Predicting the Potential Geographical Distribution of Rhodiola L. in China under Climate Change Scenarios
Abstract
1. Introduction
2. Data and Methods
2.1. Geographical Distribution Data for Rhodiola L.
2.2. Environmental Parameters
2.3. Model Simulation
2.4. Importance Assessment of Environmental Variables and Classification of Suitable Habitat
3. Results
3.1. Major Climatic Factors Affecting the Distribution of Rhodiola L.
3.2. Potential Geographical Distribution of Rhodiola across Different Periods
3.3. Spatial Pattern Changes in Potential Suitable Areas for Rhodiola Distribution during Different Periods
4. Discussion
4.1. Potential Distribution of Rhodiola
4.2. Relationship between Rhodiola and Climatic Variables
4.3. Spatial Distribution of Rhodiola under Climate Change
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Araújo, M.B.; Rahbek, C. How does climate change affect biodiversity? Science 2006, 313, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, N.J.; Austen, M.C.; Atkins, J.P.; Burdon, D.; Degraer, S.; Dentinho, T.P.; Derous, S.; Holm, P.; Horton, T.; van Ierland, E.; et al. Identification, definition and quantification of goods and services provided by marine biodiversity: Implications for the ecosystem approach. Mar. Pollut. Bull. 2007, 54, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers, Climate Change 2014: Synthesis Report. Contribution of Working Groups Ⅰ, Ⅱ and Ⅲ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Aboge Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, D.H.; Liu, H.B. An introduction to the methods of dealing with uncertainty in the Fifth Assessment Report of IPCC. Progress. Inquisitiones Mutat. Clim. 2012, 8, 150–153. (In Chinese) [Google Scholar]
- Alexander, J.M.; Kueffer, C.; Daehler, C.C.; Edwards, P.J.; Pauchard, A.; Seipel, T. Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc. Natl. Acad. Sci. USA 2011, 108, 656–661. [Google Scholar] [CrossRef]
- Marini, L.; Bertolli, A.; Bona, E.; Federici, G.; Martini, F.; Prosser, F.; Bommarco, R. Beta-diversity patterns elucidate mechanisms of alien plant invasion in mountains. Glob. Ecol. Biogeogr. 2013, 22, 450–460. [Google Scholar] [CrossRef]
- Shen, Y.P.; Wang, G.Y. Key findings and assessment results of IPCC WGI fifth assessment report. J. Glaciol. Geocryol. 2013, 35, 1068–1076. (In Chinese) [Google Scholar]
- Anderson, J.T.; Willis, J.H.; Mitchell-Olds, T. Evolutionary genetics of plant adaptation. Trends Genet. 2011, 27, 258–266. [Google Scholar] [CrossRef]
- Chen, F. What Is the Basis of Variation in Stress Tolerance in Plants? Science China Press: Beijing, China, 2017; Volume 62, pp. 3295–3301. (In Chinese) [Google Scholar]
- Tian, Z.P.; Jiang, D.P. Changes of monsoon area and monsoon precipitation in China during the Middle Holocene and the Last Glacial Maximum. Chin. Sci. Bull. 2015, 60, 400–410. (In Chinese) [Google Scholar]
- Honig, M.A.; Cowling, R.M.; Richardson, D.M. The invasive potential of Australian Banksias in South African fynbos: A comparison of the reproductive potential of Banksia ericifolia and Leucadendron laureolum. Aust. J. Ecol. 1992, 17, 305–314. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Anderson, R.P.; Lew, D.; Peterson, A.T. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A.; Rechsteiner, L. An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo-absence data. J. Appl. Ecol. 2004, 41, 263–274. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; Volume 83, pp. 655–662. [Google Scholar] [CrossRef]
- Ma, S.M.; Zhang, M.L.; Zhang, H.X.; Meng, H.H.; Chen, X. Predicting potential geographical distribution and patterns of relic plant Gymnocarpos przewalskii using maximum entropy and genetic algorithm for rule-set prediction. Chin. J. Plant Ecol. 2010, 34, 1327–1335. (In Chinese) [Google Scholar]
- Ahmed, S.E.; McInerny, G.; O’Hara, K.; Harper, R.; Salido, L.; Emmott, S.; Joppa, L.N. Scientists and software-surveying the species distribution modeling community. Divers. Distrib. 2015, 21, 258–267. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Schneck, F. Characteristics of the top-cited papers in species distribution predictive models. Ecol. Model. 2015, 313, 77–83. [Google Scholar] [CrossRef]
- Vaz, U.L.; Cunha, H.F.; Nabout, J.C. Trends and biases in global scientific literature about ecological niche models. Braz. J. Biol. 2015, 75, S17–S24. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, H.; Huang, L.; Chen, C.; Lin, X.S.; Hu, Z.J.; Li, J.L. Grassland degradation remote sensing monitoring and driving factors quantitative assessment in China from 1982 to 2010. Ecol. Indic. 2017, 83, 303–313. [Google Scholar] [CrossRef]
- Cheuk, M.L.; Fischer, G.A. The impact of climate change on the distribution of Castanopsis (Fagaceae) species in south China and Indo-China region. Glob. Ecol. Conserv. 2021, 26, e01388. [Google Scholar] [CrossRef]
- Bao, R.; Li, X.L.; Zheng, J.H. Feature tuning improves MAXENT predictions of the potential distribution of Pedicularis longiflora Rudolph and its variant. PeerJ 2022, 10, e13337. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.; Perez, V.; Gegout, J.C. Disregarding the edaphic dimension in species distribution models leads to the omission of crucial spatial information under climate change: The case of Quercus pubescens in France. Glob. Chang. Biol. 2012, 18, 2648–2660. [Google Scholar] [CrossRef]
- Wang, Y.H.; Jiang, W.M.; Comes, H.P.; Hu, F.S.; Qiu, Y.X.; Fu, C.X. Molecular phylogeography and ecological niche modeling of a widespread herbaceous climber, Tetrastigma hemsleyanum (Vitaceae): Insights into Plio-Pleistocene range dynamics of evergreen forest in subtropical China. New Phytol. 2015, 206, 852–867. [Google Scholar] [CrossRef]
- Xu, J.; Cao, B.; Bai, C.K. Prediction of potential suitable distribution of endangered plant Kingdonia uniflora in China with MaxEnt. Chin. J. Ecol. 2015, 34, 3354–3359. (In Chinese) [Google Scholar]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. MaxEnt modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Fang, Y.M. Prediction of potential suitable distribution areas of Quercus fabri in China based on an optimized MaxEnt model. Sci. Silvae Sin. 2018, 54, 153–164. (In Chinese) [Google Scholar]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Peterson, A.T.; Loiselle, B.A. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distribut Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Zhang, Y.D. Research progress and application prospect of Rhodiola rosea. J. Anhui Agric. Sci. 2015, 43, 77–79, 82. (In Chinese) [Google Scholar]
- Wu, C.Y.; Raven, P.H. Flora of China 8; Science Press: Beijing, China, 2001. [Google Scholar]
- Zhang, J.; Meng, S.; Allen, G.A.; Wen, J.; Rao, G. Rapid radiation and dispersal out of the Qinghai-Tibetan Plateau of an alpine plant lineage Rhodiola (Crassulaceae). Mol. Phylogenet. Evol. 2014, 77, 147–158. [Google Scholar] [CrossRef]
- Gao, Q.B.; Zhang, D.J.; Duan, Y.Z.; Zhang, F.Q.; Li, Y.H.; Fu, P.C.; Chen, S.L. Intraspecific divergences of Rhodiola alsia (Crassulaceae) based on plastid DNA and internal transcribed spacer fragments. Bot. J. Linn. Soc. 2012, 168, 204–215. [Google Scholar] [CrossRef]
- Hou, Y.; Lou, A. Phylogeographical patterns of an alpine plant, Rhodiola dumulosa (Crassulaceae), inferred from chloroplast DNA sequences. J. Hered. 2014, 105, 101–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Meng, S.; Rao, G. Phylogeography of Rhodiola kirilowii (Crassulaceae): A story of Miocene divergence and Quaternary expansion. PLoS ONE 2014, 9, e112923. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.; Elith, J. MaxEnt is not a presence absence method: A comment on Thibaud et al. Methods Ecol. Evol. 2014, 5, 1192–1197. [Google Scholar] [CrossRef]
- Abdelaala, M.; Foisa, M.; Fenua, G.; Bacchettaa, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Assessment of the extinction risks of medicinal plants in Egypt under climate change by integrating species distribution models and IUCN Red List criteria. J. Arid. Environ. 2019, 170, 103988. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Using species distribution models to assess the importance of Egypt’s protected areas for the conservation of medicinal plants. J. Arid. Environ. 2016, 135, 140–146. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Xiang, D.L.; Hao, Z.Q. The principle of maximum entropy and its applications in ecology. Biodivers. Sci. 2011, 7, 295–302. (In Chinese) [Google Scholar]
- Zhang, M.G.; Slik, J.W.F.; Ma, K.P. Using species distribution modeling to delineate the botanical richness patterns and phytogeographical regions of China. Sci. Rep. 2016, 6, 22400. [Google Scholar] [CrossRef]
- Katz, T.S.; Zellmer, A.J. Comparison of model selection technique performance in predicting the spread of newly invasive species: A case study with Batrachochytrium salamandrivorans. Biol. Invasions 2018, 20, 2107–2119. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- He, X.H.; Si, J.H.; Zhao, C.Y.; Wang, C.L.; Zhou, D.M. Potential distribution of Hippophae thibetana and its predicted responses to climate change. J. Desert Res. 2021, 41, 101–109. (In Chinese) [Google Scholar]
- Christmas, M.J.; Breed, M.F.; Lowe, A.J. Constraints to and conservation implications for climate change adaptation in plants. Conserv. Genet. 2016, 17, 305–320. [Google Scholar] [CrossRef]
- Morgan, J.W.; Venn, S.E. Alpine plant species have limited capacity for long-distance seed dispersal. Plant Ecol. 2017, 218, 813–819. [Google Scholar] [CrossRef]
- Herrmann, J.D.; Carlo, T.A.; Brudvig, L.A.; Damschen, E.I.; Haddad, N.M.; Levey, D.J.; Orrock, J.L.; Tewksbury, J.J. Connectivity from a different perspective: Comparing seed dispersal kernels in connected vs. unfragmented landscapes. Ecology 2016, 97, 1274–1282. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, Y.M.; Salama, M.S.; Su, Z.B. Assessment of vegetation dynamics and their response to variations in precipitation and temperature in the Tibetan Plateau. Clim. Chang. 2010, 103, 519–535. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Xia, F.; Su, Y.J. Local adaptation to temperature and precipitation in naturally fragmented populations of Cephalotaxus oliveri, an endangered conifer endemic to China. Sci. Rep. 2016, 6, 25031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Lun, Y.R.; Liu, L.; Liu, Y.X.; Li, X.; Xu, Z.X. CMIP6 evaluation and projection of climate change over the Tibetan Plateau. J. Beijing Norm. Univ. (Nat. Sci.) 2022, 58, 77–89. (In Chinese) [Google Scholar]
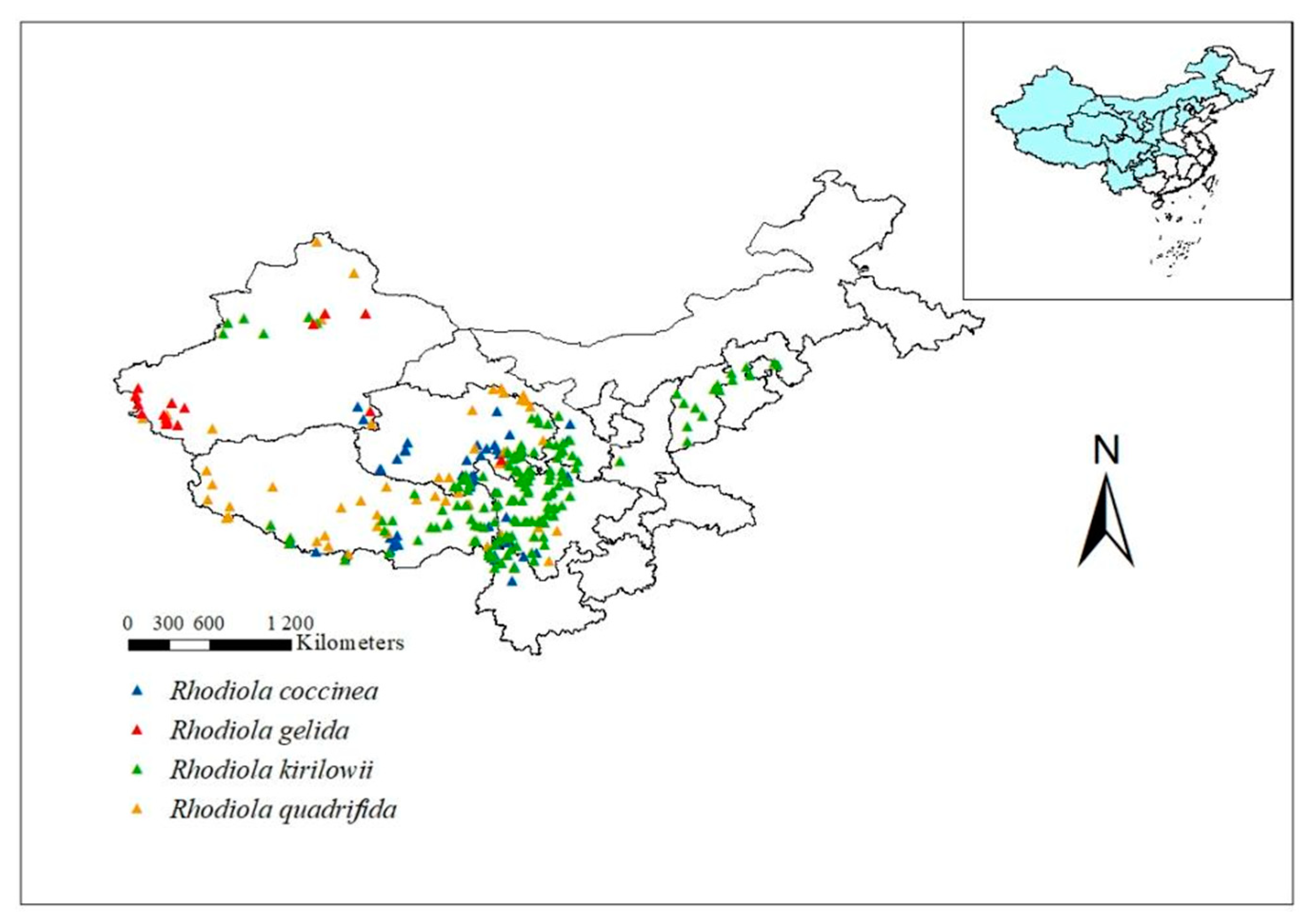
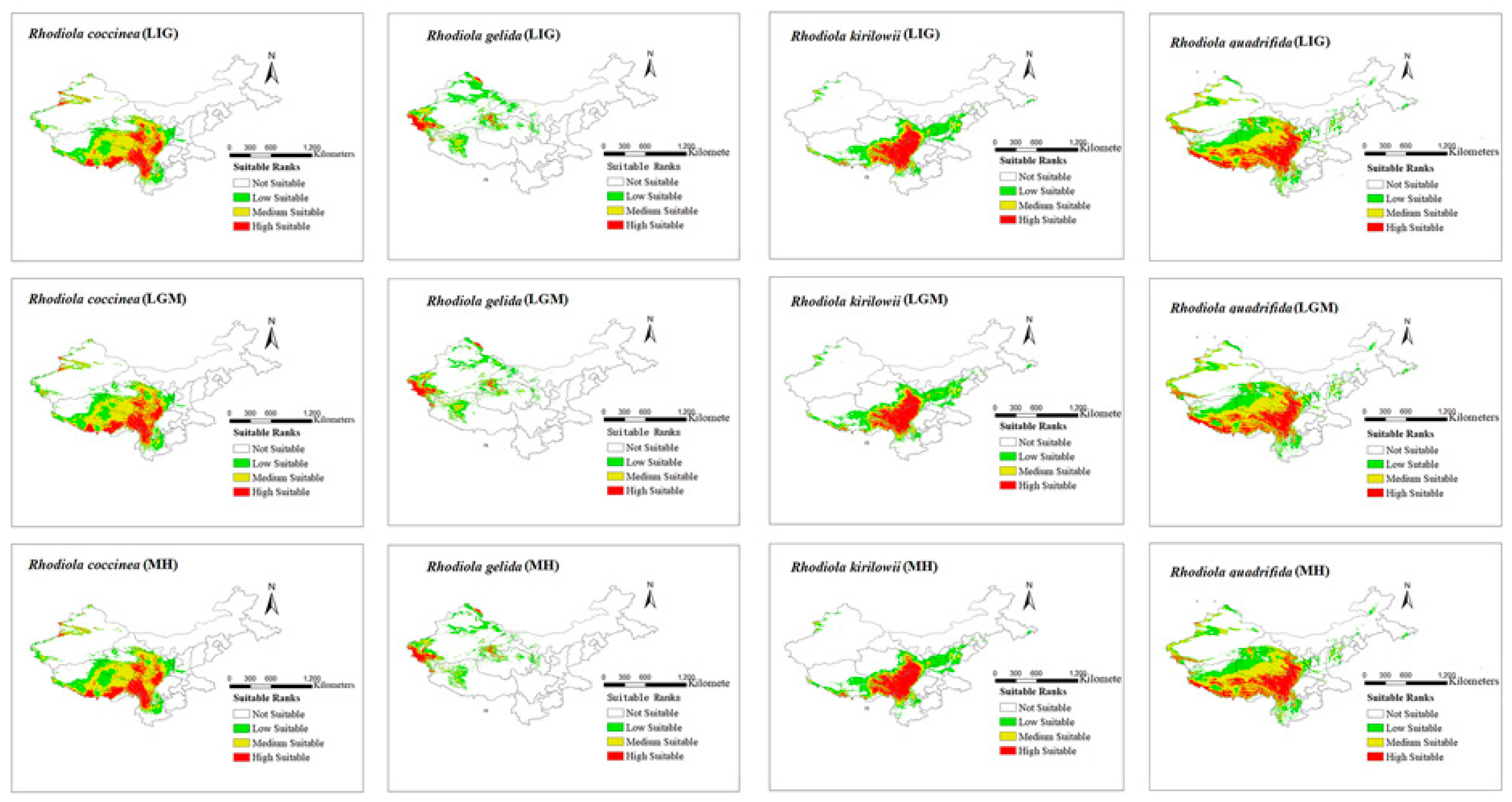
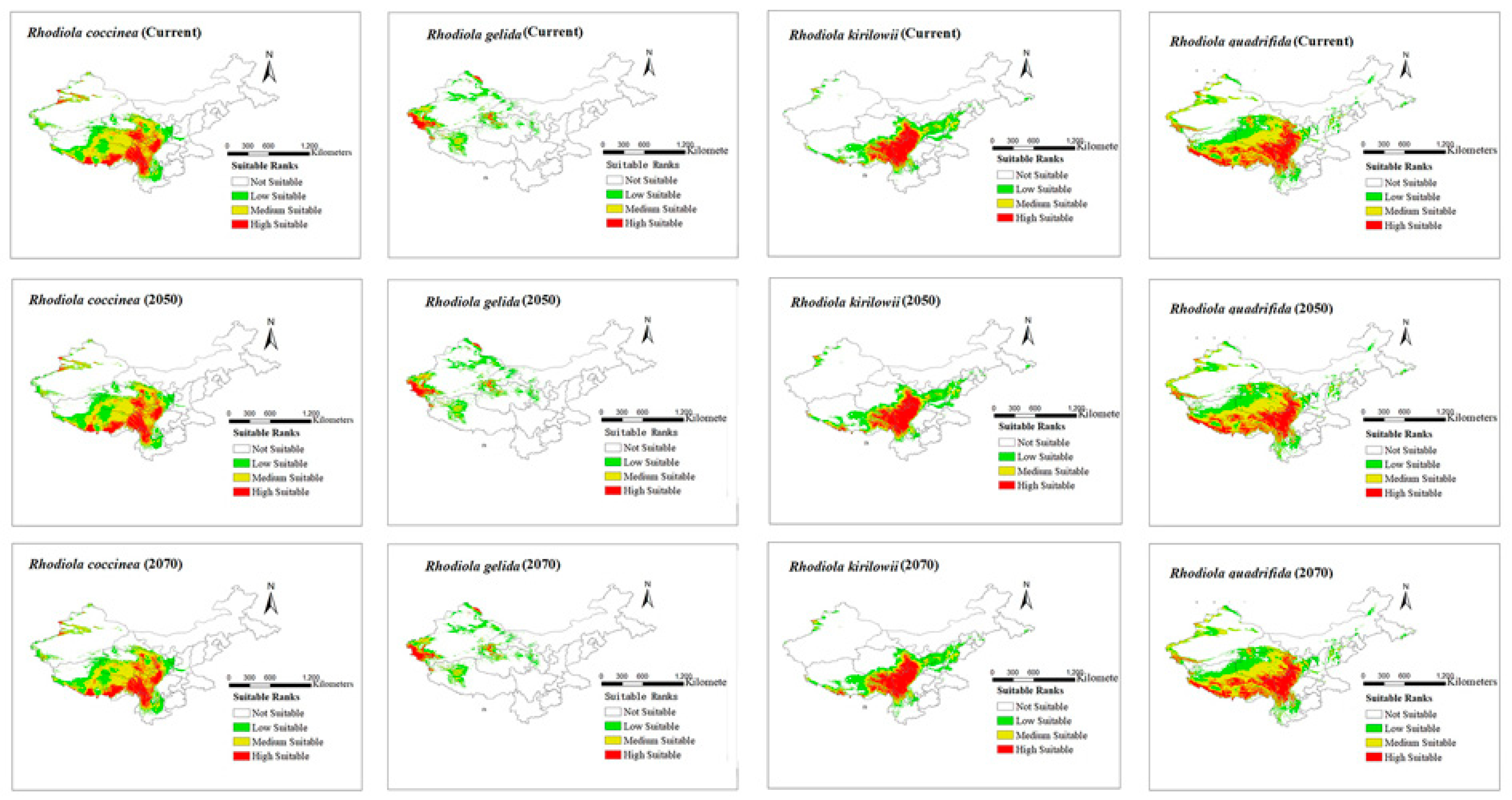
| Variable | Description | Variable | Description |
|---|---|---|---|
| Bio1 | Annual Mean Temperature | Bio11 | Mean Temperature of Coldest Quarter |
| Bio2 | Mean Diurnal Range (mean of monthly (max temp − min temp)) | Bio12 | Annual Precipitation |
| Bio3 | Isothermality (bio2/bio7) (×100) | Bio13 | Precipitation of Wettest Month |
| Bio4 | Temperature Seasonality (standard deviation × 100) | Bio14 | Precipitation of Driest Month |
| Bio5 | Max Temperature of Warmest Month | Bio15 | Precipitation Seasonality (coefficient of variation) |
| Bio6 | Min Temperature of Coldest Month | Bio16 | Precipitation of Wettest Quarter |
| Bio7 | Temperature Annual Range (bio5–bio6) | Bio17 | Precipitation of Driest Quarter |
| Bio8 | Mean Temperature of Wettest Quarter | Bio18 | Precipitation of Warmest Quarter |
| Bio9 | Mean Temperature of Driest Quarter | Bio19 | Precipitation of Coldest Quarter |
| Bio10 | Mean Temperature of Warmest Quarter |
| Species | AUC Data | Bio2 | Bio3 | Bio4 | Bio8 | Bio10 | Bio12 | Bio13 | Bio16 |
|---|---|---|---|---|---|---|---|---|---|
| R. coccineas | |||||||||
| LIG | 0.898 | 15.3 | 16.6 | 44.6 | |||||
| LGM | 0.89 | 18.9 | 14.5 | 43.7 | |||||
| MH | 0.897 | 19.7 | 13.9 | 42.5 | |||||
| Current | 0.896 | 19.5 | 13.6 | 44.1 | |||||
| 2050 | 0.899 | 21.5 | 42.6 | 12.1 | |||||
| 2070 | 0.899 | 22.6 | 43.3 | 13.6 | |||||
| R. gelida | |||||||||
| LIG | 0.905 | 18.2 | 25.2 | 35.5 | |||||
| LGM | 0.909 | 17.3 | 30.5 | 33 | |||||
| MH | 0.917 | 18 | 21.1 | 39.4 | |||||
| Current | 0.925 | 18.3 | 31 | 31 | |||||
| 2050 | 0.93 | 17.6 | 27.8 | 34 | |||||
| 2070 | 0.928 | 17.8 | 23.3 | 38.3 | |||||
| R. kirilowii | |||||||||
| LIG | 0.917 | 24.4 | 31.6 | 31.1 | |||||
| LGM | 0.917 | 21.8 | 33.3 | 31.4 | |||||
| MH | 0.915 | 20 | 34.9 | 31.6 | |||||
| Current | 0.916 | 23 | 31.3 | 30.9 | |||||
| 2050 | 0.915 | 17.2 | 36.9 | 31.8 | |||||
| 2070 | 0.918 | 16.3 | 37.9 | 30.4 | |||||
| R. quadrifida | |||||||||
| LIG | 0.86 | 11.7 | 64 | ||||||
| LGM | 0.865 | 13.1 | 61.3 | ||||||
| MH | 0.861 | 12.5 | 61.6 | ||||||
| Current | 0.866 | 13.3 | 61.1 | ||||||
| 2050 | 0.869 | 14.1 | 57.7 | ||||||
| 2070 | 0.862 | 15.7 | 62.6 |
| Species | Suitability Ranks | Species Distribution Area (km2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIG | LGM | Area change (LIG–LGM) | MH | Area change (LGM–MH) | Current | Area change (MH–Current) | 2050 | Area change (Current–2050) | 2070 | Area change (Current–2070) | ||
| R.coccinea | Not suitable | 7,375,605 | 7,389,565 | 13,960 | 7,376,330 | −13,235 | 7,403,989 | 27,659 | 7,404,598 | 609 | 7,386,625 | −17,364 |
| Low suitable | 588,549 | 595,958 | 7409 | 609,504 | 13,546 | 598,732 | −10,772 | 572,518 | −26,214 | 578,296 | −20,436 | |
| Medium suitable | 1,100,264 | 108,094 | −19,324 | 1,065,932 | −15,008 | 1,066,198 | 266 | 1,144,134 | 77,936 | 1,147,899 | 81,701 | |
| High suitable | 554,403 | 552,358 | −2045 | 567,056 | 14,697 | 549,902 | −17,153 | 497,570 | −52,332 | 506,001 | −43,901 | |
| R.gelida | Not suitable | 8,104,089 | 7,969,775 | −134,314 | 8,129,178 | 159,403 | 7,956,644 | −172,534 | 8,090,339 | 133,695 | 8,111,319 | 154,675 |
| Low suitable | 951,087 | 1,075,625 | 124,538 | 935,351 | −140,274 | 1,056,437 | 121,086 | 960,297 | −96,140 | 953,351 | −103,086 | |
| Medium suitable | 324,399 | 339,417 | 15,019 | 318,133 | −21,284 | 371,617 | 53,484 | 327,067 | −44,550 | 318,397 | −53,221 | |
| High suitable | 239,247 | 234,004 | −5243 | 236,158 | 2155 | 234,122 | −2036 | 241,117 | 6995 | 235,755 | 1633 | |
| R.kirilowii | Not suitable | 8,014,315 | 8,044,422 | 30,107 | 8,028,000 | −16,422 | 8,018,666 | −9334 | 8,003,861 | −14,805 | 8,045,777 | 27,111 |
| Low suitable | 675,553 | 647,067 | −28,487 | 679,407 | 32,340 | 674,909 | −4498 | 717,743 | 42,834 | 688,131 | 13,222 | |
| Medium suitable | 395,025 | 393,569 | −1456 | 397,809 | 4240 | 402,633 | 4824 | 372,663 | −29,971 | 364,933 | −37,700 | |
| High suitable | 533,928 | 533,763 | −165 | 513,604 | −20,159 | 522,612 | 9008 | 524,554 | 1942 | 519,979 | −2633 | |
| R.quadrifida | Not suitable | 6,529,197 | 6,588,593 | 59,396 | 6,581,346 | −7247 | 6,602,484 | 21,138 | 6,678,273 | 75,789 | 6,592,834 | −9650 |
| Low suitable | 1,128,673 | 1,151,578 | 22,905 | 1,151,659 | 81 | 1,198,135 | 46,476 | 1,134,026 | −64,109 | 1,184,936 | −13,199 | |
| Medium suitable | 1,092,051 | 1,100,747 | 8696 | 1,094,656 | −6091 | 1,015,322 | −79,334 | 1,042,712 | 27,390 | 1,053,106 | 37,784 | |
| High Suitable | 868,899 | 777,903 | −90,997 | 791,160 | 13,257 | 802,880 | 11,720 | 763,810 | −39,070 | 787,944 | −14,936 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Sun, L.; Yu, Y.; Zhang, H.; Malik, I.; Wistuba, M.; Yu, R. Predicting the Potential Geographical Distribution of Rhodiola L. in China under Climate Change Scenarios. Plants 2023, 12, 3735. https://doi.org/10.3390/plants12213735
Yang M, Sun L, Yu Y, Zhang H, Malik I, Wistuba M, Yu R. Predicting the Potential Geographical Distribution of Rhodiola L. in China under Climate Change Scenarios. Plants. 2023; 12(21):3735. https://doi.org/10.3390/plants12213735
Chicago/Turabian StyleYang, Meilin, Lingxiao Sun, Yang Yu, Haiyan Zhang, Ireneusz Malik, Małgorzata Wistuba, and Ruide Yu. 2023. "Predicting the Potential Geographical Distribution of Rhodiola L. in China under Climate Change Scenarios" Plants 12, no. 21: 3735. https://doi.org/10.3390/plants12213735
APA StyleYang, M., Sun, L., Yu, Y., Zhang, H., Malik, I., Wistuba, M., & Yu, R. (2023). Predicting the Potential Geographical Distribution of Rhodiola L. in China under Climate Change Scenarios. Plants, 12(21), 3735. https://doi.org/10.3390/plants12213735







