Creation of Rice Doubled Haploids Resistant to Prolonged Flooding Using Anther Culture
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- With meristematic foci, light shades, fine-grained, medium density (morphogenic);
- (2)
- Spherical, white, light yellow, medium density (morphogenic);
- (3)
- Dense, white, fine-grained (morphogenic);
- (4)
- Loose, moist, with vascular cords (rhizogene);
- (5)
- Granular, loose, brown, with large cells (very low ability to morphogenesis);
- (6)
- Watery, dark brown, with large shapeless cells of different sizes (non-morphogenic).
| Gene | Primer | Subsequence (5′–3′) | Size (bp) | Link |
|---|---|---|---|---|
| SUB1A | RM 7481 F | CGA CCC AAT ATC TTT CTG CC | 95 | Azarin et al., 2016 [27] |
| RM 7481 R | ATT GGT CGT GCT CAA CAA G | |||
| SNORKEL1 | 1F | ATG TGC GGA GGT TGT CTC AT | 743 | Oe et al., 2021 [28] |
| 1R | TCG TAG CGA CAG CCG TAC TG |
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, R.; Rao, G. In-vitro Androgenesis in Rice: Advantages, Constraints and Future Prospects. Rice Sci. 2016, 23, 57–68. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Muhammad, I.; Kareem, I.; AdekunleSalisu, M.; Arolu, I.W. Submergence Tolerance in Rice: Review of Mechanism, Breeding and Future. Sustainability 2020, 12, 1632. [Google Scholar] [CrossRef]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Arolu, F.; Anisuzzaman, M. Flooding tolerance in Rice: Adaptive mechanism and marker-assisted selection breeding approaches. Mol. Biol. Rep. 2023, 50, 2795–2812. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence Tolerant Rice: SUB1’s Journey from Landrace to Modern Cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Dubina, E.; Shilovsky, V.; Kostylev, P.; Garkusha, S.; Kovalev, V.; Yesaulova, L.; Balyasny, I.; Straholysova, M.; Dinh, T.; Le, L. Sub1A Gene in Rice Breeding for Tolerance to Flooding as a Factor in Weed Control. Rice Farming 2017, 2, 20–26. [Google Scholar]
- Goncharova, Y. The Use of the Anther Culture Method in rice Breeding; Research Institute of Rice: Krasnodar, Russia, 2012; p. 91. [Google Scholar]
- Savenko, E.; Korotenko, T.; Glazyrina, V.; Shundrina, L. Accelerated production of genetically stable (homozygous) forms of rice based on selectively valuable samples with target genes of resistance to pyriculariosis by anther culture in vitro method. Proc. Kuban State Agrar. Univ. 2020, 85, 213–219. [Google Scholar] [CrossRef]
- Goncharova, Y.; Kharitonov, E.; Malyuchenko, E.; Sheleg, V. Rice adaptability to abiotic stresses analysis of breeding efficiency. In Proceedings of the Achievements and Prospects for the Development of Rice Breeding and Cultivation in Temperate Countries, International scientific Internet Conference, Krasnodar, Russia, 26–27 November 2015; pp. 40–46. [Google Scholar]
- Ilyushko, M.; Romashova, M. The effect of intensity and quality of illumination on the regenerative ability of Oryza sativa L. rice callus obtained in in vitro androgenesis. Russ. Agric. Sci. 2021, 3, 41–45. [Google Scholar] [CrossRef]
- Bishnoi, U.; Jain, R.; Gupta, K.R.; Chowdhury, V.K. High frequency androgenesis in indica Basmati rice hybrids using liquid culture media. Plant Cell Tissue Organ Cult. 1995, 61, 153–159. [Google Scholar]
- Sarao, N.; Gosal, S. In vitro Androgenesis for Accelerated Breeding in Rice. Biotechnol. Crop Improv. 2018, 1, 407–435. [Google Scholar] [CrossRef]
- Malysheva, N.; Savenko, E.; Glazyrina, V.; Shundrina, L. Obtaining, evaluation and selection of dihaploid rice lines with economically valuable traits. Rice Farming 2012, 21, 14–18. [Google Scholar]
- Bushman, N.; Vereshchagina, S. The effectiveness of nutrient media for the induction of callus formation in rice hybrids. Rice Farming 2013, 1, 13–16. [Google Scholar]
- Savenko, E.; Mukhina, Z.; Glazyrina, V.; Shundrina, L. Optimization of cellular technologies in vitro for accelerated generation of rice Oryza sativa L. Bull. State Nikitsk. Bot. Gard. 2022, 144, 114–121. [Google Scholar]
- Guchenko, S. Comparative characteristics and selection of dihaploid rice lines by economically valuable traits. Far East. Agrar. Bull. 2016, 1, 10–15. Available online: https://vestnik.dalgau.ru/arkhiv_nomerov/?PAGEN_1=3 (accessed on 13 September 2023).
- Ilyushko, M.; Romashova, M. Variability of rice haploids obtained in anther culture in vitro. Russ. Agric. Sci. 2019, 2, 11–14. [Google Scholar] [CrossRef]
- Goncharova, Y.; Malyuchenko, E. The culture of anthers as a method of creating material for the study of various areas of breeding work. Proc. Kuban State Agrar. Univ. 2017, 66, 70–74. [Google Scholar] [CrossRef]
- Savenko, E.; Glazyrina, V.; Shundrina, L. Variability of traits in populations of DH rice lines. Rice Farming 2022, 2, 6–10. [Google Scholar] [CrossRef]
- Ilyushko, M.; Romashova, M.; Zhang, J.; Deng, L.; Liu, D.-J.; Zhang, R.; Guchenko, S. Intracallus variability of doubled rice haploids obtained in in vitro androgenesis. Agric. Biol. 2020, 55, 533–543. [Google Scholar] [CrossRef]
- Goncharova, Y.; Kharitonov, E.; Bushman, N.; Vereshchagina, S. Comparative analysis of the effectiveness of nutrient media for the induction of callus formation in rice hybrids. Rep. Russ. Acad. Agric. Sci. 2013, 6, 6–9. [Google Scholar]
- Romashova, M.; Ilyushko, M.; Companietz, S. Method of Obtaining Rice Regenerants in Anther Culture In Vitro. R.U. Patent 2681339 C1, 6 March 2019. [Google Scholar]
- Pattnaik, S.S.; Dash, B.; Bhuyan, S.S.; Katara, J.L.; Parameswaran, C.; Verma, R.; Ramesh, N.; Samantaray, S. Anther culture efficiency in quality hybrid rice: A comparison between hybrid rice and its ratooned plants. Plants 2020, 9, 1306. [Google Scholar] [CrossRef]
- Ilyushko, M.; Skaptsov, M.; Romashova, M. Nuclear DNA content in rice (Oryza sativa L.) regenerants obtained in anther culture in vitro. Agric. Biol. 2018, 53, 531–538. [Google Scholar] [CrossRef]
- Savenko, E.; Glazyrina, V.; Shundrina, L. Practical application of biotechnological methods for breeding rice (Oryza sativa L.), from bioproducts to bioeconomy. In Proceedings of the IV Interregional Scientific and Practical Conference (with International Participation), Barnaul, Russia, 23–24 September 2021; pp. 128–132. [Google Scholar]
- Rout, P.; Naik, N.; Ngangkham, U.; Verma, R.L.; Katara, J.L.; Singh, O.N.; Samantaray, S. Doubled Haploids generated through anther culture from an elite long duration rice hybrid, CRHR32: Method optimization and molecular characterization. Plant Biotechnol. 2016, 33, 177–186. [Google Scholar] [CrossRef]
- Ahmadi, B.; Ebrahimzadeh, H. In vitro androgenesis: Spontaneous vs. artificial genome doubling and characterization of regenerants. Plant Cell Rep. 2020, 39, 299–316. [Google Scholar] [CrossRef]
- Azarin, K.V.; Usatov, A.V.; Alabushev, A.V.; Kostylev, P.I.; Makarenko, M.S.; Kovalevich, A.A. Validation of SSR markers associated with Submergence Tolerance in Rice (Oryza sativa L.). Am. J. Agric. Biol. Sci. 2016, 11, 142–147. [Google Scholar] [CrossRef]
- Oe, S.; Sasayama, D.; Luo, Q.; Fukayama, H.; Hatanaka, T.; Azuma, T. Growth responses of seedlings under complete submergence in rice cultivars carrying both the submergence-tolerance gene sub1a-1 and the floating genes snorkels. Plant Prod. Sci. 2021, 25, 70–77. [Google Scholar] [CrossRef]
- Mayakaduwa, D.; Silva, T.D. A cytological indicator allows rapid assessment of microspore maturity, leading to improved in vitro anther response in Indica rice (Oryza sativa L.). In Vitro Cell. Dev. Biol. Plant 2017, 53, 591. [Google Scholar] [CrossRef]
- Sahoo, S.A.; Jha, Z.; Verulkar, S.B.; Srivastava, A.K.; Suprasanna, P. High-throughput cell analysis based protocol for ploidy determination in anther-derived rice callus. Plant Cell Tissue Organ Cult. 2019, 137, 187. [Google Scholar] [CrossRef]
- Palanisamy, D.; Marappan, S.; Ponnuswamy, R.D.; Mahalingam, P.S.; Bohar, R.; Vaidyanathanl, S. Accelerating hybrid rice breeding through the adoption of doubled haploid technology for R-line development. Biologia 2019, 74, 1259. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.; Bailey-Serres, J. A variable cluster of ethylene responsive-like factors regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar]
- Singh, N.; Dang, T.T.M.; Vergara, G.V.; Pandey, D.M.; Sanchez, D.; Neeraja, C.N.; Septiningsih, E.M.; Mendioro, M.; Tecson-Mendoza, E.M.; Ismail, A.M.; et al. Molecular marker survey and expression analyses of the rice submergence-tolerance gene SUB1A. Theor. Appl. Genet. 2010, 121, 1441–1453. [Google Scholar] [CrossRef]



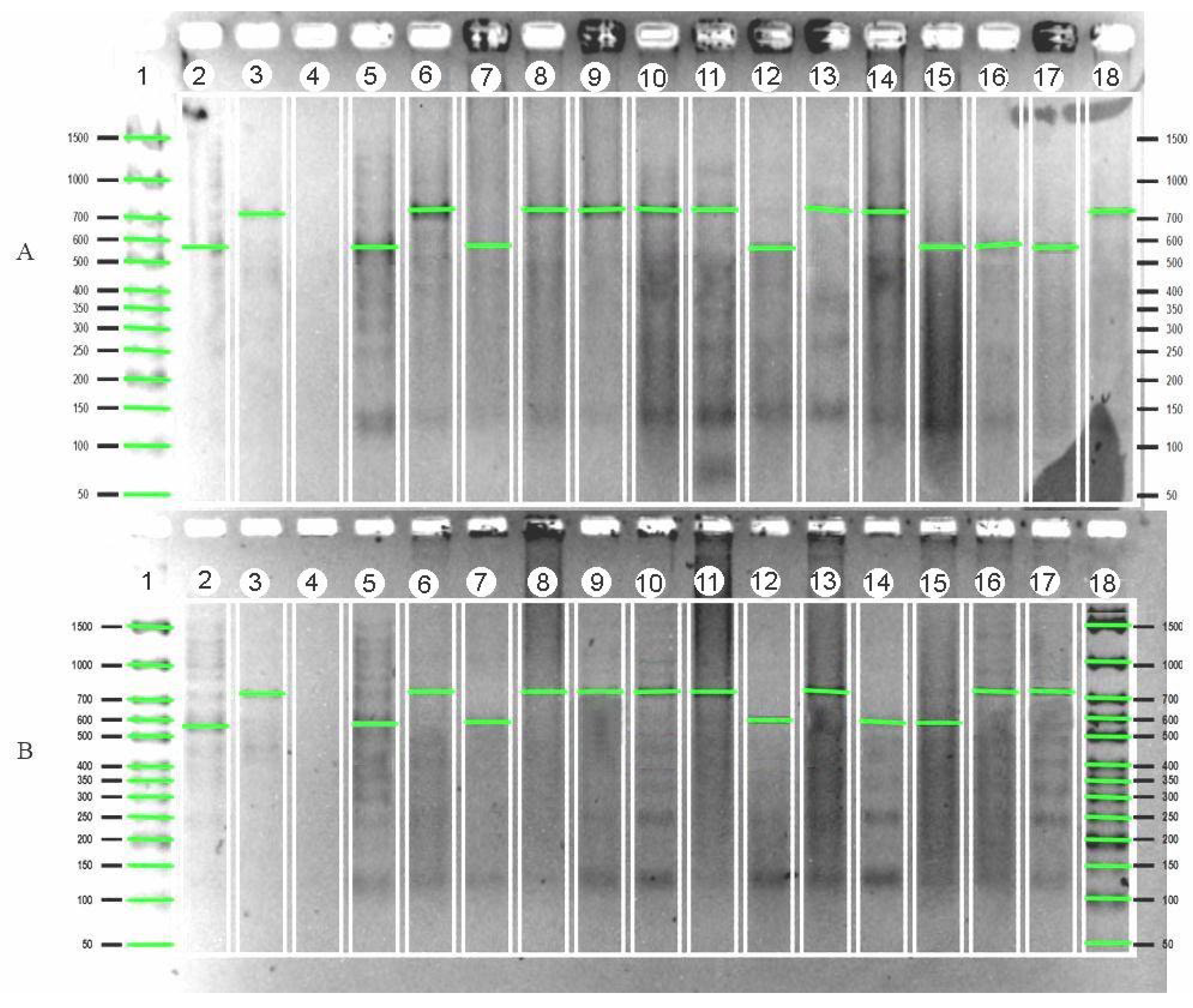



| Variety | Gene | Country of Origin |
|---|---|---|
| Novator | – | Russia |
| Magnat | – | Russia |
| Contact | – | Russia |
| Kuboyar | – | Russia |
| Br-11 | SUB1A | Bangladesh |
| CR-1009 | SUB1A | India |
| Inbara-3 | SUB1A | Indonesia |
| IR-64 | SUB1A | Philippines |
| TDK-1 | SUB1A | Laos |
| Khao Hlan On | SK1, SK2 | Myanmar |
| Kharsu 80A | SK1, SK2 | Pakistan |
| Ma-Zhan Red | SK1, SK2 | China |
| Media Components | Induction Medium (Blaydes, 1966) (mg/L) | Regeneration Medium (Muraschige and Skoog, 1964) (mg/L) |
|---|---|---|
| Macro salts | ||
| NH4NO3 | 1000 | 1650 |
| KNO3 | 1000 | 1900 |
| Ca(NO3)2 × 4 H2O | 347 | 440 |
| KH2PO4 | 300 | 170 |
| MgSO4 × 7 H2O | 35 | 370 |
| KCl | 65 | – |
| Micro salts | ||
| ZnSO4 × 7 H2O | 1.5 | 8.6 |
| H3BO3 | 1.6 | 1.6 |
| MnSO4 × 4 H2O | 4.4 | 6.92 |
| KI | 0.8 | 0.83 |
| Na2MoO4 × 2 H2O | – | 0.25 |
| CuSO4 × 5 H2O | – | 0.025 |
| CoCl2 × 6 H2O | – | 0.025 |
| Iron source | ||
| FeSO4 × 7 H2O | 27.8 | 27.8 |
| Na2EDTA | 37.2 | 37.2 |
| Vitamins | ||
| Nicotinic acid | 0.5 | 0.5 |
| Pyridoxine HCI | 0.5 | 0.5 |
| Thiamine HCI | 0.5 | 0.5 |
| Other componentss | ||
| myo-Inositol | 100 | 100 |
| Glycine | 2.0 | 2.0 |
| Agar | 8.0 | 8.0 |
| Sucrose | 30.0 | 20.0 |
| Growth regulators | ||
| 2,4-D | 2.0 | – |
| NAA | – | 1.0 |
| Kinetin | – | 5.0 |
| рН | 6.0 | 6.0 |
| № | Sample № | Plant № | Inoculated Anthers, pcs. | Number of Neoplasms, pcs. | Non-Morphogenic Callus, pcs. | Total Regenerating Plants, pcs. | Green Plants, pcs. | Albino Plants, pcs. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5022 | 1 | 243 | 4 | 4 | 0 | 0 | 0 |
| 2 | 275 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 92 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 5103 | 2 | 259 | 20 | 16 | 4 | 0 | 4 |
| 4 | 245 | 2 | 2 | 0 | 0 | 0 | ||
| 5 | 110 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 5007 | 1 | 214 | 0 | 0 | 0 | 0 | 0 |
| 3 | 152 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 114 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 5005 | 1 | 299 | 37 | 34 | 3 | 0 | 3 |
| 2 | 86 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 225 | 1 | 1 | 0 | 0 | 0 | ||
| 5 | 5029 | 3 | 270 | 0 | 0 | 0 | 0 | 0 |
| 5 | 132 | 1 | 1 | 0 | 0 | 0 | ||
| 8 | 304 | 1 | 1 | 0 | 0 | 0 | ||
| 10 | 284 | 0 | 0 | 0 | 0 | 0 | ||
| 6 | 5006 | 1 | 277 | 0 | 0 | 0 | 0 | 0 |
| 2 | 189 | 0 | 0 | 0 | 0 | 0 | ||
| 5 | 120 | 0 | 0 | 0 | 0 | 0 | ||
| 7 | 5093 | 1 | 194 | 1 | 1 | 0 | 0 | 0 |
| 3 | 82 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 272 | 0 | 0 | 0 | 0 | 0 | ||
| 8 | 5019 | 1 | 251 | 8 | 5 | 3 | 0 | 3 |
| 2 | 126 | 3 | 2 | 1 | 0 | 1 | ||
| 3 | 289 | 1 | 1 | 0 | 0 | 0 | ||
| 9 | 5003 | 1 | 306 | 0 | 0 | 0 | 0 | 0 |
| 3 | 258 | 1 | 1 | 0 | 0 | 0 | ||
| 10 | 5009 | 1 | 212 | 0 | 0 | 0 | 0 | 0 |
| 2 | 119 | 84 | 67 | 17 | 5 | 12 | ||
| 4 | 278 | 12 | 12 | 0 | 0 | 0 | ||
| 11 | 5010 | 1 | 183 | 0 | 0 | 0 | 0 | 0 |
| 2 | 277 | 94 | 87 | 7 | 5 | 2 | ||
| 12 | 5011 | 1 | 271 | 0 | 0 | 0 | 0 | 0 |
| 3 | 186 | 0 | 0 | 0 | 0 | 0 | ||
| 13 | 5008 | 1 | 86 | 3 | 3 | 0 | 0 | 0 |
| 2 | 184 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 279 | 21 | 21 | 0 | 0 | 0 | ||
| 14 | 5020 | 1 | 243 | 26 | 15 | 11 | 0 | 11 |
| 2 | 47 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 210 | 13 | 13 | 0 | 0 | 0 | ||
| 15 | 5018 | 1 | 132 | 1 | 1 | 0 | 0 | 0 |
| 2 | 298 | 5 | 4 | 1 | 0 | 1 | ||
| 3 | 297 | 23 | 20 | 3 | 0 | 3 | ||
| 16 | 4565 | 2 | 82 | 3 | 3 | 0 | 0 | 0 |
| 3 | 195 | 85 | 82 | 3 | 2 | 1 | ||
| 5 | 245 | 46 | 43 | 3 | 0 | 3 | ||
| 17 | 4773 | 1 | 47 | 4 | 4 | 0 | 0 | 0 |
| 2 | 114 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 59 | 1 | 1 | 0 | 0 | 0 | ||
| 18 | 5016 | 2 | 339 | 1 | 1 | 0 | 0 | 0 |
| 3 | 120 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 216 | 0 | 0 | 0 | 0 | 0 | ||
| 19 | 4758 | 1 | 209 | 4 | 2 | 2 | 0 | 2 |
| 20 | 5021 | 1 | 248 | 62 | 37 | 25 | 0 | 25 |
| 2 | 112 | 3 | 1 | 2 | 0 | 2 | ||
| 3 | 140 | 0 | 0 | 0 | 0 | 0 | ||
| 21 | 4641 | 1 | 193 | 5 | 4 | 1 | 0 | 1 |
| 2 | 194 | 69 | 48 | 21 | 18 | 3 | ||
| 22 | 5017 | 1 | 51 | 4 | 2 | 2 | 0 | 2 |
| 2 | 255 | 2 | 1 | 1 | 0 | 1 | ||
| 3 | 195 | 42 | 32 | 10 | 0 | 10 | ||
| 23 | 4526 | 1 | 80 | 10 | 3 | 7 | 0 | 7 |
| 24 | 4688 | 1 | 85 | 12 | 9 | 3 | 0 | 3 |
| 2 | 117 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 57 | 0 | 0 | 0 | 0 | 0 | ||
| 25 | 4617 | 1 | 83 | 1 | 1 | 0 | 0 | 0 |
| 26 | 4585 | 1 | 104 | 0 | 0 | 0 | 0 | 0 |
| 2 | 94 | 0 | 0 | 0 | 0 | 0 | ||
| Sum | 69 | 12,604 | 716 | 586 | 130 | 30 | 100 | |
| Average | 2.5 | 185.35 | 10.53 | 8.6 | 1.91 | 0.44 | 1.47 | |
| Minimum | 1 | 47 | 0 | 0 | 0 | 0 | 0 | |
| Maximum | 4 | 339 | 94 | 87 | 25 | 18 | 25 | |
| Sample № | Plant № | Number of Neoplasms/ per 100 Anthers | The Number of All Regenerants/ per 100 Anthers | Number of Green Regenerants/ per 100 Anthers | The Number of All Regenerants/ per 100 Neoplasms |
|---|---|---|---|---|---|
| 5009 | 2 | 70.6 * | 14.3 * | 4.2 | 20.2 |
| 5010 | 2 | 33.9 | 2.5 | 1.8 | 7.5 |
| 4565 | 3 | 43.6 | 1.5 | 1.0 | 3.5 |
| 4641 | 2 | 35.6 | 10.8 | 9.3 * | 30.4 * |
| Average value | 45.9 | 7.3 | 4.1 | 15.4 | |
| Standard deviation | 17.0 | 6.3 | 3.7 | 12.3 |
| Index | Haploids | Diploids | Tetraploids |
|---|---|---|---|
| Number of plants, pcs. | 9 | 11 | 5 |
| DNA content, pg: | |||
| M | 0.901 | 1.880 | 3.762 |
| ±SEM | 0.012 | 0.023 | 0.048 |
| min | 0.790 | 1.654 | 3.590 |
| max | 1.112 | 2.015 | 3.960 |
| Cv, % | 8.3 | 9.6 | 10.0 |
| Hybrid Number * | Plant No. | Ploidy | Sk1 Gene | Sub1A Gene | Plant Height, cm | Panicle Length, cm | Number of Panicles on a Plant, pcs | Number of Spikelets on a Panicle, pcs | Number of Grains on a Panicle, pcs | Weight of 1000 Grains, g |
|---|---|---|---|---|---|---|---|---|---|---|
| 4565 | 1 | 1 | 51 | 11 | 20 | 128 | 0 | 0.0 | ||
| 4565 | 2 | 1 | Sub1A | 41 | 14 | 12 | 75 | 0 | 0.0 | |
| 4641 | 1 | 2 | Sk1 | 57 | 14.5 | 15 | 96 | 39 | 25.0 | |
| 4641 | 2 | 2 | 60 | 13.5 | 9 | 116 | 52 | 22.1 | ||
| 4641 | 3 | 2 | Sk1 | 47 | 12.5 | 10 | 109 | 60 | 24.8 | |
| 4641 | 4 | 4 | 58 | 15.5 | 8 | 55 | 4 | 38.3 | ||
| 4641 | 5 | 2 | Sk1 | Sub1A | 57 | 14 | 10 | 114 | 89 | 30.1 |
| 4641 | 6 | 4 | Sk1 | 57 | 18 | 6 | 40 | 2 | 44.8 | |
| 4641 | 7 | 2 | Sub1A | 65 | 16.5 | 8 | 126 | 104 | 28.7 | |
| 4641 | 8 | 2 | Sk1 | Sub1A | 58 | 15.5 | 8 | 50 | 32 | 24.2 |
| 4641 | 9 | 4 | Sk1 | 48 | 13 | 11 | 36 | 4 | 47.2 | |
| 4641 | 10 | 2 | Sub1A | 50 | 14 | 11 | 101 | 63 | 25.6 | |
| 4641 | 11 | 2 | Sk1 | 74 | 17 | 12 | 127 | 98 | 30.1 | |
| 4641 | 12 | 2 | Sk1 | Sub1A | 51 | 14 | 12 | 43 | 25 | 27.6 |
| 4641 | 13 | 4 | Sk1 | Sub1A | 51 | 15.5 | 7 | 49 | 2 | 35.5 |
| 4641 | 14 | 4 | Sk1 | 55 | 15.5 | 3 | 64 | 4 | 51.7 | |
| 5009 | 1 | 1 | Sk1 | 53 | 14.5 | 10 | 98 | 0 | 0.0 | |
| 5009 | 2 | 2 | Sk1 | Sub1A | 33 | 10 | 7 | 51 | 18 | 28.9 |
| 5009 | 3 | 1 | 62 | 13.5 | 40 | 40 | 0 | 0.0 | ||
| 5010 | 1 | 1 | Sub1A | 26 | 5.5 | 3 | 96 | 0 | 0.0 | |
| 5010 | 2 | 1 | Sk1 | Sub1A | 31 | 7.5 | 3 | 82 | 0 | 0.0 |
| 5010 | 3 | 1 | Sk1 | Sub1A | 39 | 5 | 4 | 48 | 0 | 0.0 |
| 5010 | 4 | 1 | Sk1 | 30 | 4 | 5 | 44 | 0 | 0.0 | |
| 5010 | 5 | 1 | Sk1 | Sub1A | 44 | 13 | 10 | 117 | 0 | 0.0 |
| 5010 | 6 | 2 | 57 | 14 | 7 | 101 | 3 | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostylev, P.; Kalinina, N.; Vozhzhova, N.; Golubova, V.; Chertkova, N. Creation of Rice Doubled Haploids Resistant to Prolonged Flooding Using Anther Culture. Plants 2023, 12, 3681. https://doi.org/10.3390/plants12213681
Kostylev P, Kalinina N, Vozhzhova N, Golubova V, Chertkova N. Creation of Rice Doubled Haploids Resistant to Prolonged Flooding Using Anther Culture. Plants. 2023; 12(21):3681. https://doi.org/10.3390/plants12213681
Chicago/Turabian StyleKostylev, Pavel, Nataliya Kalinina, Nataliya Vozhzhova, Valentina Golubova, and Natalya Chertkova. 2023. "Creation of Rice Doubled Haploids Resistant to Prolonged Flooding Using Anther Culture" Plants 12, no. 21: 3681. https://doi.org/10.3390/plants12213681
APA StyleKostylev, P., Kalinina, N., Vozhzhova, N., Golubova, V., & Chertkova, N. (2023). Creation of Rice Doubled Haploids Resistant to Prolonged Flooding Using Anther Culture. Plants, 12(21), 3681. https://doi.org/10.3390/plants12213681







