Abstract
Biofertilizer as an amendment has growing awareness. Little attention has been paid to bioremediation potential of indigenous heavy-metal-resistant microbes, especially when isolated from long-term polluted soil, as a bioinoculant in biofertilizers. Biofertilizers are a type of versatile nutrient provider and soil conditioner that is cost-competitive and highly efficient with nondisruptive detoxifying capability. Herein, we investigated the effect of biofertilizers containing indigenous cadmium (Cd)-resistant microbial consortia on rice growth and physiological response. The Thai rice cultivar PSL2 (Oryza sativa L.) was grown in Cd-enriched soils amended with 3% biofertilizer. The composition of the biofertilizers’ bacterial community at different taxonomic levels was explored using 16S rRNA gene Illumina MiSeq sequencing. Upon Cd stress, the test biofertilizer had maximum mitigating effects as shown by modulating photosynthetic pigment, MDA and proline content and enzymatic antioxidants, thereby allowing increased shoot and root biomass (46% and 53%, respectively) and reduced grain Cd content, as compared to the control. These phenomena might be attributed to increased soil pH and organic matter, as well as enriched beneficial detoxifiers, i.e., Bacteroidetes, Firmicutes and Proteobacteria, in the biofertilizers. The test biofertilizer was effective in alleviating Cd stress by improving soil biophysicochemical traits to limit Cd bioavailability, along with adjusting physiological traits such as antioxidative defense. This study first demonstrated that incorporating biofertilizer derived from indigenous Cd-resistant microbes could restrict Cd contents and consequently enhance plant growth and tolerance in polluted soil.
1. Introduction
Heavy metal pollution of soils has been a concerning and persistent environmental problem worldwide. Canal irrigation water mixed together with untreated industrial and agricultural wastewater has been considered a major source of soil pollution, thereby degrading agricultural land [1,2]. Releasing large quantities of untreated wastewater containing heavily toxic metals, i.e., cadmium (Cd), arsenic (As), nickel (Ni), lead (Pb) and chromium (Cr), and letting them transfer by irrigation to water bodies and soils have led to toxicity and lower yields of unsafe crops with unsatisfactory quality [3,4]. Cd contamination of soil from various potential sources including mining and smelting, sewage sludge in agriculture and industrial releases constituted a severe environmental issue because Cd is a nonbiodegradable and highly toxic element conferring deleterious impacts on the food chain and human health [5,6]. It exhibited extreme toxicity even at low concentrations, owing to its high accumulation, mobility and persistency in living systems [7]. Cellular Cd influx in plants was mediated through calcium transport protein channels or specific membrane transport proteins involved in ion transport across plasmalemma [8]. In cereal crops, Cd had adverse effects on seed germination, growth, transpiration, anti-oxidative systems, photosynthetic rate, nitrogen assimilation and yield [5,7,8].
Tak Province in northwestern Thailand is located in close proximity to the Padaeng zinc (Zn) mining site, having areas exhibiting excessive levels of Cd in agricultural soils where health and environmental problems have been identified [9,10]. This has raised strong concerns because the International Water Management Institute discovered significant Cd contamination in rice grains and paddy soils in this province [11]. These elevated Cd levels (ranging from 3.4 to 284.0 mg kg−1 in agricultural areas) were remarkably higher relative to the European Community limit of 3 mg kg−1, posing a high risk to the environment and human health [12]. The augmented deposition of Cd in water bodies and agricultural soils threatened the health status of plants, animals and humans.
Many physicochemical methods have been widely used to reduce toxicity and recover polluted agricultural sites. Alternatively, one bioremediation method using bioadsorbents, i.e., microorganisms, to reduce Cd mobility in soils could be adopted to cope with metal pollution in soils [13,14]. The adsorption/removal of environmental pollutants via soil microbial metabolic potential provides an economical and safe technique compared with other physicochemical methods. Bioremediation using indigenous heavy-metal-resistant microorganisms conferring heavy metal removal and plant-growth-promoting potential would prove a promising choice for agricultural sustainability in metal-contaminated soil.
Biofertilizers are usually formed by the (semi-)solid-state fermentation of agro-industrial wastes and consist of both beneficial microbes and primary nutrients or plant-growth-regulating substances [13,15]. Incorporating biofertilizers in soil would help produce antibiotics and stimulate biodegradation of soil organic matter (OM), thereby increasing nutrient supplies and enhancing plant tolerance to environmental stress. Microbial strains isolated from polluted environments exhibited resistance to higher levels of metals than those isolated from unpolluted areas [16]. Through metal-stress responsive mechanisms, soil microbes applied as biofertilizers effectively promoted the growth of plants implanted in heavy-metal-enriched soils by lowering metal-mediated phytotoxicity [17,18]. In addition, other mechanisms, i.e., plant-growth-promoting bacteria (PGPB), could boost plant development. For instance, they protected colonizing plants by suppressing pathogens by producing antibiotics, hydrogen cyanide and phenazines, etc. [19,20]. Additionally, PGPB could enhance plant growth via N2 fixation [21], solubilization of insoluble phosphorus [22], formation of siderophores [23] and phytohormones [22,24], reducing ethylene content [25], synthesizing antibiotics and antifungal metabolites and inducing systemic resistance [26]. Also, PGPB could increase soil fertility and, in turn, plant yield by providing essential nutrients and growth-regulating substances [27], lowering ethylene-based stress by inducing 1-aminocyclopropane-1-carboxylate deaminase and facilitating plant resistance to abiotic contaminants, e.g., metals and pesticides [26,28]. Previous research revealed that inoculating with plant-growth-promoting rhizobacteria (PGPR) improved maize growth and limited shoot Cd contents in the presence of Cd, as compared with the untreated control [29]. Exploiting the potential of PGPB to detoxify metals as well as versatile plant advantageous characteristics constituted a potent eco-friendly metal bioremediating tool. Hence, biofertilizers have been recognized as clean and efficient soil conditioners or amendments to improve soil characteristics [30,31].
The efficiency of biofertilizers containing indigenous Cd-resistant microbial consortia isolated from Cd-contaminated soil in reducing the phytotoxicity in rice (Oryza sativa L.) has not been well documented. Accordingly, soil with long-term Cd contamination caused by the mining and smelting activities in the Tak Province of Thailand was selected as our focus. The overall objective of this study was to evaluate the effect of biofertilizer amendment on alleviating Cd phytotoxicity by assessing plant growth performance, physiological response and Cd bioaccumulation within different parts of Thai rice cultivar PSL2 until the physiological maturity growth stage. The effects of test biofertilizer on soil properties and bioavailable Cd content were also examined at the growth end stage.
2. Results
2.1. Relative Abundance and Composition Structure of the Test Biofertilizers
The relative abundance and composition of the test biofertilizers BFs were assessed and compared with the enriched bacterial culture consortia BC using a 16S RNA gene amplicon sequencing approach. Bacterial composition in the phyla of the BF was summarized (Table 1). The alteration in the diversity indices of the BF was compared with the BC (Table 2). Diversity indices of the BF were significantly higher than those of the BC, as shown by the increase in the Shannon diversity index and the Chao1 richness estimator.

Table 1.
Physicochemical properties and bacterial composition in phyla of biofertilizer.

Table 2.
Summary of 16S rRNA gene Illumina MiSeq sequencing data and diversity estimates for each sample.
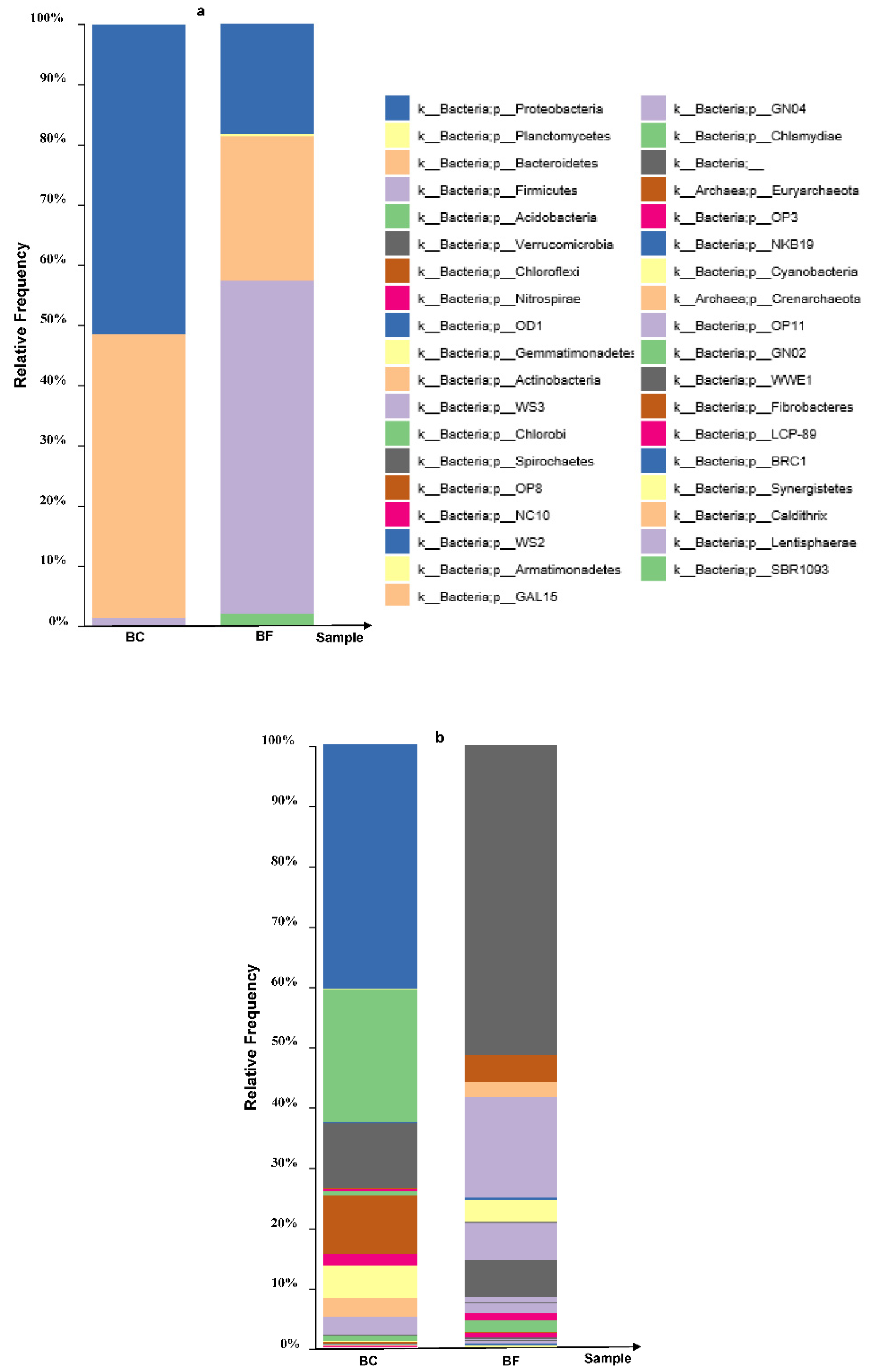
The relative abundance of bacterial phyla in the BF and the BC. Proteobacteria, Bacteroidetes, Firmicutes, Gemmatimonadetes and Acidobacteria were the most dominant phyla (Figure 1). This enriched population of Cd-resistant bacteria was inoculated into the test BF used in this study. Using 16S RNA gene amplicon sequencing, the three top main phyla including Proteobacteria, Bacteroidetes and Firmicutes were explored in the BF relative to the BC (Table 2 and Figure 1a). The relative abundance of Acidobacteria, Actinobacteria, Chloroflexi, Gemmatimonadetes, Planctomycetes and Verrucomicrobia was found to be minor. Although a decrease in Acidobacteria, Gemmatimonadetes and Planctomycetes was observed, they still somewhat remained throughout the biofertilization process.
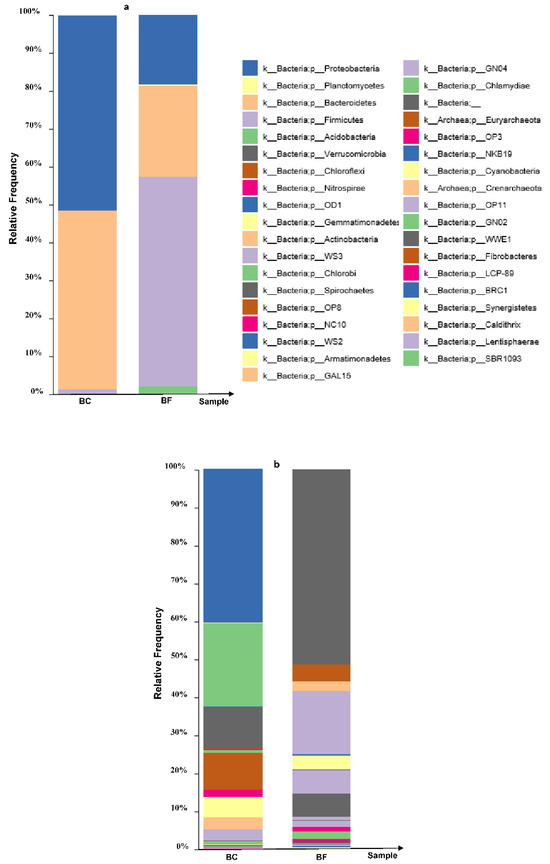
Figure 1.
Relative abundance levels of dominant bacterial phyla (a) and genera (b) in the enriched Cd-resistant bacterial consortium (BC) (cultivable Cd) and the corresponding biofertilizer (BF) based on 16S rRNA gene Illumina MiSeq sequencing. The dominant phyla in the BF and the BC include Proteobacteria, Bacteroidetes, Firmicutes, Gemmatimonadetes and Acidobacteria.
In the BF, predominant detoxifiers at a finer taxonomic level of Proteobacteria (including Comamonas sp., Pseudomonas sp., Stenotrophomonas sp., Acinetobacter sp., Arcobacter sp. and Delftia sp.), Bacteroidetes (including Wautersiella sp., Myroides sp., Cloacibacterium sp. and Paludibacter sp.) and Firmicutes (including Enterococcus sp., Lactobacillus sp. and Lactococcus sp.) were explored among other genera (Figure 1b). The biofertilizer containing the indigenous Cd-resistant bacterial consortium was successfully prepared and subjected to subsequent investigation.
2.2. Effect of Biofertilizer on Soil Physicochemical Traits
The biophysicochemical properties in the phyla of the biofertilizer BF relative to the organic fertilizer OF were summarized (Table 1). Under Cd stress, the BF amendment showed a maximum increase in soil pH, EC, CEC and OM as well as major minerals (N, P and K) and essential minor/trace elements (Ca and Mg) as Cd competitors (Table 3). This might be a result of the intrinsic pH and nutrients of the BF (Table 1). The amendments, by promoting soil pH (Table 3) and OM content (Table 3) and limiting tissue Cd content (Table 4), were ranked in the order of BF > OF. The increase in soil pH and OM content (Table 3) by the test amendments could lower soil bioavailable and tissue contents of Cd (Table 3 and Table 4, respectively), as compared with the control CD.

Table 3.
Effect of biofertilizer containing indigenous Cd-resistant bacterial consortium on soil physicochemical properties and metal contents (N, P, K, Ca, Mg, Cd and Zn) in potting system after plantation under Cd stress.

Table 4.
Effect of biofertilizer containing indigenous Cd-resistant microbial consortia on mineral nutrition (tissue N, P, K, Ca and Mg content, as expressed in mg kg−1) and tissue Cd content (shoot, root and panicle, as expressed in mg kg−1) of PSL2 Thai rice cultivar grown in Cd-enriched soils in potting system.
2.3. Effect of Biofertilizer on Rice Growth under Cd Stress
Cd exposure could impair plant growth, resulting in limited shoot and root length as well as biomass accumulation. All the amendments increased the biomass and length of rice shoot and root under Cd stress, as compared to the non-amended stressed control CD (Table 5). BF amendment in the presence of Cd showed a maximum increase in shoot and root dry weight (46% and 53%, respectively) as well as shoot and root length (27% and 45%, respectively), as compared to the control CD.

Table 5.
Effect of biofertilizer containing indigenous Cd-resistant microbial consortia on biomass and length of shoot and roots of PSL2 Thai rice cultivar grown in Cd-enriched soils in potting system over 4 months.
2.4. Effect of Biofertilizer on Mineral Homeostasis and Cd Bioaccumulation toward Cd Stress
Due to the extent of stress and growth depending on nutrient uptake and translocation, Cd toxicity caused a reduction in the major mineral (N, P and K) and minor/trace element (Ca and Mg) content of rice tissues (Table 4).
The Cd-mediated reduction in shoot N and P contents as well as shoot and root K contents were suppressed by all test amendments (Table 4). Similarly, the losses in the Ca and Mg content of the shoot and root were suppressed by the test amendments (Table 4). Among the other amendments, the BF amendment in the presence of Cd was effective in elevating the maximum levels of tissue N, P, K, Ca and Mg (Table 4).
Cd exposure increased the Cd content of rice parts, with it being much higher in the root than in the shoot (Table 4). The BF amendment in the presence of Cd remarkably reduced tissue Cd contents especially in rice grain (p < 0.05).
2.5. Effect of Biofertilizer on Photosynthetic Pigments, MDA and Proline Content in Response to Cd Stress
Cd toxicity caused a loss of photosynthetic pigments, resulting in chlorotic symptoms. Herein, Cd stress caused a remarkable decrease in chlorophyll (Chl) a, Chl b and carotenoid contents, whereas the BF amendment effectively suppressed these losses of pigments in the presence of Cd (Table 6). Among other amendments, the BF amendment showed a maximum increase in Chl a, Chl b, total Chl and carotenoid synthesis (150%, 125%, 144% and 114%, respectively), in the presence of Cd.

Table 6.
Effect of biofertilizer containing indigenous Cd-resistant microbial consortia on photosynthetic pigments (chlorophyll a and b and carotenoid content), MDA and proline content of PSL2 Thai rice cultivar grown in Cd-enriched soils in potting system.
Malondialdehyde (MDA), a by-product of lipid peroxidation due to oxidative stress, was increased (59%) in the rice leaves under Cd stress (Table 6). Intriguingly, the BF amendment was effective in suppressing the Cd-mediated increase in MDA content.
Cd stress disturbed the water balance, leading to osmolyte accumulation in the rice leaves, as indicated by a great increase in proline content (Table 6). Under Cd toxicity, all the amendments except organic fertilizer OF prevented the accumulation of shoot proline. The BF amendment was effective in suppressing the Cd-mediated accumulation of proline by 39%.
2.6. The Effect of Biofertilizer on Enzymatic Antioxidant Activity upon Cd Stress
Among the enzymatic antioxidants, the activities of the antioxidant enzymes superoxide radical decomposer SOD (35%, 46%) and hydrogen peroxide scavenger APX (40%, 32%) of leaves and roots, respectively, were stimulated by Cd stress, as compared to the non-stressed control seedling CK (Table 7). In the presence of Cd, all the amendments further increased SOD and APX activity, as compared to the control CD. The BF amendment was more effective in promoting Cd-induced SOD (44%, 34%) and APX (38%, 39%) activity both in leaves and roots, respectively.

Table 7.
Effect of biofertilizer containing indigenous Cd-resistant microbial consortia on enzymatic antioxidant activities (SOD, APX, GPX, GR, GST and CAT, as expressed in EU mg−1 protein) of PSL2 Thai rice seedling grown in Cd-enriched soils in potting system.
Similarly, Cd stress induced the activities of the antioxidant enzymes GPX (40%, 33%) and GR (38%, 43%) of leaves and roots, respectively. The BF amendment showed a substantial increase in the GPX (23%, 33%) and GR (23%, 36%) activity of leaves and roots, respectively, as compared to the control CD (Table 7). Among other amendments, the BF amendment showed maximum activities of SOD, APX, GPX and GR, as compared to the control CD (Table 7). By contrast, the activities of the antioxidant enzymes CAT (56%, 49%) and GST (53%, 51%) of leaves and roots, respectively, were decreased due to Cd stress, but BF amendment in the presence of Cd suppressed these losses in CAT (38%, 43%) and GST (48%, 47%) activity, compared to the control CD (Table 7). It is worth noting that the tested biofertilizer was effective in alleviating the Cd toxicity by modulating the activities of SOD, APX, GPX, GR, CAT and GST in the rice tissues with a likely strong impact on roots.
3. Discussion
Among metals, Cd is recognized as a highly toxic metal that impacts the growth and physiological processes, particularly during the early stages of growth and development of the plant, thereby leading to significant yield loss [5,7,8]. Exposure of rice plants to Cd stress negatively affected seed germination [32]. To reduce toxicity and remediate polluted agricultural areas, soil amendments of biofertilizer containing bioagent and fertilizer are renowned with multi-modalities to enhance plant tolerance to environmental stress, nutrient supplies and biodegradation of organic matter [13]. Indeed, indigenous microbial strains isolated from a long-term polluted paddy site displayed resistance to Cd at higher levels than those isolated from unpolluted soil [32]. The efficiency of biofertilizers derived from indigenous Cd-resistant microbial consortia isolated from Cd-contaminated soil in mitigating Cd phytotoxicity has not been well elucidated yet.
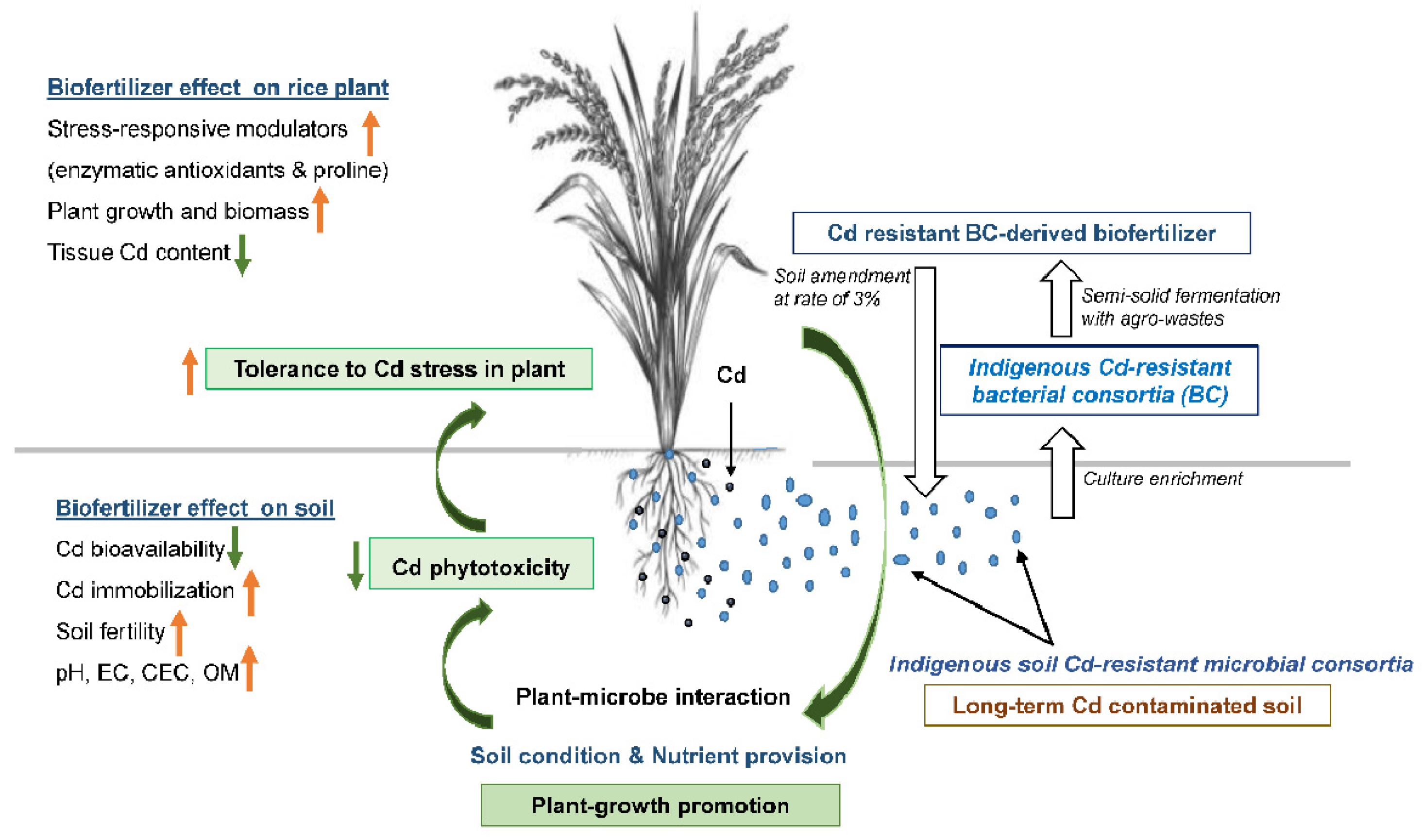
Our study firstly demonstrated that the soil amendment of biofertilizer containing indigenous cadmium-resistant microbial consortia could mitigate the adverse effect of Cd and improve plant growth by restoring photosynthetic pigment losses and supplying essential nutrients in Thai rice cultivars (PSL2). Additionally, the test biofertilizer consisting of bioagent and fertilizer was effective in overcoming water imbalance and oxidative stress by adjusting the content of proline and the activities of antioxidant enzymes, respectively, and limiting Cd bioaccumulation by manipulating soil physicochemical traits and mineral homeostasis (Figure 2).
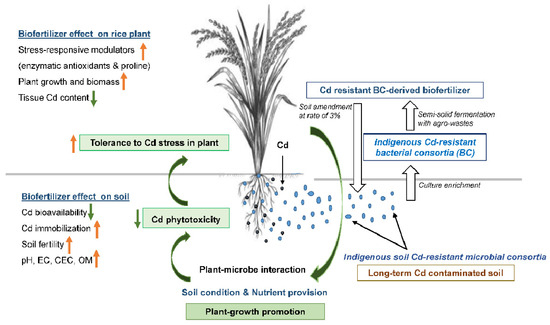
Figure 2.
Scheme showing mitigating potential of indigenous Cd-resistant soil-microbe-derived biofertilizer to Cd toxicity. Application of biofertilizer containing indigenous Cd-resistant soil microorganisms could alleviate Cd stress in PSL2 Thai rice cultivar grown in contaminated soil, due to improved soil biophysicochemical and nutrient availability, thereby leading to lower tissue Cd content and restored growth. Especially, soil pH increased after applying biofertilizer could limit soil Cd bioavailability and toxicity to rice roots. Beneficial phyla detected in the biofertilizer, i.e., Bacteroidetes, Firmicutes and Proteobacteria, could help plants cope with Cd-induced oxidative stress by adjusting antioxidative defense, water relation and photosynthetic systems. Orange-and green-colored small arrows indicate increase and decrease of that parameters, respectively.
In the biofertilizers, Proteobacteria, Bacteroidetes, Firmicutes and Gemmatimonadetes were determined as the most dominant bacterial phyla using 16S rRNA gene Illumina MiSeq sequencing (Figure 1). In agreement with findings from related studies, the biofertilizer pH and organic carbon could affect the abundance of Bacteroidetes, Gemmatimonadetes and Proteobacteria, and these phyla were also dominant in biofertilizer [33]. It suggested that the biofertilizer pH and organic carbon played key roles in shaping the enriched microbial communities. Moreover, soil Cd bioavailability could positively influence the microbial communities in the biofertilizer-treated soils, and some Cd-coexistence bacteria, i.e., Chloroflexi, Acidobacteria and Saccharibacteria, might have become dominant due to excess Cd in rhizosphere soils [33]. In addition, the solubility and availability of soil phosphate were determined by specific microbial activities, while soil phosphate concentration became a determinant for Cd phytotoxicity [33].
One promising method for alleviating Cd stress and promoting plant growth is the bioaugmentation of microorganisms. For instance, the application of the amendment of Pseudomonas aeruginosa, Bacillus subtilis, Cupriavidus taiwanensis and Beauveria bassiana to soil significantly restricted tissue Cd content in rice (Oryza sativa L.) and improved plant growth performance under Cd stress, owing to the biotransformation of the toxic Cd form to nontoxic insoluble form of Cd sulfide (CdS) and adsorption by Cd-binding proteins [34,35]. Another related study showed that a multifunctional biofertilizer containing PGPB Pseudomonas putida PT promoted maize growth and reduced shoot Cd contents in Cd-contaminated soils [29,36]. Very recent findings indicated that highly Cd-tolerant bacterial strains, Pseudomonas putida 23483 and Bacillus sp. GY16, had Cd removal efficiency in solution via biosorption and passivated soil via Fe-Mn binding in the rhizosphere of rice plants [37]. Related findings revealed that adding Cd-resistant Comamonas sp. XL8 mitigated Cd toxicity and oxidative stress in rice seedlings via intracellular formation of Cd nanoparticles [38]. Moreover, co-inoculation of Comamonas and Enterobacter species improved rice defense response, alleviated Cd stress and reduced Cd content in rice grain via Cd immobilization in soils under a pot experiment [39]. Although soil microorganisms have been extensively used to promote plant performance and restrict soil Cd, adding microorganisms into fields together with an appropriate organic substrate is recommended to maintain their substantial activities over a long period [40]. The combined advantages of bioagents and fertilizers would be an alternative to mitigate Cd phytotoxicity.
Our study revealed that the application of the biofertilizer BF increased soil chemical properties such as pH, EC, CEC and OM content (Table 3), in addition to a decrease in DTPA-extractable Cd and Zn (Table 3). Mineral nutrition containing basic cations (K, Ca and Mg) in the biofertilizer could contribute to the increase in soil pH, EC and CEC, while organic residues derived from agricultural wastes were involved in the nourishment of soil organic carbon. An increase in soil EC and CEC could also be regarded as a key factor in modulating heavy metal exchangeability and bioavailability [41,42]. Increasing soil pH after applying biofertilizer could increase the negatively charged surfaces as well as alkaline conditions, such as hydroxide and carbonate groups, which could support active substances for surface sorption, precipitation and complexation, thus reducing heavy metal bioavailability with less possibility to enter the food chain [43]. In addition, soil OM could stabilize soil Cd via microbial reduction of native oxidized soil constituents upon watering submergence [44]. OM harboring negatively charged surfaces could form stable complexes with metal ions, leading to limiting metal solubility and bioavailability to plants [45]. OM showed an effect on lowering soil Cd bioavailability and bioaccumulation in rice via Cd adsorption and the formation of stable complexes with Cd [14].
Compared to the control, the BF amendment remarkably increased soil pH, but the OF amendment had less impact on increasing soil pH (Table 3). This could be due to the initial pH of the amendments (Table 1). Although applying the OF amendment had a slight effect on soil pH, this amendment still decreased Cd contents in rice tissues compared to the control (Table 3 and Table 4). These results indicated that besides pH, the mineral nutrients, e.g., N, P, K, Ca, Mg, S and Fe, and microorganisms in the biofertilizer might play crucial roles in controlling Cd uptake and bioaccumulation [46]. Related studies reported that soil pH, DTPA-extractable Cd, total phosphorus and organic carbon were determined as the most pivotal environmental factors contributing to the changes in microbial community composition [33,47,48]. Additionally, soil organic carbon serving as the carbon source for bacteria was considered crucial. Indeed, soil pH and OM were considered important factors for limiting Cd availability [33,49]. Moreover, potential parameters other than soil pH and OM, including the mineral nutrition, e.g., N, P, K, Ca, Mg, S and Fe, and microorganisms in the biofertilizer itself remained effective in suppressing Cd bioaccumulation [14,33,50].
The retarded plant growth toward Cd stress could be due to various factors, which adversely affect water status, nutrient assimilation, mineral homeostasis, photosynthesis, oxidative stress response, etc. Our study indicated that the biofertilizer BF was effective in promoting growth performance in rice plants under Cd stress (Table 5). This might be elucidated by the fact that beneficial microorganisms, as well as mineral nutrition, in the biofertilizer could facilitate Cd immobilization in the soil and lower its toxicity to roots, possibly allowing plants to assimilate more nutrients [13,51].
Among the other amendments, the biofertilizer BF exhibited the lowest level of tissue Cd as shown by the decrease in grain Cd content, compared to the non-amended control (Table 4). This implied that the test biofertilizer had a mitigating effect on limiting Cd uptake and accumulation in rice due to the higher pH and OM content of the biofertilizer BF (Table 1 and Table 3), both of which were responsible for restricting Cd in soils by facilitating the generation of stable metalo-organo complexes at elevated pH levels [49]. Cadmium is able to form Cd hydroxide at high soil pH (>7), resulting in the promotion of Cd adsorption to soils. Indeed, rice has been regarded as a Cd-sensitive plant and an accumulator of Cd, often containing >0.1 mg Cd kg−1 dry biomass [52]. Indeed, the nutrients in the biofertilizer itself could also promote native microbial activity in the amended soils, thereby leading to stimulated nutrient cycling, hormone production, plant symbioses and ultimately enhanced plant tolerance to stress [13,14].
One of the adverse effects of metal stress is nutrient deficiency, as toxic metal elements compete with essential minerals for plant uptake [53]. Hence, nutrient homeostasis plays a role, in part, in preventing metal accumulation, thereby reducing their toxicity and further facilitating physiological mechanisms under Cd stress. Our study indicated that Cd stress disturbed the mineral homeostasis in rice plant, as shown by the decrease in shoot and root K, Ca and Mg content (Table 4), in agreement with other previous work [54,55,56] that has demonstrated a hermetic effect of Cd on mineral homeostasis in several plant species. Furthermore, Cd-mediated oxidative stress caused membrane damage, probably leading to reduced nutrient content in roots. Herewith, the BF amendment in the presence of Cd could restore the mineral balance, suggesting that BF-related nutrient provision could mitigate the toxic effects of Cd bioaccumulation and adjust the plant’s ability to maintain its physiological functions. Similarly, organic fertilizer amendments effectively promoted rice yield in Cd-polluted soil and reduced Cd content in rice grain [57].
One of the deleterious effects due to metal stress is the remarkable destruction of the photosynthetic apparatus. The reduction in chlorophyll biosynthesis and its content was observed in various plant species under Cd stress [55,58,59]. Our study revealed a remarkable decrease in photosynthetic pigment content in terms of the Chl (a + b) and carotenoid content in rice seedling leaves under Cd stress. These losses of pigments due to Cd toxicity resulted from the suppression of relevant enzymes, thereby leading to impaired pigment biosynthesis. In addition, the peroxidative breakdown of photosynthetic pigments and the chloroplast membrane lipid responded to abiotic stress due to excessive ROS [60]. However, these photosynthetic pigment losses could be suppressed when applying the biofertilizer (Table 6), in consistence with a previous report [61]. Alleviating Chl and carotenoid content would be linked with an increase in Cd sequestration or pigment biosynthesis and/or decrease in the breakdown of pigment complexes [62].
Similar to other xenobiotic stresses, Cd stress induces the formation of free radicals (e.g., superoxide, hydrogen peroxide) that are capable of damaging the cell membrane and biomolecules (e.g., DNA, proteins) [62]. Our study reported that a by-product of membrane lipid peroxidation MDA, a main oxidative stress indicator, was noticeably increased in response to Cd stress (Table 6). This response would be attributed to an excessive level of ROS by Cd stress. However, the BF amendment suppressed the Cd-mediated generation of MDA (Table 6).
Upon Cd stress, plants exhibited a range of secondary stress symptoms, including osmotic changes [63]. Under stress, the plants utilize crucial strategies by adjusting osmolytes to alleviate the water imbalance. For instance, biosynthesis and accumulation of proline, glycine betaine and trehalose led to osmotic adjustment of Na+ stress inside cells to equilibrate water balance [63,64]. Our study showed an increased proline content in rice tissues under Cd stress, but this accumulation was alleviated by the BF amendment (Table 7). The exogenous application of biofertilizer could mitigate the water imbalance in Cd-stressed rice plants by modulating the biosynthesis of proline.
Plants have vital strategies for the avoidance of Cd toxicity. For the prime line on counteracting excessive ROS and serving as cellular redox buffers, the plant’s antioxidant defense system consists of antioxidants that are enzymatic (e.g., superoxide dismutase SOD, ascorbate peroxidase APX, catalase CAT, glutathione peroxidase GPX, glutathione reductase GR and glutathione-S-transferase GST) and non-enzymatic (ascorbic acid, glutathione, tocopherol and phenolic compounds) [60,63]. Both enzymatic and non-enzymatic antioxidants work simultaneously to combat Cd-induced oxidative stress. Upon exposure to metal stress, the plant’s enzymatic antioxidant activity gradually rises with increasing metal concentration while these activities drop, and the enzymatic defense system is ultimately disrupted with extremely high metal concentration.
Recent findings indicated that APX and SOD, GR and GPX, and GST and CAT were regarded as prominent enzymatic antioxidants in the plant’s defense system for scavenging toxic O2.− radicals and converting them to H2O2 [65]. The dramatical change in APX and SOD activity toward oxidative stress was due to overproduced O2.− and H2O2 content, which was endured by the test biofertilizer (Table 7). Our findings were also in line with another previous study that reported remarkable changes in the activities of APX and SOD as well as other enzymatic and non-enzymatic antioxidants in plant responses to Cd stress [59]. Similarly, recent research documented an increase in the activities of potent antioxidant enzymes APX and SOD and GR and GPX in a concentration-related manner (1.0 mM and 2.0 mM CdCl2, respectively), as compared to the control rice seedlings (Oryza sativa L. cv. BRRI dhan54) [65].
In addition, this Cd-related increase in GPX and GR activity (Table 7) was responsible for scavenging H2O2 and GSSG to GSH recycling, in accordance with other previous work [66]. A substantial increase in GR and GPX activity against Cd-mediated oxidative stress was due to overproduced H2O2 and GSH content (Table 7), which was endured by the test biofertilizer, similar to previous findings [65]. Detoxifying H2O2 was mediated with the AsA-GSH cycle via the GPX/GST and GR machinery system for the conversion of GSH to GSSG for AsA recycling and the recycling of GSSG to GSH, respectively, while detoxifying xenobiotics via GST [66]. By contrast, the GST activity was reduced in the Cd-stressed seedlings (Table 7), presumably because the increased antioxidant activity hampered the overproduction of H2O2. The CAT activity was also reduced under Cd stress (Table 7), similar to another previous report [67]. However, the BF amendment suppressed the Cd-mediated reduction in CAT activity (Table 7).
In summary, the biofertilizer containing the indigenous Cd-resistant bacterial consortium therefore appeared to enhance plant growth and tolerance to Cd stress. In our investigation, the soil supplementation of the test biofertilizer at a 3% applied rate in a Thai rice cultivar (PSL2) could mitigate Cd phytotoxicity by modulating the antioxidative defense system, water and nutrient homeostasis and restricting soil Cd bioavailability. These responses most likely reflected multi-modalities of the biofertilizer harboring such microbial detoxifiers and organic residues, which could help adjust the plant’s antioxidative defense and water relation together with the improvement of soil biophysicochemical traits in coping with Cd toxicity. Furthermore, the test biofertilizer would offer the development of an eco-friendly sustainable strategy for improving plant fitness and soil quality rather than those using foreign strains, with practicability and simplicity.
4. Materials and Methods
4.1. Collection and Analysis of Soil Samples
The long-term polluted top soils (<20 cm in depth) used for greenhouse experiments were sampled from an agricultural area in Pha Dei Village, Mae Sot District, Tak Province, Thailand (N 16°40′35.9″ E 98°37′37.4″), at an altitude of 197 m. The soil at this site was tilled for either rice-corn or rice-bean crops in one cropping year. The selected physicochemical characteristics of the soil are shown in Table 4. Soil samples were divided into two main portions: one for physicochemical characterizations and the other for enriched culture following biofertilizer preparation.
Soil material was homogenized, air-dried, crushed and sieved (2 mm mesh size). The following physicochemical properties of the soil were determined: pH and electrical conductivity (EC) (1:5 soil/water suspensions) using a pH meter and an EC meter, respectively; OM content by wet oxidization and titration according to the modified Walkley–Black procedure [68]; cation exchange capacity (CEC) using 1 N ammonium chloride pH 7.0 after pretreatment to remove soluble salts [69]; total N by using the Kjeldahl method [70]; extractable P by using Bray II method [71]; and extractable K using an atomic absorption spectrophotometer (Perkin Elmer AAnalyst 200, Waltham, MA, USA) after ammonium acetate extraction at pH 7.
4.2. Preparation and Analysis of Cd-Resistant Biofertilizer
The biofertilizers used as amendments for remediation of Cd-contaminated soils were prepared using repeated culture enrichment of the soil Cd-resistant bacteria as previously described [32], followed by semi-solid fermentation/biofertilization conditions. Topsoil (<20 cm in depth) was collected from a long-term Cd- and Zn-contaminated agricultural area in Pha Dei Village, Mae Sot District, Tak Province (N 16° 40′ 35.9″ E 98° 37′ 37.4″), at an altitude of 197 m for culture enrichment. To enrich Cd-resistant bacteria (BC), the first 5 g of each topsoil sample was added to 95 mL of nutrient broth (NB, 0.5% peptone, 0.3% meat extract, pH7.0) containing 50 or 100 ppm Cd chloride (CdCl2). After two weeks of consecutive incubation at 30 °C, the bacteria were cultured on nutrient agar plates (NA, nutrient broth and 1.5% agar) supplemented with CdCl2 for 72 h at 30 °C. The colonies of Cd-resistant bacteria were quantified as colony-forming units per milliliter (CFU ml−1). The test biofertilizer (BF) was prepared under aerobic conditions, using enriched BC with rice bran supplemented with micronutrients and mineral additives to stimulate fermentation. The organic fertilizer (OF) was produced by fermenting the rice bran supplemented with micronutrients and mineral additives as aforementioned in an aerobic environment, in absence of BC. The biofertilizers were stored at 4 °C prior to use in greenhouse experiments. Hence, the treatments used in this study are listed in Table 8. The main components and bacterial compositions of each amendment are shown in Table 1.

Table 8.
Experimental treatments and their nomenclature used in this study.
For physicochemical analyses, properties of the biofertilizers as soil amendments or conditioners were determined: pH using a pH meter and OM content using wet oxidization and titration according to the modified Walkley–Black procedure [68]. Total contents of metal elements including Cd, Zn, Ca, Mg, S, Fe and Mn in biofertilizer samples were determined using microwave digestion and quantification using an atomic absorption spectrophotometer (Perkin Elmer AAnalyst 200, Waltham, MA, USA).
Bacterial diversity and composition of the test biofertilizers compared with the enriched consortia were analyzed using 16S rRNA gene Illumina MiSeq sequencing as previously described [32]. Total genomic DNA was extracted from 10 mL of the enriched culture and the biofertilizers (three biological replicates per treatment) using QIAamp® DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions with some modifications. The 16S rDNA (V3-V4) bacterial primers containing the Illumina overhang adapter sequences (as underlined) 341F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG) and 805R (5′- GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) were used for PCR amplification [72]. The PCR mixtures (25 µL) contained 12.5 µL of 2x KAPA HiFi Hot Start Readymix (KAPA Biosystems, Wilmington, MA, USA), 5 µL of each primer (1 µmol l−1) and 2.5 µL of target DNA (5 ng µL−1). The PCR cycling conditions consisted of an initial denaturation step at 94 °C (3 min), followed by 25 cycles of 98 °C (20 s), 55 °C (30 s) and 72 °C (30 s) and a final elongation at 72 °C (5 min). The PCR products were cleaned up on AMPure XP beads (Agencourt Bioscience, Indianapolis, IN, USA). The purified amplicons (550-bp fragments) were submitted to the Omics Sciences and Bioinformatics Center (Chulalongkorn University, Bangkok, Thailand) for paired-end sequencing on the Illumina MiSeq platform. Subsequently, the purified 16S RNA gene amplicons were then indexed using 2× KAPA hot-start ready mix and 5 µL of each Nextera XT index primer in a 50 µL PCR reaction, followed by 8 to 10 cycles of PCR amplification. The PCR cycling was set as aforementioned. Next, the indexed 16S RNA gene amplicons were purified on AMPure XP beads (Agencourt Bioscience, Indianapolis, IN, USA), pooled and diluted to a final loading concentration of 4 pM. Cluster generation and 250-bp paired-end read sequencing were performed on an Illumina MiSeq using the MiSeq Reagent Kit. Amplicon sequence analysis was performed with QIIME version 1.9.0. [73]. All sequence reads were sorted based on their unique barcodes, trimmed for sequence quality and clustered at 97% identity for operational taxonomic units (OTUs). The UCHIME algorithm was used to discard chimera sequences [74].
The microbial diversity index in terms of diversity (Shannon index) and richness (Chao1 index) was subsequently computed using MOTHUR [75]. To investigate the microbial composition and diversity, the Shannon diversity index, an estimator of species richness and diversity using a natural logarithm, accounts for both abundance and evenness of the taxa present, while the Chao1 richness estimator reflects diversity from abundance data and the number of rare taxa missed from under-sampling.
4.3. Greenhouse Experimental Design
All the experiments involving plants adhered to the relevant ethical guidelines on plant usage. Table 1 presents the treatments used in this study. A 2000 g soil sample was crushed, sieved (2 mm mesh size) and placed in a plastic pot as previously described with some modifications [33]. Biofertilizers at a rate of 3% were mixed in long-term Cd-contaminated soil. This rate was chosen since it could contribute to optimal growth performance when varying BF concentrations (1%, 3% and 5%). Hence, NPK basal fertilizer containing 0.25 g urea kg−1 soil, 0.15 g KH2PO4 kg−1 soil and 0.04 KCl kg−1 soil was initially dissolved in deionized water and thoroughly mixed with the soil in each pot. Subsequently, all pots were incubated with moisture at 75% of water holding capacity for five weeks to allow the nutrients in biofertilizers to be released in the soil, as well as promote the microbes in biofertilizers to work and to functionally act toward Cd stress. Thai rice seeds (PSL2) were sterilized with 5% hydrogen peroxide (H2O2) for 5 min, rinsed with distilled water and placed in a Petri dish containing two pieces of filter paper. After germination, eight rice seedlings were transplanted in each plastic pot. The pots were arranged in a randomized complete block design with six replicates for each treatment. During rice growth, each pot was irrigated every three days with distilled water to maintain soil moisture at ca. 60 to 70% of water holding capacity. Greenhouse conditions were as follows: temperature 26 to 40 °C, 55 to 70% relative humidity, 5500 to 50,000 lx light intensity and a 12/12 h photoperiod.
After 30 days of treatment, the roots and shoots were collected separately per biological replicate and stored at 4 °C for measuring proline and malondialdehyde (MDA) content, photosynthetic pigments and different antioxidant enzymes.
Four months after transplantation, the plant samples were washed with tap water and rinsed with deionized water several times until all excess soil was removed, and then the shoots and roots were harvested. Plant materials were oven-dried at 80 °C for four days before determining weight. Soil material was collected from each pot and allowed to air-dry for five days. Soil and plant samples were subjected to chemical analyses.
4.4. Measurement of Total Protein Content
The total protein content of rice leaves was quantified as previously described [76]. Plant leaves (0.5 g) were ground and added with phosphate buffer. The mixture was centrifuged at 2500× g for 10 min. The resulting supernatant (0.1 mL) was added with distilled water to make the volume up to 1 mL. This solution was added with an equal volume of alkaline CuSO4 reagent and shaken for 10 min. Finally, the Follin reagent was added and then incubated for 30 min at 28 ± 2 °C. Readings were measured at 650 nm. Bovine serum albumin (BSA) was taken as a reference for the calculation of total protein contents.
4.5. Estimation of Photosynthetic Pigments
Photosynthetic pigments (chlorophyll (Chl) a and b and carotenoids) of rice leaves were estimated as previously mentioned [77]. Plant leaves (0.5 g) were added with 10 mL of dimethyl sulfoxide (DMSO) and then heated at 65 °C in water bath for 4 h. The supernatant was separated, and its absorbance was recorded at 663 nm and 645 nm for Chl a and Chl b, respectively, and 480 nm for carotenoids.
4.6. Estimation of Proline Content
Proline contents were determined by using previous protocol [78]. Rice leaves (0.5 g) were ground in 80% ethanol and then heated at 80 °C for 1 h in a water bath. After centrifugation, 0.5 mL supernatant was taken into a new test tube, added with 0.5 mL dH2O and 1 mL of 5% phenol and placed in an incubator for 1 h. After incubation, 2.5 mL sulfuric acid was added, and the readings were measured at 490 nm.
4.7. Estimation of Malondialdehyde (MDA) Content
MDA content as thiobarbituric acid (TBA) reactive substances in rice tissues was estimated by using previous protocol [79]. Initially, fresh harvested rice leaves (0.5 g) were extracted by 5% trichloroacetic acid (TCA) using a chilled mortar and pestle and then centrifuged at 11,500× g for 15 min. The supernatants were mixed with TBA for reaction to generate the MDA, which was subsequently measured by the absorbance difference at 532 and 600 nm and calculated using an extinction coefficient of 155 mM−1 cm−1 expressed as nmol g−1 FW.
4.8. Determination of Enzymatic Antioxidant Activities
4.8.1. Enzyme Extracts
For preparing enzyme extracts, 0.5 g leaves and roots were ground in 3 mL phosphate buffer (pH 7.8) and subjected to homogenization on ice. The solution was made to 5 mL and centrifuged at 12,500× g for 20 min at 4 °C. The supernatant was covered with aluminum foil to avoid light exposure and stored at 4 °C for subsequent enzyme assays.
4.8.2. Ascorbate Peroxidase (APX) Activity
APX (EC# 1.11.1.11) activity was quantified by examining the rate of ascorbate oxidation at a wavelength of 290 nm as the previous method [80]. The reaction mixture consisted of 50 mM phosphate buffer pH 7.0, 0.1 mM H2O2, 0.5 mM ascorbic acid and 100 µL of enzyme crude extract.
4.8.3. Superoxide Dismutase (SOD) Activity
SOD (EC# 1.15.1.1) activity was subjected to assess the inhibition in the photoreduction of nitro blue tetrazolium (NBT) as previous procedure [81]. Reaction mixture was taken with 50 mM sodium phosphate buffer (pH 7.6), 0.1 mM EDTA, 50 mM sodium carbonate, 12 mM L-methionine, 50 µM NBT, 10 µM riboflavin and 100 µL of enzyme crude extract. For comparison, a set of reactions with all components except the crude extract was taken as control. To start the reactions, the reaction tubes were exposed to white light for 15 min. Reactions were terminated by switching off the lights, and readings were recorded at 560 nm.
4.8.4. Glutathione Peroxidase (GPX) Activity
Glutathione peroxidase (EC# 1.11.1.9) activity was determined according to the previous procedure [82], where the reaction buffer contained 100 mM potassium phosphate buffer (pH 7.0), 1 mM EDTA, 1 mM sodium azide (NaN3), 0.12 mM NADPH, 2 mM GSH and 1 unit of GR. The reaction used 0.6 mM H2O2 as substrate, and the activity was calculated using extinction coefficient of 6.6 mM−1 cm−1.
4.8.5. Glutathione Reductase (GR) Activity
Glutathione reductase (EC# 1.6.4.2) activity was determined according to previous protocol [83] by observing the decline in absorbance at 340 nm, where reaction mixture consisted of 100 mM potassium phosphate buffer (pH 7.0) and 1 mM EDTA. The reaction was NADPH-dependent and initiated with GSSG. Glutathione reductase activity was finally calculated using 6.2 mM−1 cm−1 as extinction coefficient.
4.8.6. Glutathione S-Transferase (GST) Activity
Glutathione S-transferase (EC# 2.5.1.18) activity was spectrophotometrically examined according to previous protocol [84], where the reaction mixture contained 1.5 mM GSH, 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) and enzyme crude extract. The increase in absorbance was read at 340 nm for 1 min and the enzyme activity was computed using the CDNB extinction coefficient of 9.6 mM−1 cm−1.
4.8.7. Catalase (CAT) Activity
Catalase (EC# 1.11.1.6) activity was determined according to the previous method [85] by observing the decrease in absorbance at 240 nm for 1 min (as conversion of H2O2 to water and O2), where the enzyme extract was used to initiate the reaction. The activity of enzyme was computed using 39.4 M−1 cm−1 as extinction coefficient.
4.9. Sampling and Metal Analyses of Soil and Plant Tissue
Total and extractable contents of metal elements in soil and plant samples were determined using microwave digestion and diethylenetriamine pentaacetate (DTPA) extraction, respectively. Total (strong acid-extractable) Cd and Zn in soil before and after planting were estimated by digesting approximately 1 g of air-dried soil with 4.5 mL of 37% hydrochloric acid (HCl), 1.5 mL of 65% nitric acid and 1 mL of 30% H2O2 in a microwave digestion system (Milestone ETHOS One, Twinsburg, OH, USA). A similar procedure was employed to digest plant materials but without HCl addition. The amount of DTPA-extractable Cd and Zn in soil was determined using the mixture (0.005M DTPA, 0.01 M CaCl2 and 0.1 M triethanolamine, pH 7.30) at a soil-to-solution ratio (w/v) of 1:2. The metal concentrations in both the digests and extracts were quantified using an atomic absorption spectrophotometer (Perkin Elmer AAnalyst 200, Waltham, MA, USA).
4.10. Statistical Analyses
Data were subjected to statistical analysis using two-way ANOVA (SPSS Software version 18) to detect significant differences with 95% confidence level (p-value ≤ 0.05).
5. Conclusions
Amending with biofertilizer derived from indigenous Cd-resistant microbes was effective in mitigating the Cd phytotoxicity by modifying the soil biophysicochemical traits to restrict Cd bioavailability and enhance plant tolerance toward environmental stress. The promoting effect of biofertilizer could be due to a rise in soil pH, nutrient homeostasis and its enrichment of beneficial detoxifiers such as Bacteroidetes, Firmicutes and Proteobacteria, which stabilized soil Cd and limited its bioavailability, in addition to triggering stress-responsive modulators such as antioxidative enzymes and proline osmolyte. Our results indicated that the test biofertilizer as combined bioagents with fertilizer could not only nourish soil fertility but also serve as an effective amendment, especially at an applied rate to immobilize Cd in the polluted soil. These findings introduced a promising means for the sustainable development of strategies in the bioremediation of Cd-contaminated soils and plant growth improvement. Further study should be designed in the field setting to determine the efficiency of other organic residues in the heavy metal decontamination in soils to reduce the health risk of exposure to excessive toxic metals via the food chain resulting from anthropogenic environments.
Author Contributions
The funding acquisition was prepared by P.K. The experimental conceptualization and design were conducted by P.K. Methodology, material preparation, data collection and analysis were performed by L.S.-O. and P.K. The first draft of the manuscript was written by P.K. with suggestion by W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from Mahidol University, Thailand, under the program titled “Stabilization and bioremediation of cadmium- and zinc-contaminated soil for sustainable rice cultivation” from the National Research Council of Thailand.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and information files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rafique, M.; Ortas, I.; Rizwan, M.; Sultan, T.; Chaudhary, H.J.; Işik, M.; Aydin, O. Effects of Rhizophagus clarus and biochar on growth, photosynthesis, nutrients, and cadmium (Cd) concentration of maize (Zea mays) grown in Cd-spiked soil. Environ. Sci. Pollut. Res. 2019, 26, 20689–20700. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in Cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308. [Google Scholar] [CrossRef] [PubMed]
- Din, B.U.; Rafique, M.; Javed, M.T.; Kamran, M.A.; Mehmood, S.; Khan, M.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Assisted phytoremediation of chromium spiked soils by Sesbania sesban in association with Bacillus xiamenensis PM14: A biochemical analysis. Plant Physiol. Biochem. 2020, 146, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.; Gill, M.B.; Mao, B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ. Sci. Pollut. Res. 2016, 23, 20483–20496. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Javed, M.T.; Tanwir, K.; Akram, M.S.; Shahid, M.; Niazi, N.K.; Lindberg, S. Phytoremediation of cadmium-polluted water/sediment by aquatic macrophytes: Role of plant-induced pH changes. In Cadmium Toxicity and Tolerance in Plants from Physiology to Remediation; Academic Press: Cambridge, MA, USA, 2019; pp. 495–529. [Google Scholar] [CrossRef]
- Sarwar, N.; Ishaq, W.; Farid, G.; Shaheen, M.R.; Imran, M.; Geng, M.; Hussain, S. Zinc–cadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotoxicol. Environ. Saf. 2015, 122, 528–536. [Google Scholar] [CrossRef]
- Sriprachote, A.; Kanyawongha, P.; Ochiai, K.; Mato, T. Current situation of cadmium-polluted paddy soil, rice and soybean in the Mae Sot District, Tak Province, Thailand. Soil Sci. Plant Nutr. 2012, 58, 349–359. [Google Scholar] [CrossRef]
- Sriprachote, A.; Pengprecha, S.; Pengprecha, P.; Kanyawongha, P.; Ochiai, K.; Matoh, T. Assessment of Cadmium and Zinc Contamination in the Soils Around Pha Te Village, Mae Sot District, Tak Province, Thailand. Appl. Envir. Res. 2014, 36, 67–79. [Google Scholar] [CrossRef]
- Simmons, R.W.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implications for public health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: A five-year follow-up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef]
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Ullah, A.; Tahir, A.; Rashid, H.U.; Rehman, T.U.; Danish, S.; Hussain, B.; Akca, H. Strategies for reducing Cd concentration in paddy soil for rice safety. J. Clean. Prod. 2021, 316, 128116. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Raza, W.; Luo, J.; Zhang, F.; Shen, Q. Solid-state fermentation of agro-industrial wastes to produce bioorganic fertilizer for the biocontrol of Fusarium wilt of cucumber in continuously cropped soil. Biores. Technol. 2011, 102, 3900–3910. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S. Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem. Toxicol. 2010, 48, 3262–3267. [Google Scholar] [CrossRef]
- Cazorla, F.; Romero, D.; Pérez-García, A.; Lugtenberg, B.; Vicente, A.d.; Bloemberg, G. Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J. Appl. Microbiol. 2007, 103, 1950–1959. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Vijayakumar, C.; Kumar, N.; Samiyappan, R. PGPR-induced defense responses in the tea plant against blister blight disease. CropPro 2007, 26, 556–565. [Google Scholar] [CrossRef]
- Jha, P.N.; Kumar, A. Endophytic colonization of Typha australis by a plant growth-promoting bacterium Klebsiella oxytoca strain GR-3. J. Appl. Microbiol. 2007, 103, 1311–1320. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Effect of fungicides on plant growth promoting activities of phosphate solubilizing Pseudomonas putida isolated from mustard (Brassica compestris) rhizosphere. Chemosphere 2012, 86, 945–950. [Google Scholar] [CrossRef]
- Jahanian, A.; Chaichi, M.; Rezaei, K.; Rezayazdi, K.; Khavazi, K. The effect of plant growth promoting rhizobacteria (PGPR) on germination and primary growth of artichoke (Cynara scolymus). Int. Agric. Crop Sci. 2012, 4, 923–929. [Google Scholar]
- Ahemad, M.; Khan, M.S. Ecological assessment of biotoxicity of pesticides towards plant growth promoting activities of pea (Pisum sativum)-specific rhizobium sp. STRAINMRP1. Emir. J. Food Agric. 2012, 24, 334–343. [Google Scholar]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Khan, M.S. Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek] using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi J. Biol. Sci. 2012, 19, 451–459. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Evaluation of plant-growth-promoting activities of rhizobacterium Pseudomonas putida under herbicide stress. Ann. Microbiol. 2012, 62, 1531–1540. [Google Scholar] [CrossRef]
- Moreira, H.; Marques, A.P.; Franco, A.R.; Rangel, A.O.; Castro, P.M. Phytomanagement of Cd-contaminated soils using maize (Zea mays L.) assisted by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2014, 21, 9742–9753. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef]
- Shen, Z.; Zhong, S.; Wang, Y.; Wang, B.; Mei, X.; Li, R.; Ruan, Y.; Shen, Q. Induced soil microbial suppression of banana Fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur. J. Soil Biol. 2013, 57, 1–8. [Google Scholar] [CrossRef]
- Seang-On, L.; Meeinkuirt, W.; Saengwilai, P.; Saminpanya, S.; Koedrith, K. Alleviation of cadmium stress in Thai rice cultivar (PSL2) by inoculation of indigenous cadmium-resistant microbial consortia. Appl. Ecol. Environ. Res. 2019, 17, 14679–14697. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Chen, S.; Meng, N.; Li, X.; Zheng, H.; Zhao, C.; Wang, D. Manipulation of the rhizosphere bacterial community by biofertilizers is associated with mitigation of cadmium phytotoxicity. Sci. Total Environ. 2019, 649, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Siripornadulsil, S.; Siripornadulsil, W. Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotoxicol. Environ. Saf. 2013, 94, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Suksabye, P.; Pimthong, A.; Dhurakit, P.; Mekvichitsaeng, P.; Thiravetyan, P. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Khashei, S.; Etemadifar, Z.; Rahmani, H.R. Multifunctional biofertilizer from Pseudomonas putida PT: A potential approach for simultaneous improving maize growth and bioremediation of cadmium-polluted soil. Biol. J. Microorg. 2019, 8, 117–129. [Google Scholar]
- Li, J.; Guo, Y.-K.; Zhao, Q.-X.; He, J.-Z.; Zhang, Q.; Cao, H.-Y.; Liang, C.-Q. Microbial cell wall sorption and Fe-Mn binding in rhizosphere contribute to the obstruction of cadmium from soil to rice. Front. Microbiol. 2023, 14, 1162119. [Google Scholar] [CrossRef]
- Shi, Z.; Qi, X.; Zeng, X.; Lu, Y.; Zhou, J.; Cui, K.; Zhang, L. A newly isolated bacterium Comamonas sp. XL8 alleviates the toxicity of cadmium exposure in rice seedlings by accumulating cadmium. J. Hazard. Mater. 2021, 403, 123824. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Hu, K.; Wang, G.; Shi, K. A Coculture of Enterobacter and Comamonas species reduces cadmium accumulation in rice. Mol. Plant Microbe Interact. 2023, 36, 95–108. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Chao, X.; Zhang, J.; Li, R.; Shen, Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fertil. Soils 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Li, J.; Abbas, M.H. Feasibility of biochar manufactured from organic wastes on the stabilization of heavy metals in a metal smelter contaminated soil. Chemosphere 2014, 117, 66–71. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Younis, U.; Qayyum, M.F.; Shah, M.H.R.; Danish, S.; Shahzad, A.N.; Malik, S.A.; Mahmood, S. Growth, survival, and heavy metal (Cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J. Plant Nutr. Soil Sci. 2015, 178, 209–217. [Google Scholar] [CrossRef]
- Yuan, C.; Li, F.; Cao, W.; Yang, Z.; Hu, M.; Sun, W. Cadmium solubility in paddy soil amended with organic matter, sulfate, and iron oxide in alternative watering conditions. J. Hazard Mater. 2019, 378, 120672. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Usman, A.R.A.; Lee, S.S.; Abd El-Azeem, S.A.M.; Choi, B.; Hashimoto, Y.; Yang, J.E. Effects of rapeseed residue on lead and cadmium availability and uptake by rice plants in heavy metal contaminated paddy soil. Chemosphere 2011, 85, 677–682. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-Y.; Zhang, X.-Y.; Dai, Z.-Q.; Fu, X.-L.; Yang, F.-T.; Liu, X.-Y.; Sun, X.-M.; Wen, X.-F.; Schaeffer, S. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Song, X.; Tao, B.; Guo, J.; Li, J.; Chen, G. Changes in the microbial community structure and soil chemical properties of vertisols under different cropping systems in Northern China. Front. Environ. Sci. 2018, 13, 132. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. J. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Haider, F.U.; Maqsood, M.F.; Mohy-Ud-Din, W.; Shabaan, M.; Ahmad, M.; Kaleem, M.; Ishfaq, M.; Aslam, Z.; Shahzad, B. Recent advances in microbial-assisted remediation of cadmium-contaminated soil. Plants 2023, 12, 3147. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Jung, M.C.; Kim, K.-H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef]
- Grant, C.A.; Clarke, J.M.; Duguid, S.; Chaney, R. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci. Total Environ. 2008, 390, 301–310. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Singh, S.; Srivastava, P.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Exogenous mineral regulation under heavy metal stress: Advances and prospects. Biochem. Pharmacol. 2016, 5, 220. [Google Scholar] [CrossRef]
- Luo, Q.; Bai, B.; Xie, Y.; Yao, D.; Chen, Z.; Zhang, D.; Liu, Y.; Xiao, Y.; Yu, Y.; Wu, J. Spatial ionomics provides new insights into the accumulation and transport of mineral ions in rice (Oryza sativa L.) under cadmium stress. Environ. Exp. Bot. 2023, 208, 105267. [Google Scholar] [CrossRef]
- Shanying, H.E.; Xiaoe, Y.; Zhenli, H.E.; Virupax, C.B. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Gonçalves, J.F.; Antes, F.G.; Maldaner, J.; Pereira, L.B.; Tabaldi, L.A.; Rauber, R.; Rossato, L.V.; Bisognin, D.A.; Dressler, V.L.; de Moraes Flores, É.M.; et al. Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol. Biochem. 2009, 47, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, L.; Yang, Y.; Deng, X.; Zhou, X.; Zeng, Q. Basal and foliar treatment using an organic fertilizer amendment lowers cadmium availability in soil and cadmium uptake by rice on field micro-plot experiment planted in contaminated acidic paddy soil. Soil Sedim. Contam. Int. J. 2019, 28, 1–14. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Lv, X.; Zhang, Y.; Wang, W. Effects of biochar and biofertilizer on cadmium-contaminated cotton growth and the antioxidative defense system. Sci. Rep. 2020, 10, 20112. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Applications of metabolomics for the elucidation of abiotic stress tolerance in plants: A special focus on osmotic stress and heavy metal toxicity. Plants 2023, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.; Gupta, M. Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J. Hazard. Mater. 2015, 287, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.H.M.B.; Parvin, K.; Mohsin, S.M.; Mahmud, J.A.; Hasanuzzaman, M.; Fujita, M. Modulation of cadmium tolerance in rice: Insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants 2020, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidativedamage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, C.; Khan, E.; Panthri, M.; Gupta, M. Silicon mediated genotoxic alterations in Brassica juncea under arsenic stress: A comparative study of biochemical and molecular markers. Pedosphere 2020, 30, 517–527. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Oorts, K.; Ghesquiere, U.; Smolders, E. Leaching and aging decrease nickel toxicity to soil microbial processes in soils freshly spiked with nickel chloride. Environ. Toxicol. Chem. Int. J. 2007, 26, 1130–1138. [Google Scholar] [CrossRef]
- Black, G.R. Bulk density: Method of soil analysis. In Monograph No. 9 Part I; American Society of Agronomy Inc.: Washington, DC, USA, 1965. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Burnison, B.K. Modified dimethyl sulfoxide (DMSO) extraction for chlorophyll analysis of phytoplankton. Can. J. Fish Aquat. Sci. 1980, 37, 729–733. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of active oxygen in photosynthesis. In Photoinhibition; Kyle, D.J., Osmond, C.B., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dörr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Booth, J.; Boyland, E.; Sims, A.P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem. J. 1961, 79, 516–524. [Google Scholar] [CrossRef]
- Patra, H.K.; Kar, M.; Mishra, D. Catalase activity in leaves and cotyledons during plant development and senescence. Biochem. Physiol. Pflanz. 1978, 172, 385–390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).