The Morphological Parameters and Cytosolic pH of Cells of Root Zones in Tobacco Plants (Nicotiana tabacum L.): Nonlinear Effects of NaCl Concentrations
Abstract
:1. Introduction
2. Results
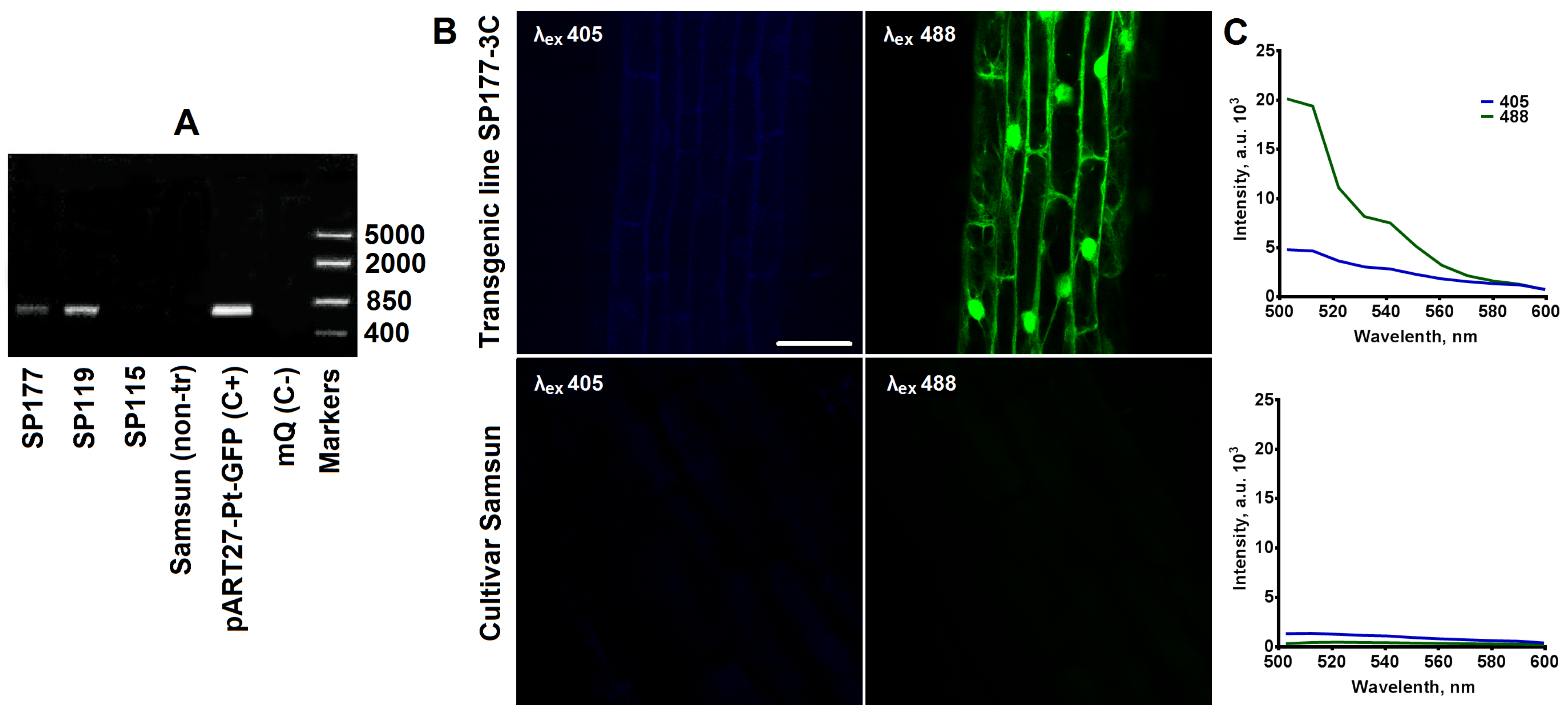
2.1. Obtaining and Characterisation of Transgenic Tobacco Plants Expressing the pH-Sensitive Pt-GFP
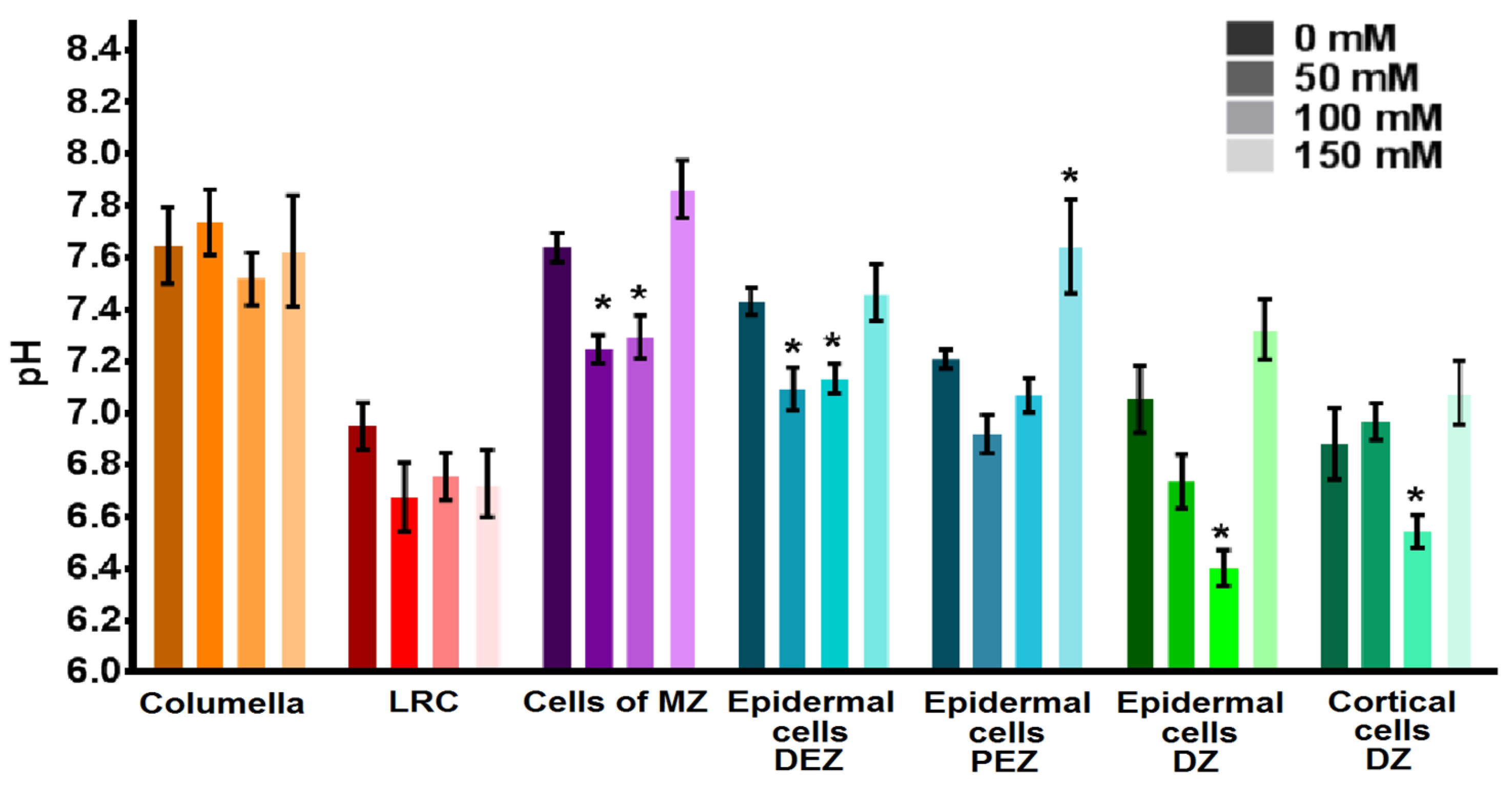
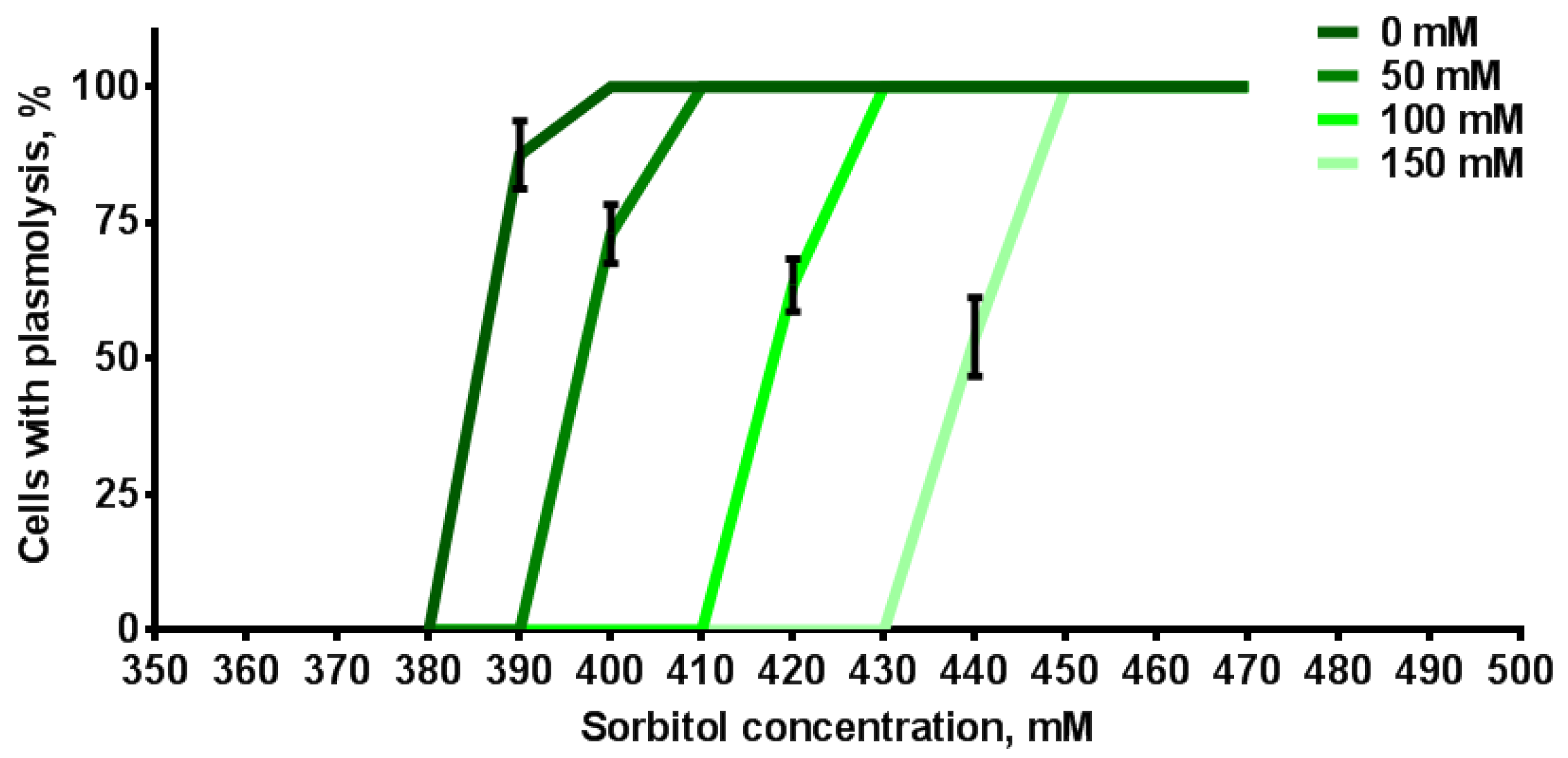
2.2. Cytosolic pH of Root Cells under Salinity
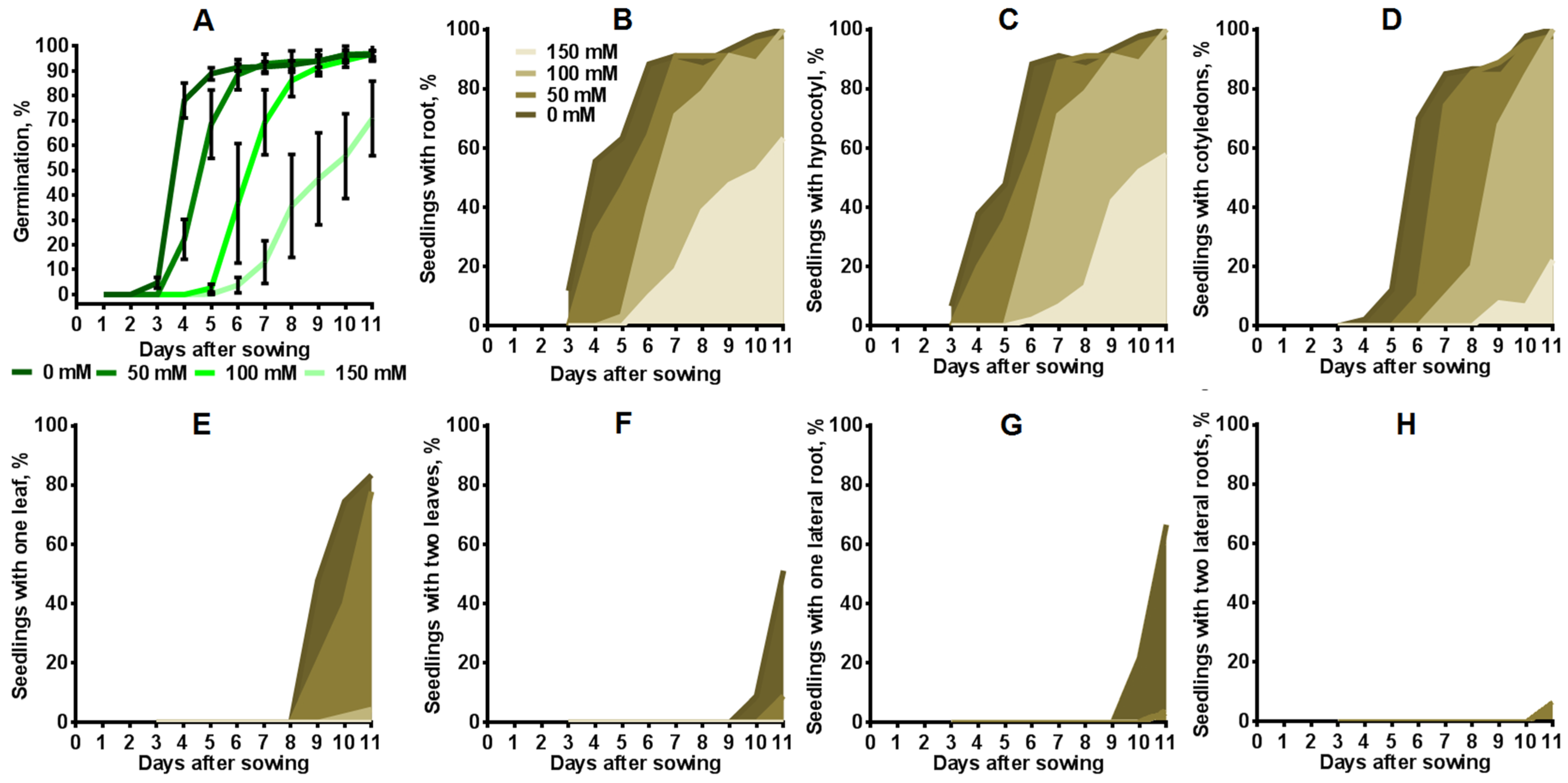
2.3. Influence of Salinity on Seed Germination and Seedling Development
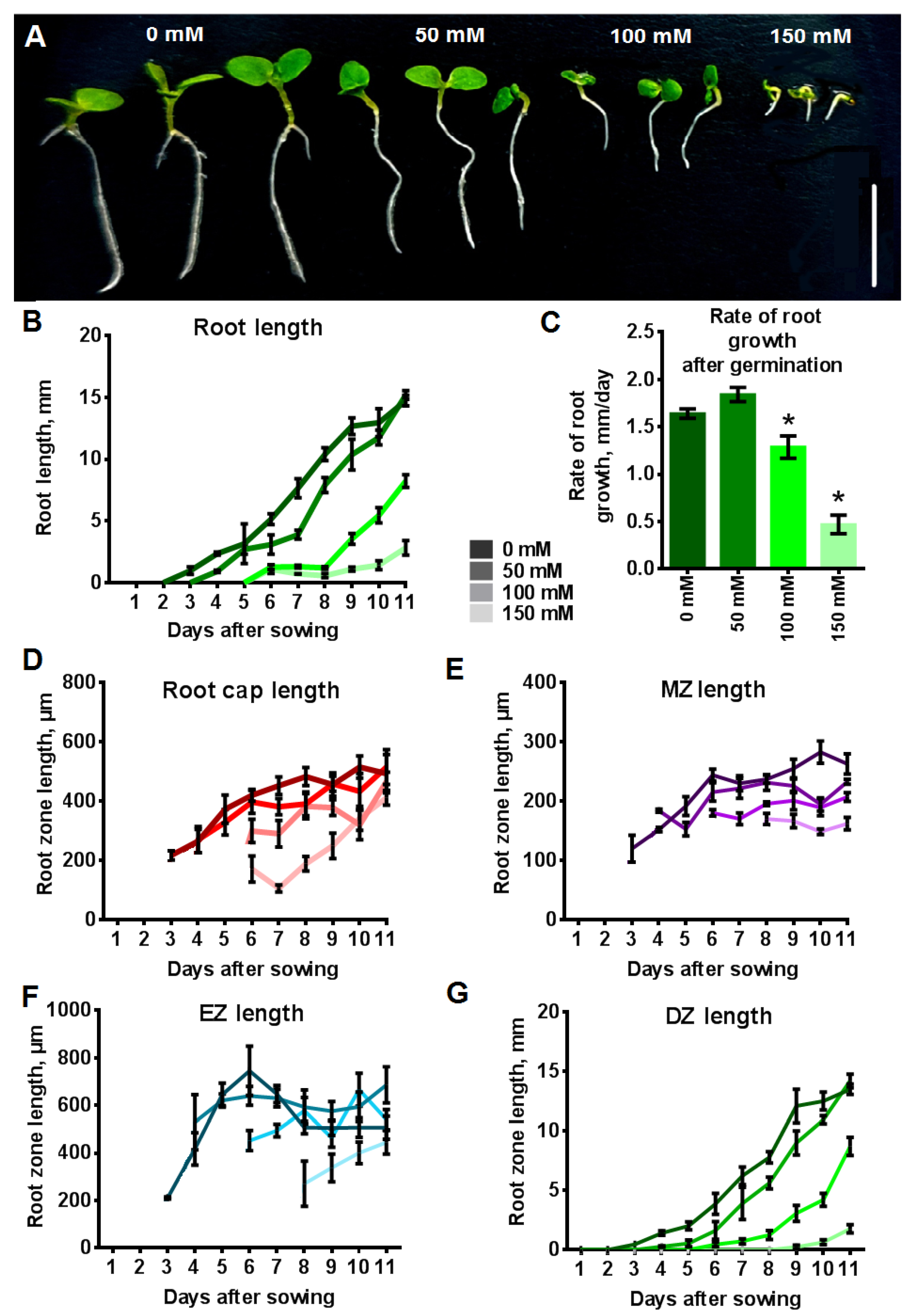
2.4. Influence of NaCl on the Length of Primary Root and the Length of Root Zones
2.5. Effect of NaCl on the Cell Length and the Cell Number of Root Zones
3. Discussion
4. Materials and Methods
4.1. Genetic Transformation of Tobacco Plants
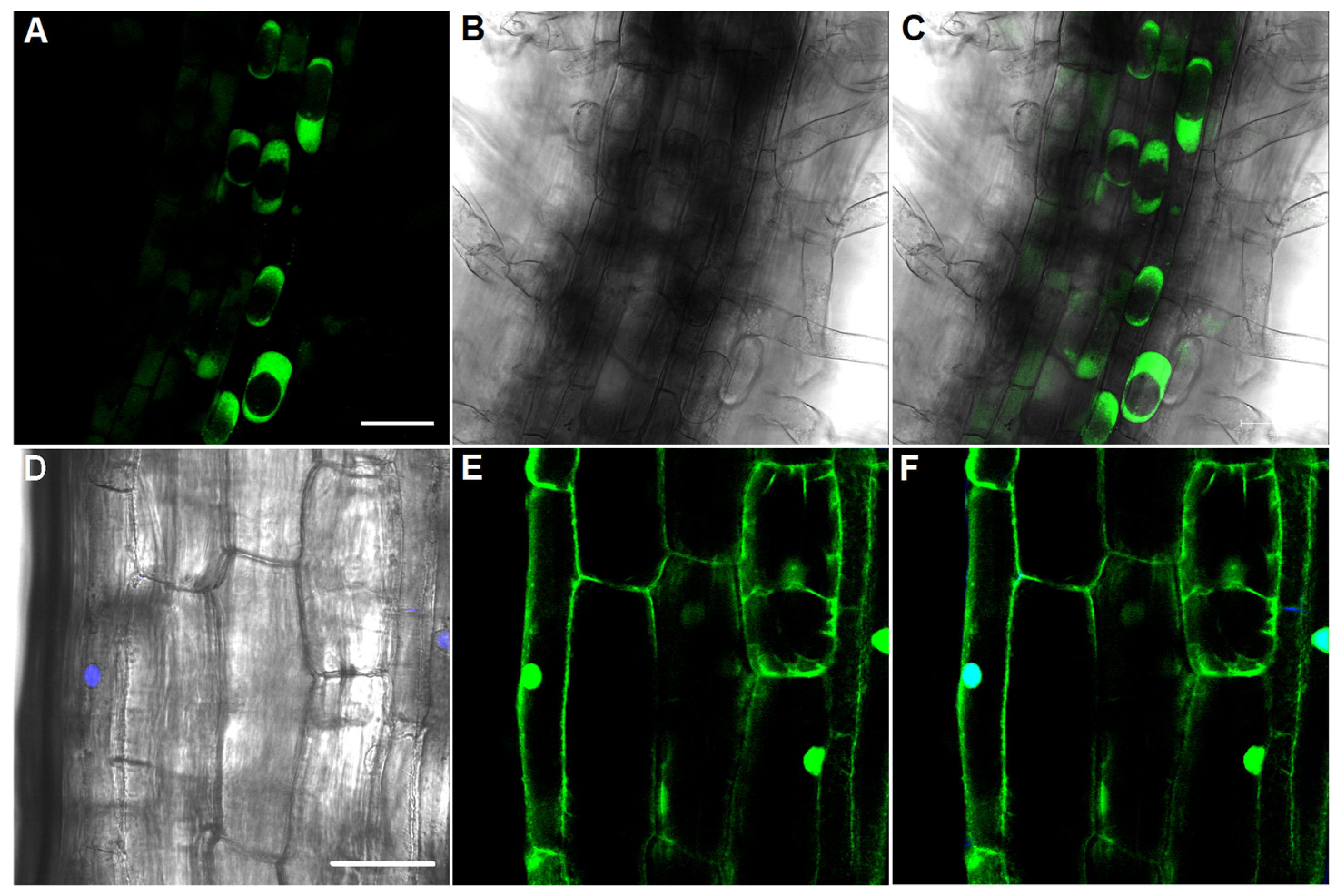
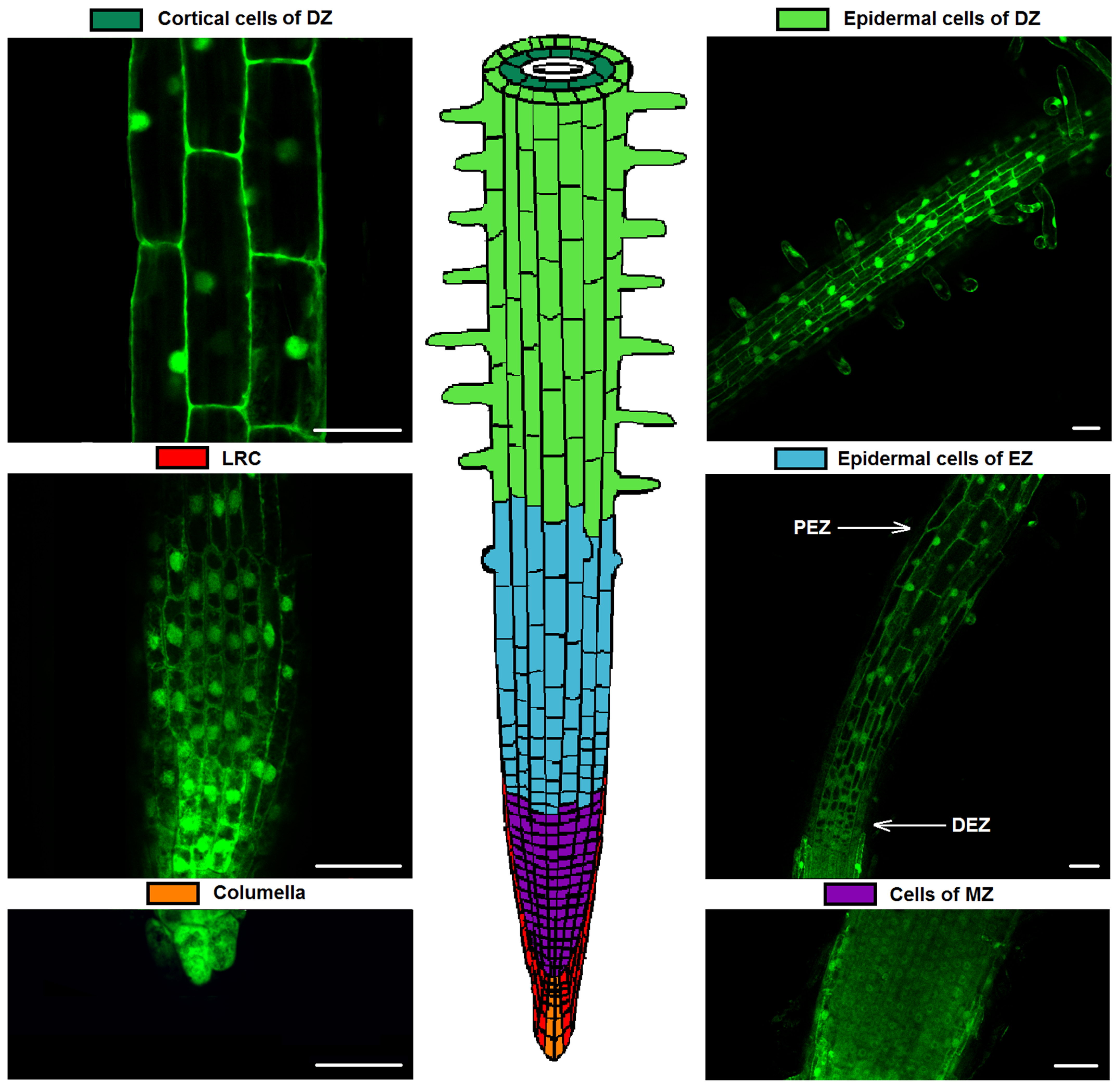
4.2. LSM-Microscopy of Root Cells of Tobacco: Ratiometric Analysis
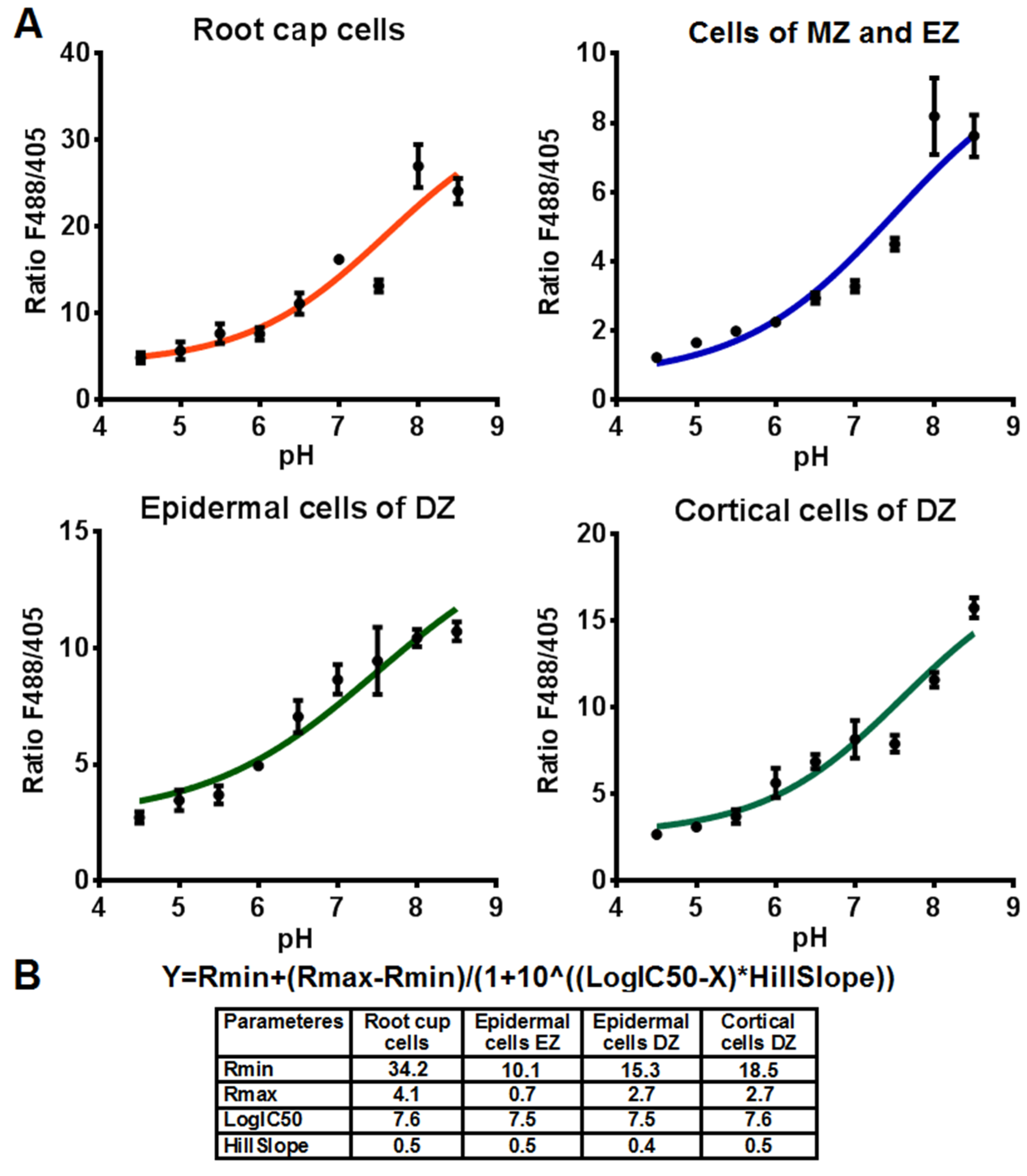
4.3. In Vivo Calibration of Pt-GFP Signal in Tobacco Roots
4.4. Assessment of the Effect of NaCl on pHcyt of Tobacco Root Cells, Tobacco Plant Development and Morphometric Parameters of Tobacco Roots
4.5. Plasmolysis of Root Cells in the Sorbitol Solutions
4.6. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trușcă, M.; Gâdea, S.; Vidican, R.; Stoian, V.; Vâtcă, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vâtcă, S. Exploring the Research Challenges and Perspectives in Ecophysiology of Plants Affected by Salinity Stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Litalien, A.; Zeeb, B. Curing the Earth: A Review of Anthropogenic Soil Salinization and Plant-Based Strategies for Sustainable Mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef]
- Mustafa, G.; Akhtar, M.S.; Abdullah, R. Global Concern for Salinity on Various Agro-Ecosystems. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Akhtar, M.S., Ed.; Springer Singapore: Singapore, 2019; pp. 1–19. ISBN 9789811388002. [Google Scholar]
- Yang, S.; Lee, H. Salinity-Triggered Responses in Plant Apical Meristems for Developmental Plasticity. Int. J. Mol. Sci. 2023, 24, 6647. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Role of Vacuolar Membrane Transport Systems in Plant Salinity Tolerance. J. Plant Growth Regul. 2023, 42, 1364–1401. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Hao, D.-L.; Yang, G.-Z. Regulation of Cytosolic pH: The Contributions of Plant Plasma Membrane H+-ATPases and Multiple Transporters. Int. J. Mol. Sci. 2021, 22, 12998. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Han, G.-L.; Yang, Z.-R.; Li, Y.-X.; Wang, B.-S. Plant Salinity Sensors: Current Understanding and Future Directions. Front. Plant Sci. 2022, 13, 859224. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shabala, L.; Azzarello, E.; Huang, Y.; Pandolfi, C.; Su, N.; Wu, Q.; Cai, S.; Bazihizina, N.; Wang, L.; et al. Na+ Extrusion from the Cytosol and Tissue-Specific Na+ Sequestration in Roots Confer Differential Salt Stress Tolerance between Durum and Bread Wheat. J. Exp. Bot. 2018, 69, 3987–4001. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shabala, L.; Liu, X.; Azzarello, E.; Zhou, M.; Pandolfi, C.; Chen, Z.-H.; Bose, J.; Mancuso, S.; Shabala, S. Linking Salinity Stress Tolerance with Tissue-Specific Na+ Sequestration in Wheat Roots. Front. Plant Sci. 2015, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N. Effects of Salinity on Root Growth. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; p. 784. [Google Scholar]
- Julkowska, M.M.; Hoefsloot, H.C.J.; Mol, S.; Feron, R.; De Boer, G.-J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis Root Architecture Dynamics with root-fit Reveals Diversity in Responses to Salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Ampudia, C.S.; Testerink, C. Salt Stress Signals Shape the Plant Root. Curr. Opin. Plant Biol. 2011, 14, 296–302. [Google Scholar] [CrossRef]
- Galvan-Ampudia, C.S.; Julkowska, M.M.; Darwish, E.; Gandullo, J.; Korver, R.A.; Brunoud, G.; Haring, M.A.; Munnik, T.; Vernoux, T.; Testerink, C. Halotropism Is a Response of Plant Roots to Avoid a Saline Environment. Curr. Biol. 2013, 23, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Moe-Lange, J.; Hennet, L.; Feldman, L.J. Salt Stress Affects the Redox Status of Arabidopsis Root Meristems. Front. Plant Sci. 2016, 7, 81. [Google Scholar] [CrossRef]
- Felle, H.H. pH: Signal and Messenger in Plant Cells. Plant Biol. 2001, 3, 577–591. [Google Scholar] [CrossRef]
- Gjetting, S.K.; Ytting, C.K.; Schulz, A.; Fuglsang, A.T. Live Imaging of Intra- and Extracellular pH in Plants Using pHusion, a Novel Genetically Encoded Biosensor. J. Exp. Bot. 2012, 63, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle pH in the Arabidopsis Endomembrane System. Mol. Plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef]
- Cosse, M.; Seidel, T. Plant Proton Pumps and Cytosolic pH-Homeostasis. Front. Plant Sci. 2021, 12, 672873. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, H.; Xu, F.; Yan, F.; Xu, W. H+-ATPases in Plant Growth and Stress Responses. Annu. Rev. Plant Biol. 2022, 73, 495–521. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Yang, Y. The Molecular Mechanism of Plasma Membrane H+-ATPases in Plant Responses to Abiotic Stress. J. Genet. Genomics 2022, 49, 715–725. [Google Scholar] [CrossRef]
- Barnes, A.C.; Benning, C.; Roston, R.L. Chloroplast Membrane Remodeling during Freezing Stress Is Accompanied by Cytoplasmic Acidification Activating SENSITIVE TO FREEZING2. Plant Physiol. 2016, 171, 2140–2149. [Google Scholar] [CrossRef]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zdobnova, T.; Ladeynova, M.; Yudintsev, A.; Vodeneev, V.; Brilkina, A. Whole-Plant Measure of Temperature-Induced Changes in the Cytosolic pH of Potato Plants Using Genetically Encoded Fluorescent Sensor Pt-GFP. Agriculture 2021, 11, 1131. [Google Scholar] [CrossRef]
- Kesten, C.; Gámez-Arjona, F.M.; Menna, A.; Scholl, S.; Dora, S.; Huerta, A.I.; Huang, H.; Tintor, N.; Kinoshita, T.; Rep, M.; et al. Pathogen-induced pH Changes Regulate the Growth-defense Balance in Plants. EMBO J. 2019, 38, e101822. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-Reporting Arabidopsis Expressing pH and [Ca2+] Indicators Unveil Ion Dynamics in the Cytoplasm and in the Apoplast under Abiotic Stress. Plant Physiol. 2004, 134, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Lorenzen, I.; Böttcher, M.; Plieth, C. A Novel Fluorescent pH Probe for Expression in Plants. Plant Methods 2006, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Ageyeva, M.; Veselov, A.; Vodeneev, V.; Brilkina, A. Cell-Type-Specific Length and Cytosolic pH Response of Superficial Cells of Arabidopsis Root to Chronic Salinity. Plants 2022, 11, 3532. [Google Scholar] [CrossRef]
- Rombolá-Caldentey, B.; Andrés, Z.; Waadt, R.; Quintero, F.J.; Schumacher, K.; Pardo, J.M. Salinity-Induced Cytosolic Alkaline Shifts in Arabidopsis Roots Require the SOS Pathway. Int. J. Mol. Sci. 2023, 24, 3549. [Google Scholar] [CrossRef]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zanegina, D.; Akinchits, E.; Brilkina, A.; Vodeneev, V. Salt-Induced Changes in Cytosolic pH and Photosynthesis in Tobacco and Potato Leaves. Int. J. Mol. Sci. 2022, 24, 491. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Lindberg, S. Sodium Induces Simultaneous Changes in Cytosolic Calcium and pH in Salt-Tolerant Quince Protoplasts. J. Plant Physiol. 2009, 166, 1755–1763. [Google Scholar] [CrossRef]
- Morgan, S.H.; Maity, P.J.; Geilfus, C.-M.; Lindberg, S.; Mühling, K.H. Leaf Ion Homeostasis and Plasma Membrane H+-ATPase Activity in Vicia Faba Change after Extra Calcium and Potassium Supply under Salinity. Plant Physiol. Biochem. 2014, 82, 244–253. [Google Scholar] [CrossRef]
- Sun, Y.; Lindberg, S.; Shabala, L.; Morgan, S.; Shabala, S.; Jacobsen, S.-E. A Comparative Analysis of Cytosolic Na+ Changes under Salinity between Halophyte Quinoa (Chenopodium quinoa) and Glycophyte Pea (Pisum sativum). Environ. Exp. Bot. 2017, 141, 154–160. [Google Scholar] [CrossRef]
- Kader, M.A.; Lindberg, S.; Seidel, T.; Golldack, D.; Yemelyanov, V. Sodium Sensing Induces Different Changes in Free Cytosolic Calcium Concentration and pH in Salt-Tolerant and -Sensitive Rice (Oryza sativa) Cultivars. Physiol. Plant. 2007, 130, 99–111. [Google Scholar] [CrossRef]
- Waadt, R.; Kudla, J.; Kollist, H. Multiparameter in Vivo Imaging in Plants Using Genetically Encoded Fluorescent Indicator Multiplexing. Plant Physiol. 2021, 187, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Kaneko, Y.; Miyazawa, Y.; Fujii, N.; Higashitani, N.; Wada, S.; Ishida, H.; Yoshimoto, K.; Shirasu, K.; Yamada, K.; et al. A Possible Involvement of Autophagy in Amyloplast Degradation in Columella Cells during Hydrotropic Response of Arabidopsis Roots. Planta 2012, 236, 999–1012. [Google Scholar] [CrossRef]
- Geilfus, C.-M.; Mühling, K.H.; Kaiser, H.; Plieth, C. Bacterially Produced Pt-GFP as Ratiometric Dual-Excitation Sensor for in Planta Mapping of Leaf Apoplastic pH in Intact Avena Sativa and Vicia Faba. Plant Methods 2014, 10, 31. [Google Scholar] [CrossRef]
- Grinberg, M.; Nemtsova, Y.; Ageyeva, M.; Brilkina, A.; Vodeneev, V. Effect of Low-Dose Ionizing Radiation on Spatiotemporal Parameters of Functional Responses Induced by Electrical Signals in Tobacco Plants. Photosynth. Res. 2023, 157, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Lindberg, S. Cytosolic Calcium and pH Signaling in Plants under Salinity Stress. Plant Signal. Behav. 2010, 5, 233–238. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Schmidt, W. The Enigma of Environmental pH Sensing in Plants. Nat. Plants 2021, 7, 106–115. [Google Scholar] [CrossRef]
- Guo, K.; Babourina, O.; Rengel, Z. Na + /H + Antiporter Activity of the SOS1 Gene: Lifetime Imaging Analysis and Electrophysiological Studies on Arabidopsis Seedlings. Physiol. Plant. 2009, 137, 155–165. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant Cell-Surface GIPC Sphingolipids Sense Salt to Trigger Ca2+ Influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z.; Ahmad, P. Ion Homeostasis for Salinity Tolerance in Plants: A Molecular Approach. Physiol. Plant. 2021, 171, 578–594. [Google Scholar] [CrossRef]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular Ion Homeostasis: Emerging Roles of Intracellular NHX Na+/H+ Antiporters in Plant Growth and Development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.-K. Regulation of SOS1, a Plasma Membrane Na + /H + Exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef]
- Shabala, L.; Cuin, T.A.; Newman, I.A.; Shabala, S. Salinity-Induced Ion Flux Patterns from the Excised Roots of Arabidopsis Sos Mutants. Planta 2005, 222, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E.; Poole, R.J. Na + /H + Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985, 78, 163–167. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabała, K. The Role of Plasma Membrane H+-ATPase in Salinity Stress of Plants. In Progress in Botany; Lüttge, U., Beyschlag, W., Eds.; Progress in Botany; Springer International Publishing: Cham, Switzerland, 2015; Volume 76, pp. 77–92. ISBN 978-3-319-08806-8. [Google Scholar]
- Xu, L.; Song, J.-Q.; Wang, Y.-L.; Liu, X.-H.; Li, X.-L.; Zhang, B.; Li, A.-J.; Ye, X.-F.; Wang, J.; Wang, P. Thymol Improves Salinity Tolerance of Tobacco by Increasing the Sodium Ion Efflux and Enhancing the Content of Nitric Oxide and Glutathione. BMC Plant Biol. 2022, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Damsz, B.; Kononowicz, A.K.; Bressan, R.A.; Hasegawa, P.M. NaCl-Induced Alterations in Both Cell Structure and Tissue-Specific Plasma Membrane H+ -ATPase Gene Expression. Plant Physiol. 1996, 111, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Vera-Estrella, R.; Barkla, B.J.; Bohnert, H.J.; Pantoja, O. Salt Stress in Mesembryanthemum crystallinum L. Cell Suspensions Activates Adaptive Mechanisms Similar to Those Observed in the Whole Plant. Planta 1999, 207, 426–435. [Google Scholar] [CrossRef]
- Lijuan, C.; Huiming, G.; Yi, L.; Hongmei, C. Chalcone Synthase EaCHS1 from Eupatorium adenophorum Functions in Salt Stress Tolerance in Tobacco. Plant Cell Rep. 2015, 34, 885–894. [Google Scholar] [CrossRef]
- Wang, M.; Ren, T.; Huang, R.; Li, Y.; Zhang, C.; Xu, Z. Overexpression of an Apocynum Venetum Flavonols Synthetase Gene Confers Salinity Stress Tolerance to Transgenic Tobacco Plants. Plant Physiol. Biochem. 2021, 162, 667–676. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Kong, X.; Kang, H.; Ren, Y.; Wang, W. Ectopic Expression of Wheat Expansin Gene TaEXPA2 Improved the Salt Tolerance of Transgenic Tobacco by Regulating Na+ /K+ and Antioxidant Competence. Physiol. Plant. 2017, 159, 161–177. [Google Scholar] [CrossRef]
- Pandey, G.K.; Kanwar, P.; Singh, A.; Steinhorst, L.; Pandey, A.; Yadav, A.K.; Tokas, I.; Sanyal, S.K.; Kim, B.-G.; Lee, S.-C.; et al. Calcineurin B-Like Protein-Interacting Protein Kinase CIPK21 Regulates Osmotic and Salt Stress Responses in Arabidopsis. Plant Physiol. 2015, 169, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Wolny, E.; Skalska, A.; Braszewska, A.; Mur, L.A.J.; Hasterok, R. Defining the Cell Wall, Cell Cycle and Chromatin Landmarks in the Responses of Brachypodium Distachyon to Salinity. Int. J. Mol. Sci. 2021, 22, 949. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Khah, E.M.; Pavli, O.I. Germination Profiling of Lentil Genotypes Subjected to Salinity Stress. Plant Biol. 2019, 21, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Szewczyk, A.; Ptak, A. Melatonin Promotes Seed Germination under Salinity and Enhances the Biosynthesis of Steviol Glycosides in Stevia Rebaudiana Bertoni Leaves. PLoS ONE 2020, 15, e0230755. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyeva, L.I.; Baranova, E.N.; Chaban, I.A.; Dilovarova, T.A.; Vanyushin, B.F.; Kononenko, N.V. Elongating Effect of the Peptide AEDL on the Root of Nicotiana Tabacum under Salinity. Plants 2022, 11, 1352. [Google Scholar] [CrossRef]
- Zidan, I.; Azaizeh, H.; Neumann, P.M. Does Salinity Reduce Growth in Maize Root Epidermal Cells by Inhibiting Their Capacity for Cell Wall Acidification? Plant Physiol. 1990, 93, 7–11. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef]
- Bogoutdinova, L.R.; Baranova, E.N.; Baranova, G.B.; Kononenko, N.V.; Lazareva, E.M.; Smirnova, E.A.; Khaliluev, M.R. Morpho-Biological and Cytological Characterization of Tomato Roots (Solanum lycopersicum L., Cv. Rekordsmen) Regenerated under NaCl Salinity in Vitro. Cell Tissue Biol. 2020, 14, 228–242. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T.S. Cell Cycle Modulation in the Response of the Primary Root of Arabidopsis to Salt Stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef]
- Kurth, E.; Cramer, G.R.; Läuchli, A.; Epstein, E. Effects of NaCl and CaCl 2 on Cell Enlargement and Cell Production in Cotton Roots. Plant Physiol. 1986, 82, 1102–1106. [Google Scholar] [CrossRef]
- Azaizeh, H.; Gunse, B.; Steudle, E. Effects of NaCl and CaCl2 on Water Transport across Root Cells of Maize (Zea mays L.) Seedlings. Plant Physiol. 1992, 99, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Onodera, M.; Nakajima, T.; Nanzyo, M.; Takahashi, T.; Xu, D.; Homma, K.; Kokubun, M. Regulation of Root-to-Leaf Na and Cl Transport and Its Association with Photosynthetic Activity in Salt-Tolerant Soybean Genotypes. Plant Prod. Sci. 2019, 22, 262–274. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Shabala, S.; Chen, G.; Chen, Z.; Pottosin, I. The Energy Cost of the Tonoplast Futile Sodium Leak. New Phytol. 2020, 225, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root Cell Wall Solutions for Crop Plants in Saline Soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Ketehouli, T.; Idrice Carther, K.F.; Noman, M.; Wang, F.-W.; Li, X.-W.; Li, H.-Y. Adaptation of Plants to Salt Stress: Characterization of Na+ and K+ Transporters and Role of CBL Gene Family in Regulating Salt Stress Response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. The Importance of Cl− Exclusion and Vacuolar Cl− Sequestration: Revisiting the Role of Cl− Transport in Plant Salt Tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- An, G.; Ebert, P.R.; Mitra, A.; Ha, S.B. Binary Vectors. In Plant Molecular Biology Manual; Klawcr Academic Publishers: Dordrect, Belgium, 1988; pp. 1–19. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Raldugina, G.N.; Evsukov, S.V.; Bogoutdinova, L.R.; Gulevich, A.A.; Baranova, E.N. Morpho-Physiological Testing of NaCl Sensitivity of Tobacco Plants Overexpressing Choline Oxidase Gene. Plants 2021, 10, 1102. [Google Scholar] [CrossRef]
- Raldugina, G.N.; Bogoutdinova, L.R.; Shelepova, O.V.; Kondrateva, V.V.; Platonova, E.V.; Nechaeva, T.L.; Kazantseva, V.V.; Lapshin, P.V.; Rostovtseva, H.I.; Aniskina, T.S.; et al. Heterologous codA Gene Expression Leads to Mitigation of Salt Stress Effects and Modulates Developmental Processes. Int. J. Mol. Sci. 2023, 24, 13998. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Method 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageyeva, M.N.; Zdobnova, T.A.; Nazarova, M.S.; Raldugina, G.N.; Beliaev, D.V.; Vodeneev, V.A.; Brilkina, A.A. The Morphological Parameters and Cytosolic pH of Cells of Root Zones in Tobacco Plants (Nicotiana tabacum L.): Nonlinear Effects of NaCl Concentrations. Plants 2023, 12, 3708. https://doi.org/10.3390/plants12213708
Ageyeva MN, Zdobnova TA, Nazarova MS, Raldugina GN, Beliaev DV, Vodeneev VA, Brilkina AA. The Morphological Parameters and Cytosolic pH of Cells of Root Zones in Tobacco Plants (Nicotiana tabacum L.): Nonlinear Effects of NaCl Concentrations. Plants. 2023; 12(21):3708. https://doi.org/10.3390/plants12213708
Chicago/Turabian StyleAgeyeva, Maria N., Tatiana A. Zdobnova, Mariia S. Nazarova, Galina N. Raldugina, Denis V. Beliaev, Vladimir A. Vodeneev, and Anna A. Brilkina. 2023. "The Morphological Parameters and Cytosolic pH of Cells of Root Zones in Tobacco Plants (Nicotiana tabacum L.): Nonlinear Effects of NaCl Concentrations" Plants 12, no. 21: 3708. https://doi.org/10.3390/plants12213708
APA StyleAgeyeva, M. N., Zdobnova, T. A., Nazarova, M. S., Raldugina, G. N., Beliaev, D. V., Vodeneev, V. A., & Brilkina, A. A. (2023). The Morphological Parameters and Cytosolic pH of Cells of Root Zones in Tobacco Plants (Nicotiana tabacum L.): Nonlinear Effects of NaCl Concentrations. Plants, 12(21), 3708. https://doi.org/10.3390/plants12213708





