A First Report of Sclerotinia sclerotiorum Causing Forsythia Twig Blight in Romania
Abstract
:1. Introduction
2. Results
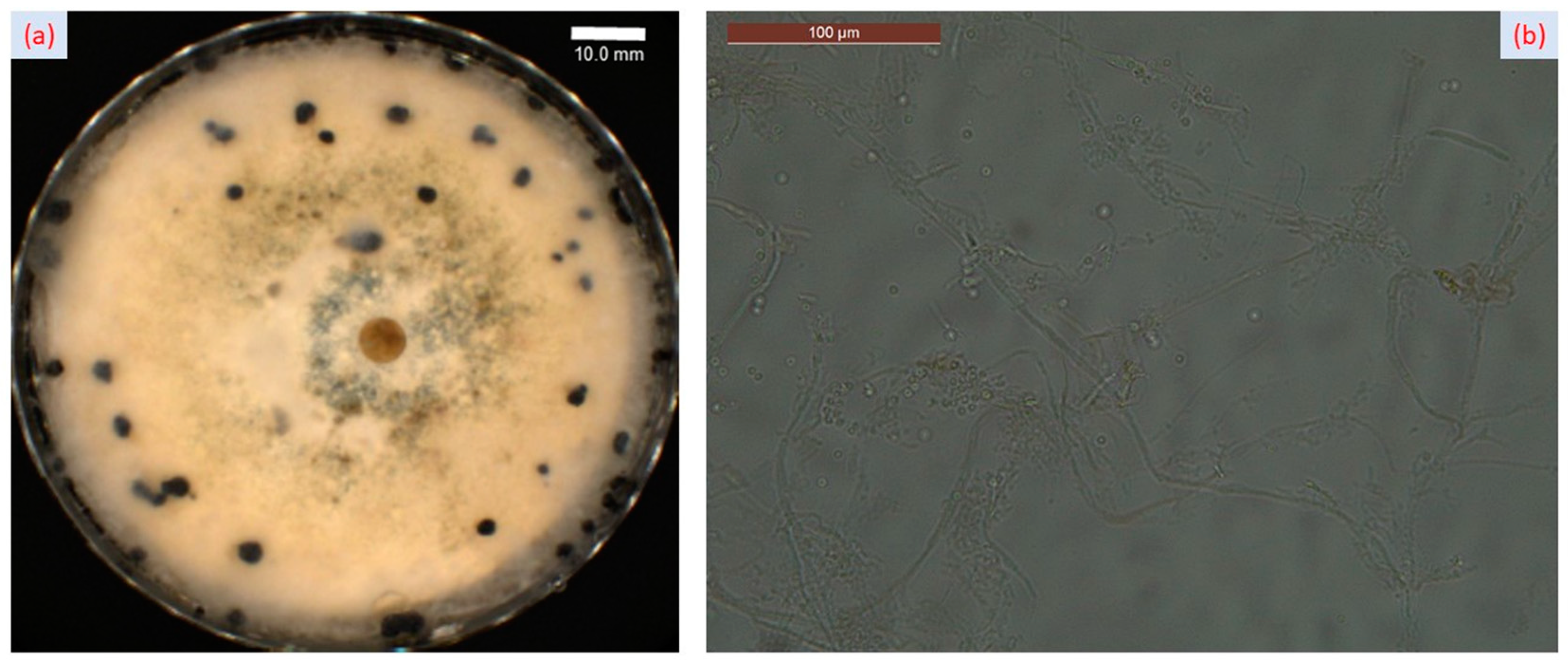
2.1. Fungal Infection and Symptoms
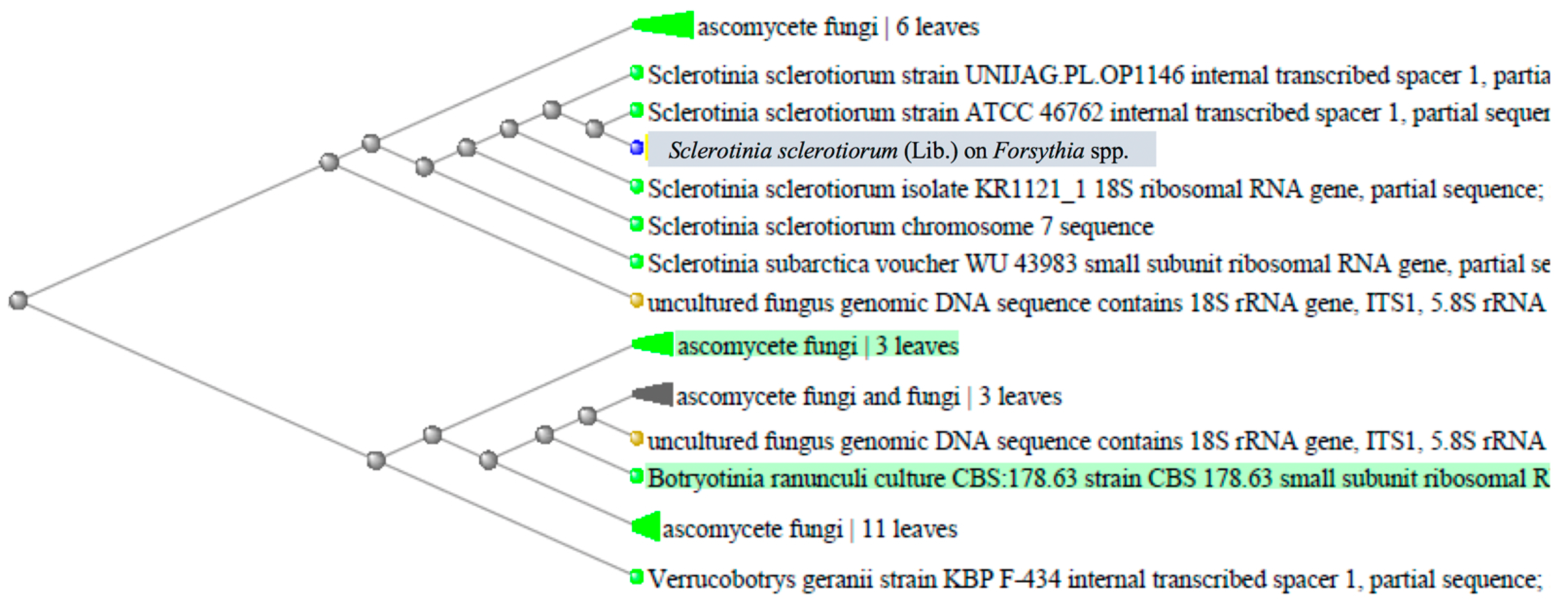
2.2. Sequence Alignment and Phylogenetic Analysis/Molecular Characterization
3. Discussion
4. Materials and Methods
4.1. Diseased Sample Collection and Fungus Isolation
4.2. Pathogen Identification—Morphologic
4.3. Molecular Characterization of Sclerotinia Sclerotiorum Isolates
4.4. Sequencing and Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purdy, L.H. Sclerotinia sclerotiorum: History, Diseases and Symptomatology, Host Range, Geographic Distribution, and Impact. Phytopathology 1979, 69, 875. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the Pathogenomic Features of a Global Pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef]
- Jahan, R.; Siddique, S.S.; Jannat, R.; Hossain, M.M. Cosmos white rot: First characterization, physiology, host range, disease resistance, and chemical control. J. Basic Microbiol. 2022, 62, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akanda, A.M.; Hossain, M.M.; Hossain, M.M. First characterization of a newly emerging phytopathogen, Sclerotinia sclerotiorum causing white mold in pea. J. Basic Microbiol. 2021, 61, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Prova, A.; Akanda, A.M.; Hossain, M.M. First report of white mould caused by Sclerotinia sclerotiorum on pea in Bangladesh. J. Plant. Pathol. 2020, 102, 941. [Google Scholar] [CrossRef]
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.O.; Chen, T.; Zhao, Y.; Gao, Z.; Yang, P.; Barbetti, M.J.; et al. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef]
- Cohen, S.D. Estimating the Climate Niche of Sclerotinia sclerotiorum Using Maximum Entropy Modeling. J. Fungi 2023, 9, 892. [Google Scholar] [CrossRef]
- Borah, T.R.; Dutta, S.; Barman, A.R.; Helim, R.; Sen, K. Variability and host range of Sclerotinia sclerotiorum in Eastern and North Eastern India. J. Plant Pathol. 2021, 103, 809–822. [Google Scholar] [CrossRef]
- Sesan, T.E.; Crisan, A. Putregaiul alb al plantelor de cultura-Sclerotinia sclerotiorum-Prevenire si combatere, Edit. Ceres Bucur. 1998, 15–27. [Google Scholar]
- Bontea, V. Parasitic and Saprophytic Fungi of Romania; Academia Republici Socialiste România: Bucuresti, Romania, 1985. [Google Scholar]
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases; U.S. National Fungus Collections; ARS, USDA: Washington, DC, USA, 2017. Available online: https://nt.arsgrin.gov/fungaldatabases/ (accessed on 15 May 2023).
- O’Sullivan, C.A.; Belt, K.; Thatcher, L.F. Tackling Control of a Cosmopolitan Phytopathogen: Sclerotinia. Front. Plant Sci. 2021, 12, 707509. [Google Scholar] [CrossRef] [PubMed]
- Ekins, M.G.; Hayden, H.L.; Aitken, E.A.B.; Goulter, K.C. Population structure of Sclerotinia sclerotiorum on sunflower in Australia. Australas. Plant Pathol. 2011, 40, 99–108. [Google Scholar] [CrossRef]
- Kim, H.J.; Ono, E.; Morimoto, K.; Yamagaki, T.; Okazawa, A.; Kobayashi, A.; Satake, H. Metabolic engineering of lignan biosynthesis in Forsythia cell culture. Plant Cell Physiol. 2009, 50, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Daughtrey, M.L.; Wick, R.L.; Peterson, J.L. Compendium of Flowering Potted Plant Diseases; APS Press: Saint Paul, MN, USA, 1995. [Google Scholar]
- Grabowski, M.A. Exploring the host range of Sclerotinia sclerotiorum in herbaceous ornamental plants. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2017. Available online: https://conservancy.umn.edu/handle/11299/201043 (accessed on 10 May 2023).
- Jones, R.K.; Benson, D.M. Diseases of Woody Ornamentals and Trees in Nurseries; APS Press: Saint Paul, MN, USA, 2001; pp. 100–125. [Google Scholar]
- Muñoz, M.; Behnke, L.E.; Faust, J.E.; Schnabel, G. First Report of Rose BentNeck Caused by Sclerotinia sclerotiorum on Commercial Cut Roses (Rosa hybrida L.). Horticulturae 2023, 9, 646. [Google Scholar] [CrossRef]
- Rahman, M.M.E.; Suzuki, K.; Islam, M.M.; Dey, T.K.; Harada, N.; Hossain, D.M. Molecular characterization, mycelial compatibility grouping, and aggressiveness of a newly emerging phytopathogen, Sclerotinia sclerotiorum, causing white mold disease in new host crops in Bangladesh. J. Plant Pathol. 2020, 102, 775–785. [Google Scholar] [CrossRef]
- Florea, A.M.; Gafencu, A.M.; Lipșa, F.D.; Ulea, E. New host for Sclerotinia sclerotiorum in the NE region of Romania, Scientific Papers. Series, A. Agronomy 2022, 65, 69–72. [Google Scholar]
- Zanoschi, V.; Sârbu, I.; Toniuc, A. Flora Lemnoasă Spontană și Cultivată din România; Universitatea Alexandru Ioan Cuza: Iași, Romania, 2004; Volume 4, p. 290. [Google Scholar]
- Heffer Link, V.; Johnson, K.B. White Mold. Plant Health Instr. 2007. Available online: https://www.apsnet.org/edcenter/disandpath/fungalasco/pdlessons/Pages/WhiteMoldPort.aspx (accessed on 10 January 2023). [CrossRef]
- Abawi, G.S.; Grogan, R.G. Epidemiology of diseases caused by Sclerotinia Species. Am. Phytopathol. Soc. 1979, 69, 899–904. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 1988. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 6 June 2023).
- Dutta, S.; Borah, T.; Roy Barman, A.; Hansda, S.; Ghosh, P. Overview of Sclerotinia sclerotiorum in India with special reference to its emerging severity in Eastern Region. Indian Phytopathol. 2016, 69, 236–239. [Google Scholar]
- Firoz, M.J.; Xiao, X.; Zhu, F.-X.; Fu, Y.-P.; Jiang, D.-H.; Schnabel, G.; Luo, C.-X. Exploring mechanisms of resistance to dimethachlone in Sclerotinia sclerotiorum. Pest Manag. Sci. 2016, 72, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Mei, J.; Chai, Y.; Yang, W.; Mao, Y.; Yan, B.; Yu, Y.; Disi, J.O.; Rana, K.; Li, J.; et al. Sclerotinia sclerotiorum utilizes host-derived copper for ROS detoxification and infection. PLOS Pathog. 2020, 16, e1008919. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Mei, J.; Chai, Y.; Yu, Y.; Shao, C.; Wu, Q.; Disi, J.O.; Li, Y.; Wan, H.; Qiam, W. Simultaneous transcriptome analysis of host and pathogen highlights the interaction between Brassica oleracea and Sclerotinia sclerotiorum. Phytopathology 2019, 109, 542–550. [Google Scholar] [CrossRef]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Floryszak-Wieczorek, J.; Sobieszczuk-Nowicka, E.; Mattoo, A.; Arasimowicz-Jelonek, M. Fungal and oomycete pathogens and heavy metals: An inglorious couple in the environment. IMA Fungus 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Saharan, G.S.; Naresh, M. Chapter: Infection and Pathogenesis. In Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–217. [Google Scholar] [CrossRef]
- Wang, A.R.; Lin, W.W.; Chen, X.T.; Lu, G.D.; Zhou, J.; Wang, Z.H. Isolation and identification of Sclerotinia stem rot causal pathogen in Arabidopsis thaliana. J. Zhejiang Univ. Sci. B 2008, 9, 818–822. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W.; Innis, M.A.; Gelfand, D.H.; Sninsky, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, A.-M.; Gafencu, A.-M.; Lipșa, F.-D.; Gabur, I.; Ulea, E. A First Report of Sclerotinia sclerotiorum Causing Forsythia Twig Blight in Romania. Plants 2023, 12, 3516. https://doi.org/10.3390/plants12203516
Florea A-M, Gafencu A-M, Lipșa F-D, Gabur I, Ulea E. A First Report of Sclerotinia sclerotiorum Causing Forsythia Twig Blight in Romania. Plants. 2023; 12(20):3516. https://doi.org/10.3390/plants12203516
Chicago/Turabian StyleFlorea, Andreea-Mihaela, Andrei-Mihai Gafencu, Florin-Daniel Lipșa, Iulian Gabur, and Eugen Ulea. 2023. "A First Report of Sclerotinia sclerotiorum Causing Forsythia Twig Blight in Romania" Plants 12, no. 20: 3516. https://doi.org/10.3390/plants12203516
APA StyleFlorea, A.-M., Gafencu, A.-M., Lipșa, F.-D., Gabur, I., & Ulea, E. (2023). A First Report of Sclerotinia sclerotiorum Causing Forsythia Twig Blight in Romania. Plants, 12(20), 3516. https://doi.org/10.3390/plants12203516






