.-H.; Seo, H.-S.; Chun, S.-C.; Oh, J.; Choi, E.-H.; Park, G. Enhancement of Vitality and Activity of a Plant Growth-Promoting Bacteria (PGPB) by Atmospheric Pressure Non-Thermal Plasma. Sci. Rep. 2019, 9, 1044. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [Green Version]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant Protection and Growth Stimulation by Microorganisms: Biotechnological Applications of Bacilli in Agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Prévost, D. Development of Emulsion from Rhizobial Fermented Starch Industry Wastewater for Application as Medicago Sativa Seed Coat. Eng. Life Sci. 2010, 10, 248–256. [Google Scholar] [CrossRef]
- McIntyre, H.J.; Davies, H.; Hore, T.A.; Miller, S.H.; Dufour, J.-P.; Ronson, C.W. Trehalose Biosynthesis in Rhizobium Leguminosarum Bv. Trifolii and Its Role in Desiccation Tolerance. Appl. Environ. Microbiol. 2007. [Google Scholar] [CrossRef] [Green Version]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Zvinavashe, A.T.; Mardad, I.; Mhada, M.; Kouisni, L.; Marelli, B. Engineering the Plant Microenvironment to Facilitate Plant-Growth-Promoting Microbe Association. J. Agric. Food Chem. 2021, 69, 13270–13285. [Google Scholar] [CrossRef]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical Farming: A Summary of Approaches to Growing Skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]
- Artemis State of Indoor Farming. 2020. Available online: https://artemisag.com/state-of-indoor-farming-2020/ (accessed on 1 January 2023).
- Kalantari, F.; Mohd tahir, O.; Akbari Joni, R.; Fatemi, E. Opportunities and Challenges in Sustainability of Vertical Farming: A Review. J. Landsc. Ecol. 2017, 11, 35–60. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, C.; Adenaeuer, L. Up, up and away! The Economics of Vertical Farming. J. Agric. Stud. 2014, 2, 40. [Google Scholar] [CrossRef]
- Stein, E.W. The Transformative Environmental Effects Large-Scale Indoor Farming May Have on Air, Water, and Soil. Air Soil Water Res. 2021, 14, 1178622121995819. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future Food-Production Systems: Vertical Farming and Controlled-Environment Agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.J.; Behe, B.K.; Currey, C.J.; Lopez, R.G. Historical, Current, and Future Perspectives for Controlled Environment Hydroponic Food Crop Production in the United States. HortScience 2020, 55, 758–767. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Bonnett, G.D.; McIntyre, C.L.; Hochman, Z.; Wasson, A.P. Strategies to Improve the Productivity, Product Diversity and Profitability of Urban Agriculture. Agric. Syst. 2019, 174, 133–144. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The Vertical Farm: A Review of Developments and Implications for the Vertical City. Buildings 2018, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Zabel, P.; Zeidler, C.; Vrakking, V.; Dorn, M.; Schubert, D. Biomass Production of the EDEN ISS Space Greenhouse in Antarctica During the 2018 Experiment Phase. Front. Plant Sci. 2020, 11, 656. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial Bacteria and Fungi in Hydroponic Systems: Types and Characteristics of Hydroponic Food Production Methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O. Hydroponics as an Advanced Technique for Vegetable Production: An Overview. J. Soil Water Conserv. 2019, 17, 364–371. [Google Scholar] [CrossRef]
- Bugbee, B. Nutrient management in recirculating hydroponic culture. Acta Hortic. 2004, 648, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.S.; Ma, N.L.; Jusoh, A.; Ambak, M.A. Biological Nutrient Removal by Recirculating Aquaponic System: Optimization of the Dimension Ratio between the Hydroponic & Rearing Tank Components. Int. Biodeterior. Biodegrad. 2015, 102, 107–115. [Google Scholar] [CrossRef]
- AlShrouf, A. Hydroponics, Aeroponic and Aquaponic as Compared with Conventional Farming. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; van Os, E.; Anseeuw, D.; Havermaet, R.V.; Junge, R. Hydroponic Technologies. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 77–110. ISBN 978-3-030-15943-6. [Google Scholar]
- Hibar, K.; Daami-Remadi, M.; Hamada, W.; El-Mahjoub, M. Bio-Fungicides as an Alternative for Tomato Fusarium Crown and Root Rot Control. Tunis. J. Plant Prot. 2006, 1, 19–29. [Google Scholar]
- Aydinalp, C.; Cresser, M.S. The Effects of Global Climate Change on Agriculture. J. Agric. Environ. Sci. 2008, 3, 672–676. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Vikram, P.; Swamy, B.P.M.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought Susceptibility of Modern Rice Varieties: An Effect of Linkage of Drought Tolerance with Undesirable Traits. Sci. Rep. 2015, 5, 14799. [Google Scholar] [CrossRef] [Green Version]
- Lubna, F.A.; Lewus, D.C.; Shelford, T.J.; Both, A.-J. What You May Not Realize about Vertical Farming. Horticulturae 2022, 8, 322. [Google Scholar] [CrossRef]
- Perez, V.M. Study of the Sustainbility Issues of Food Production Using Vertical Farm Methods in an Urban Environment within the State of Indiana. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2014. [Google Scholar]
- Janick, J. Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 43, ISBN 978-1-119-10776-7. [Google Scholar]
- Eaves, J.; Eaves, S. Comparing the Profitability of a Greenhouse to a Vertical Farm in Quebec. Can. J. Agric. Econ./Rev. Can. D’agroeconomie 2018, 66, 43–54. [Google Scholar] [CrossRef]
- Mattson, N.; Lieth, J.H. Chapter 12—Liquid Culture Hydroponic System Operation. In Soilless Culture, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier: Boston, MA, USA, 2019; pp. 567–585. ISBN 978-0-444-63696-6. [Google Scholar]
- Shimizu, K.; Matsuda, Y.; Nonomura, T.; Ikeda, H.; Tamura, N.; Kusakari, S.; Kimbara, J.; Toyoda, H. Dual Protection of Hydroponic Tomatoes from Rhizosphere Pathogens Ralstonia Solanacearum and Fusarium oxysporum f.Sp. Radicis-Lycopersici and Airborne Conidia of Oidium Neolycopersici with an Ozone-Generative Electrostatic Spore Precipitator. Plant Pathol. 2007, 56, 987–997. [Google Scholar] [CrossRef]
- Roberts, J.M.; Bruce, T.J.A.; Monaghan, J.M.; Pope, T.W.; Leather, S.R.; Beacham, A.M. Vertical Farming Systems Bring New Considerations for Pest and Disease Management. Ann. Appl. Biol. 2020, 176, 226–232. [Google Scholar] [CrossRef]
- Lam, K.-L.; Kong, W.-P.; Ling, P.-Y.; Lau, T.-H.; Ho, K.-H.; Lee, F.W.-F.; Chan, P.-L. Antibiotic-Resistant Bacteria in Hydroponic Lettuce in Retail: A Comparative Survey. Foods 2020, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, X.; Shi, C.; Yan, W.; Zhang, L.; Wang, G. Ecotoxicological Effects and Accumulation of Ciprofloxacin in Eichhornia Crassipes under Hydroponic Conditions. Environ. Sci. Pollut. Res. 2019, 26, 30348–30355. [Google Scholar] [CrossRef] [PubMed]
- Aryal, N.; Reinhold, D. Phytoaccumulation of Antimicrobials by Hydroponic Cucurbita Pepo. Int. J. Phytoremediation 2013, 15, 330–342. [Google Scholar] [CrossRef]
- Shahanaz, E. Use of Antibiotics Leading the Occurrence of Antibiotic Resistant Bacteria on Hydroponically Grown Mung Bean Sprouts. Undergraduate Thesis, BRAC University, Dhaka, Bangladesh, 2021. [Google Scholar]
- Vallance, J.; Déniel, F.; Floch, G.L.; Guérin-Dubrana, L.; Blancard, D.; Rey, P. Pathogenic and Beneficial Microorganisms in Soilless Cultures. In Sustainable Agriculture; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; Volume 2, pp. 711–726. ISBN 978-94-007-0394-0. [Google Scholar]
- Cirou, A.; Raffoux, A.; Diallo, S.; Latour, X.; Dessaux, Y.; Faure, D. Gamma-Caprolactone Stimulates Growth of Quorum-Quenching Rhodococcus Populations in a Large-Scale Hydroponic System for Culturing Solanum Tuberosum. Res. Microbiol. 2011, 162, 945–950. [Google Scholar] [CrossRef]
- Yu, H.; Liang, H.; Qu, F.; He, J.; Xu, G.; Hu, H.; Li, G. Biofouling Control by Biostimulation of Quorum-Quenching Bacteria in a Membrane Bioreactor for Wastewater Treatment: Biofouling Control by Biostimulation. Biotechnol. Bioeng. 2016, 113, 2624–2632. [Google Scholar] [CrossRef]
- Verdoliva, S.G.; Gwyn-Jones, D.; Detheridge, A.; Robson, P. Controlled Comparisons between Soil and Hydroponic Systems Reveal Increased Water Use Efficiency and Higher Lycopene and β-Carotene Contents in Hydroponically Grown Tomatoes. Sci. Hortic. 2021, 279, 109896. [Google Scholar] [CrossRef]
- Abdullah, M.J.; Zhang, Z.; Matsubae, K. Potential for Food Self-Sufficiency Improvements through Indoor and Vertical Farming in the Gulf Cooperation Council: Challenges and Opportunities from the Case of Kuwait. Sustainability 2021, 13, 12553. [Google Scholar] [CrossRef]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent Advances and Perspectives in the Treatment of Hydroponic Wastewater: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Chen, C.; Bélanger, R.R.; Benhamou, N.; Paulitz, T.C. Role of Salicylic Acid in Systemic Resistance Induced by Pseudomonas Spp. Against Pythium Aphanidermatum in Cucumber Roots. Eur. J. Plant Pathol. 1999, 105, 477–486. [Google Scholar] [CrossRef]
- Chen, C.; Bélanger, R.R.; Benhamou, N.; Paulitz, T.C. Defense Enzymes Induced in Cucumber Roots by Treatment with Plant Growth-Promoting Rhizobacteria (PGPR) and Pythium Aphanidermatum. Physiol. Mol. Plant Pathol. 2000, 56, 13–23. [Google Scholar] [CrossRef]
- Gravel, V.; Martinez, C.; Antoun, H.; Tweddell, R.J. Control of Greenhouse Tomato Root Rot [Pythium ultimum] in Hydroponic Systems, Using Plant-Growth-Promoting Microorganisms. Can. J. Plant Pathol. 2006, 28, 475–483. [Google Scholar] [CrossRef]
- Renault, D.; Déniel, F.; Benizri, E.; Sohier, D.; Barbier, G.; Rey, P. Characterization of Bacillus and Pseudomonas Strains with Suppressive Traits Isolated from Tomato Hydroponic-Slow Filtration Unit. Can. J. Microbiol. 2007, 53, 784–797. [Google Scholar] [CrossRef]
- Chinta, Y.D.; Kano, K.; Widiastuti, A.; Fukahori, M.; Kawasaki, S.; Eguchi, Y.; Misu, H.; Odani, H.; Zhou, S.; Narisawa, K.; et al. Effect of Corn Steep Liquor on Lettuce Root Rot (Fusarium oxysporum f.Sp. Lactucae) in Hydroponic Cultures. J. Sci. Food Agric. 2014, 94, 2317–2323. [Google Scholar] [CrossRef]
- Liu, W.; Sutton, J.C.; Grodzinski, B.; Kloepper, J.W.; Reddy, M.S. Biological Control of Pythium Root Rot of Chrysanthemum in Small-Scale Hydroponic Units. Phytoparasitica 2007, 35, 159. [Google Scholar] [CrossRef]
- Sopher, C.R.; Sutton, J.C. Quantitative Relationships of Pseudomonas Chlororaphis 63-28 to Pythium Root Rot and Growth in Hydroponic Peppers. Trop. Plant Pathol. 2011, 36, 214–224. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, K.; He, X.; Li, S.; Zhang, Z.; Shen, B.; Yang, X.; Zhang, R.; Huang, Q.; Shen, Q. A New Bioorganic Fertilizer Can Effectively Control Banana Wilt by Strong Colonization with Bacillus Subtilis N11. Plant Soil 2011, 344, 87–97. [Google Scholar] [CrossRef]
- Gül, A.; Kidoglu, F.; Tüzel, Y. Effects of Nutrition and Bacillus Amyloliquefaciens on Tomato (Solanum lycopersicum, L.) Growing in Perlite. Span. J. Agric. Res. 2008, 6, 422–429. [Google Scholar] [CrossRef]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The Plant Microbiota: Systems-Level Insights and Perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Voges, M.J.E.E.E.; Bai, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-Derived Coumarins Shape the Composition of an Arabidopsis Synthetic Root Microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and Organic Growing Media Have Distinct Community Structure, Stability and Functionality in Soilless Culture Systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial Activity in Peat-Reduced Plant Growing Media: Identifying Influential Growing Medium Constituents and Physicochemical Properties Using Fractional Factorial Design of Experiments. J. Clean. Prod. 2020, 256, 120323. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Dixon, M. An Upper Limit for Elevated Root Zone Dissolved Oxygen Concentration for Tomato. Sci. Hortic. 2007, 113, 162–165. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Hartman, K.; Tringe, S.G. Interactions between Plants and Soil Shaping the Root Microbiome under Abiotic Stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef] [Green Version]
- Bodelier, P.L.E. Interactions Between Oxygen-Releasing Roots and Microbial Processes in Flooded Soils and Sediments. In Root Ecology; de Kroon, H., Visser, E.J.W., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2003; pp. 331–362. ISBN 978-3-662-09784-7. [Google Scholar]
- Hamonts, K.; Clough, T.J.; Stewart, A.; Clinton, P.W.; Richardson, A.E.; Wakelin, S.A.; O’Callaghan, M.; Condron, L.M. Effect of Nitrogen and Waterlogging on Denitrifier Gene Abundance, Community Structure and Activity in the Rhizosphere of Wheat. FEMS Microbiol. Ecol. 2013, 83, 568–584. [Google Scholar] [CrossRef]
- Mittelstrass, J.; Sperone, F.G.; Horton, M.W. Using Transects to Disentangle the Environmental Drivers of Plant-Microbiome Assembly. Plant Cell Environ. 2021, 44, 3745–3755. [Google Scholar] [CrossRef]
- van der Voort, M.; Kempenaar, M.; van Driel, M.; Raaijmakers, J.M.; Mendes, R. Impact of Soil Heat on Reassembly of Bacterial Communities in the Rhizosphere Microbiome and Plant Disease Suppression. Ecol. Lett. 2016, 19, 375–382. [Google Scholar] [CrossRef]
- Chave, M.; Dabert, P.; Brun, R.; Godon, J.-J.; Poncet, C. Dynamics of Rhizoplane Bacterial Communities Subjected to Physicochemical Treatments in Hydroponic Crops. Crop Prot. 2008, 27, 418–426. [Google Scholar] [CrossRef]
- Xu, G.; Wolf, S.; Kafkafi, U. Interactive Effect of Nutrient Concentration and Container Volume on Flowering, Fruiting, and Nutrient Uptake of Sweet Pepper. J. Plant Nutr. 2001, 24, 479–501. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Carroll, T.L.; Sundin, G.W. The Role of Pigmentation, Ultraviolet Radiation Tolerance, and Leaf Colonization Strategies in the Epiphytic Survival of Phyllosphere Bacteria. Microb. Ecol. 2005, 49, 104–113. [Google Scholar] [CrossRef]
- Kadivar, H.; Stapleton, A.E. Ultraviolet Radiation Alters Maize Phyllosphere Bacterial Diversity. Microb. Ecol. 2003, 45, 353–361. [Google Scholar] [CrossRef]
- Zhang, W.; Tu, J.C. Effect of Ultraviolet Disinfection of Hydroponic Solutions on Pythium Root Rot and Non-Target Bacteria. Eur. J. Plant Pathol. 2000, 106, 415–421. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Alsanius, B.W.; Bergstrand, K.-J.; Hartmann, R.; Gharaie, S.; Wohanka, W.; Dorais, M.; Rosberg, A.K. Ornamental Flowers in New Light: Artificial Lighting Shapes the Microbial Phyllosphere Community Structure of Greenhouse Grown Sunflowers (Helianthus annuus L.). Sci. Hortic. 2017, 216, 234–247. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Postma, J.; Willemsen-de Klein, M.J.E.I.M.; van Elsas, J.D. Effect of the Indigenous Microflora on the Development of Root and Crown Rot Caused by Pythium aphanidermatum in Cucumber Grown on Rockwool. Phytopathology 2000, 90, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, J.W.; Sackett, J.D.; Lomprey, H.; Hudson, H.L.; Moser, D.P. The Aeroponic Rhizosphere Microbiome: Community Dynamics in Early Succession Suggest Strong Selectional Forces. Antonie Van Leeuwenhoek 2020, 113, 83–99. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the Roots of Aeroponic Indoor Farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.P.; He, J.; Lee, S.K. Effects of Root-Zone Temperature on the Root Development and Nutrient Uptake of Lactuca sativa L. “Panama” Grown in an Aeroponic System in the Tropics. J. Plant Nutr. 2002, 25, 297–314. [Google Scholar] [CrossRef]
- Haase, S.; Neumann, G.; Kania, A.; Kuzyakov, Y.; Römheld, V.; Kandeler, E. Elevation of Atmospheric CO2 and N-Nutritional Status Modify Nodulation, Nodule-Carbon Supply, and Root Exudation of Phaseolus vulgaris L. Soil Biology and Biochemistry 2007, 39, 2208–2221. [Google Scholar] [CrossRef]
- Phillips, D.A.; Fox, T.C.; Six, J. Root Exudation (Net Efflux of Amino Acids) May Increase Rhizodeposition under Elevated CO2. Glob. Chang. Biol. 2006, 12, 561–567. [Google Scholar] [CrossRef]
- Phillips, R.P.; Bernhardt, E.S.; Schlesinger, W.H. Elevated CO2 Increases Root Exudation from Loblolly Pine (Pinus taeda) Seedlings as an N-Mediated Response. Tree Physiol. 2009, 29, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Usyskin-Tonne, A.; Hadar, Y.; Yermiyahu, U.; Minz, D. Elevated CO2 and Nitrate Levels Increase Wheat Root-Associated Bacterial Abundance and Impact Rhizosphere Microbial Community Composition and Function. ISME J. 2021, 15, 1073–1084. [Google Scholar] [CrossRef]
- Berkelmann, B.; Wohanka, W.; Wolf, G.A. Characterization of the bacterial flora in circulating nutrient solutions of a hydroponic system with rockwool. Acta Hortic. 1994, 361, 372–381. [Google Scholar] [CrossRef]
- Calvo-Bado, L.A.; Petch, G.; Parsons, N.R.; Morgan, J.A.W.; Pettitt, T.R.; Whipps, J.M. Microbial Community Responses Associated with the Development of Oomycete Plant Pathogens on Tomato Roots in Soilless Growing Systems. J. Appl. Microbiol. 2006, 100, 1194–1207. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Skandamis, P.; Tzortzakis, N. Salmonella Enteritidis Survival in Different Temperatures and Nutrient Solution PH Levels in Hydroponically Grown Lettuce. Food Microbiol. 2022, 102, 103898. [Google Scholar] [CrossRef]
- Fukami, T. Historical Contingency in Community Assembly: Integrating Niches, Species Pools, and Priority Effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Debray, R.; Herbert, R.A.; Jaffe, A.L.; Crits-Christoph, A.; Power, M.E.; Koskella, B. Priority Effects in Microbiome Assembly. Nat. Rev. Microbiol. 2022, 20, 109–121. [Google Scholar] [CrossRef]
- Boyle, J.A.; Simonsen, A.K.; Frederickson, M.E.; Stinchcombe, J.R. Priority Effects Alter Interaction Outcomes in a Legume–Rhizobium Mutualism. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202753. [Google Scholar] [CrossRef]
- Burr, A.A.; Woods, K.D.; Cassidy, S.T.; Wood, C.W. Priority Effects Alter the Colonization Success of a Host-Associated Parasite and Mutualist. Ecology 2022, 103, e3720. [Google Scholar] [CrossRef]
| Plant Host | Endophytic Partner | Function | Reference |
|---|---|---|---|
| Solanum lycopersicum | Micrococcus luteus | Improved seedling growth. | [24] |
| Arabidopsis thaliana | Escherichia coli | Increased expression of cell wall modification genes. Downregulation of heat shock proteins. | [25,31] |
| Leersia oryzoides/Oryza sativa | Pseudomonas sp. Pantoea sp | Improved root gravitropism. Improved root and shoot growth. Improved root hair formation. | [32] |
| Phragmites australis/Poa annua | Pseudomonas sp. | Improved seed germination. Improved root branching. | [24] |
| Poa reptans | Pseudomonas fluorescens | Production of ethylene. Improved root cell growth. | [26] |
| Panicum virgatum | Burkholderia sp. | Nitrogen fixation. | [33] |
| Gossypium sp. | Bacillus amyloliquefaciens | Improved seedling growth. Increased expression of nitrate transport genes. | [34,35] |
| Vanilla phaeantha | Bacillus amyloliquefaciens | Fungal inhibition. Improved seedling growth. | [36] |
| Saccharum officinarum x spontaneum L. | Burkholderia australis | Nitrogen fixation. Improved seedling growth. | [37] |
| Hedera helix | Bacillus amyloliquefaciens | IAA synthesis. Fungal inhibition via lipopeptide production. | [38] |
| Digitaria ischaemum | Pantoea sp. | Antagonism of competitor Taraxacum officinale. | [39] |
| Cynodon dactylon | Bacillus sp. | Improved root hair formation. | [40] |
| Saccharum officinarum | Gluconacetobacter diazotrophicus | Nitrogen fixation. Phytohormone production. Siderophore production. Bacteriocin production. | [41] |
| Factor | Type of Agriculture | References | |||
|---|---|---|---|---|---|
| Monetary or technological investment | Soil-based, field | Hydroponic, glasshouse | Vertical, glasshouse | Vertical, CEA | [155] |
| Low | Medium | High | Highest | ||
| Energy use | Low | Medium | High | Highest | [156,157,158,159] |
| Potential crop productivity | Lowest | Medium | High | Highest | [156,157] |
| Considerations for farm placement | -Climate -Soil fertility -Access to sunlight -High amount of acreage | -Climate -Access to sunlight -High amount of acreage | -Climate -Access to sunlight -Lower amount of acreage | -Lower amount of acreage | [154,155] |
| Crop traits that limit feasibility | -None | -Extensive roots -Tall height | -Extensive roots -Tall height -Slow growth -Low ratio of marketable plant parts | -Extensive roots -Tall height -Slow growth -Low ratio of marketable plant parts | [13] |
| Commonly produced crops | -Any | -Lettuce -Tomatoes -Herbs -Microgreens -Other leafy greens | -Leafy greens -Microgreens | -Leafy greens -Microgreens | [155,160] |
| Issues | Advantages | Challenges | References |
|---|---|---|---|
| Water Use | -No soil runoff in closed hydroponic systems. -Improved water use efficiency. | -Production can be constrained by freshwater resources. | [165,168,188,189] |
| Nutrition and Fertilization | -Fewer nutrients wasted to runoff. -Fine control of nutrient concentrations. | -Closed loop systems can increase the risk of nutrient toxicity, if mismanaged. | [57,165,166,167] |
| Disease and pests | -Exclusion of pests, pathogens from closed environments. -Sanitation of tools, equipment, growing area. | -High humidity and temperature may be suitable for pathogens. -Rapid spread if pathogen is not excluded. | [57,164,179] |
| Crop productivity | -Consistent, high yields, depending on crop. | -Major staple crops (rice, wheat, corn) are not feasible to grow in a vertical farm. | [13,165] |
| Costs | -Produce transportation savings and minimization of spoilage. -Reduced pesticide requirements. | -High setup and operational costs. | [159,177] |
| Environmental impact | -Minimization of fertilizer runoff and downstream eutrophication. -Reduced use of synthetic fertilizers and pesticides. | -Wastewater accumulation can be high in salts and organic matter. -Intensive energy use from LEDs. | [175,190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaranunt, P.; White, J.F. Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems. Plants 2023, 12, 400. https://doi.org/10.3390/plants12020400
Chiaranunt P, White JF. Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems. Plants. 2023; 12(2):400. https://doi.org/10.3390/plants12020400
Chicago/Turabian StyleChiaranunt, Peerapol, and James F. White. 2023. "Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems" Plants 12, no. 2: 400. https://doi.org/10.3390/plants12020400
APA StyleChiaranunt, P., & White, J. F. (2023). Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems. Plants, 12(2), 400. https://doi.org/10.3390/plants12020400






