Crop-Livestock Integration Improves Physical Soil, Agronomic and Environmental Aspects in Soybean Cultivation
Abstract
1. Introduction
2. Results and Discussion
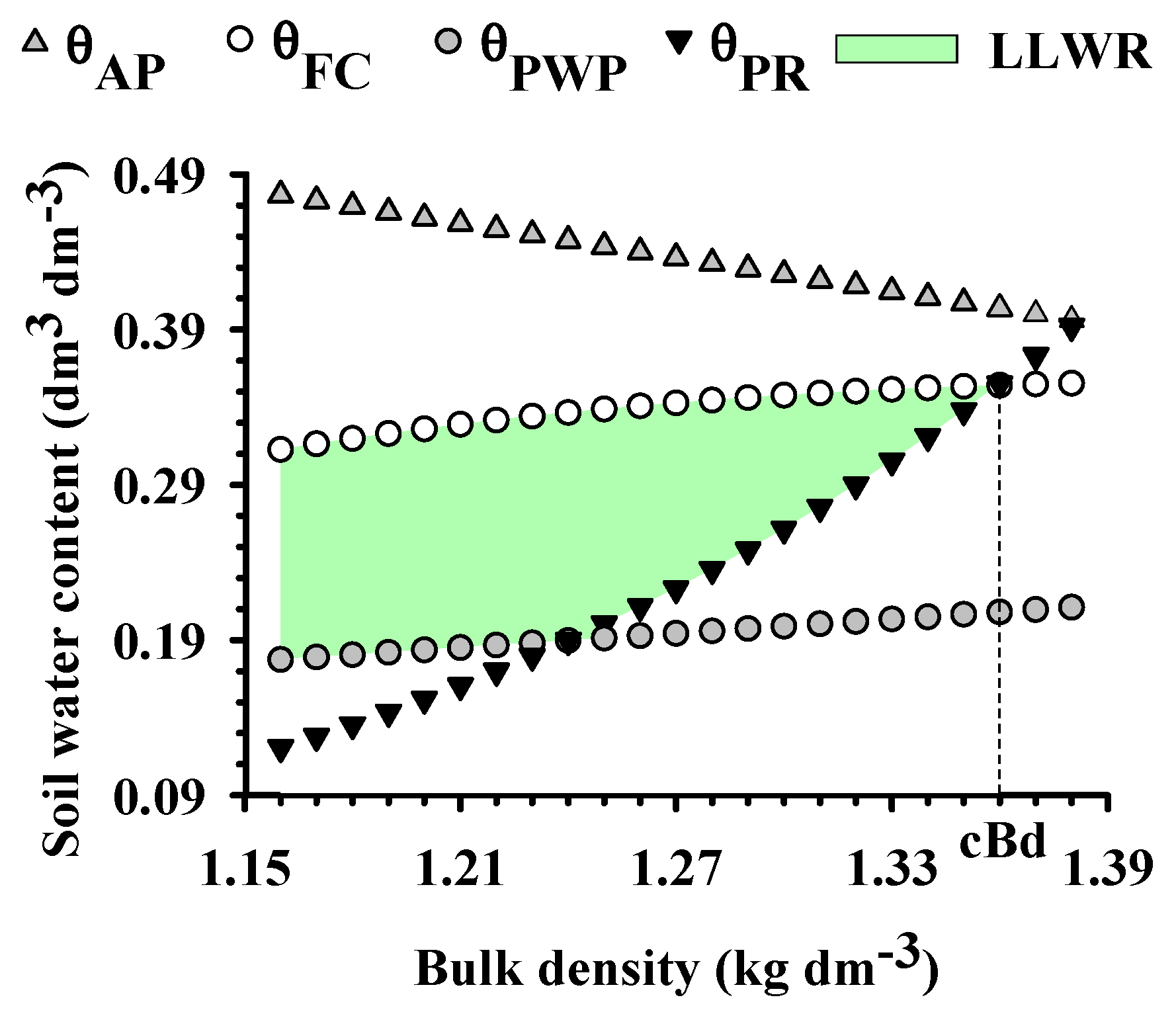
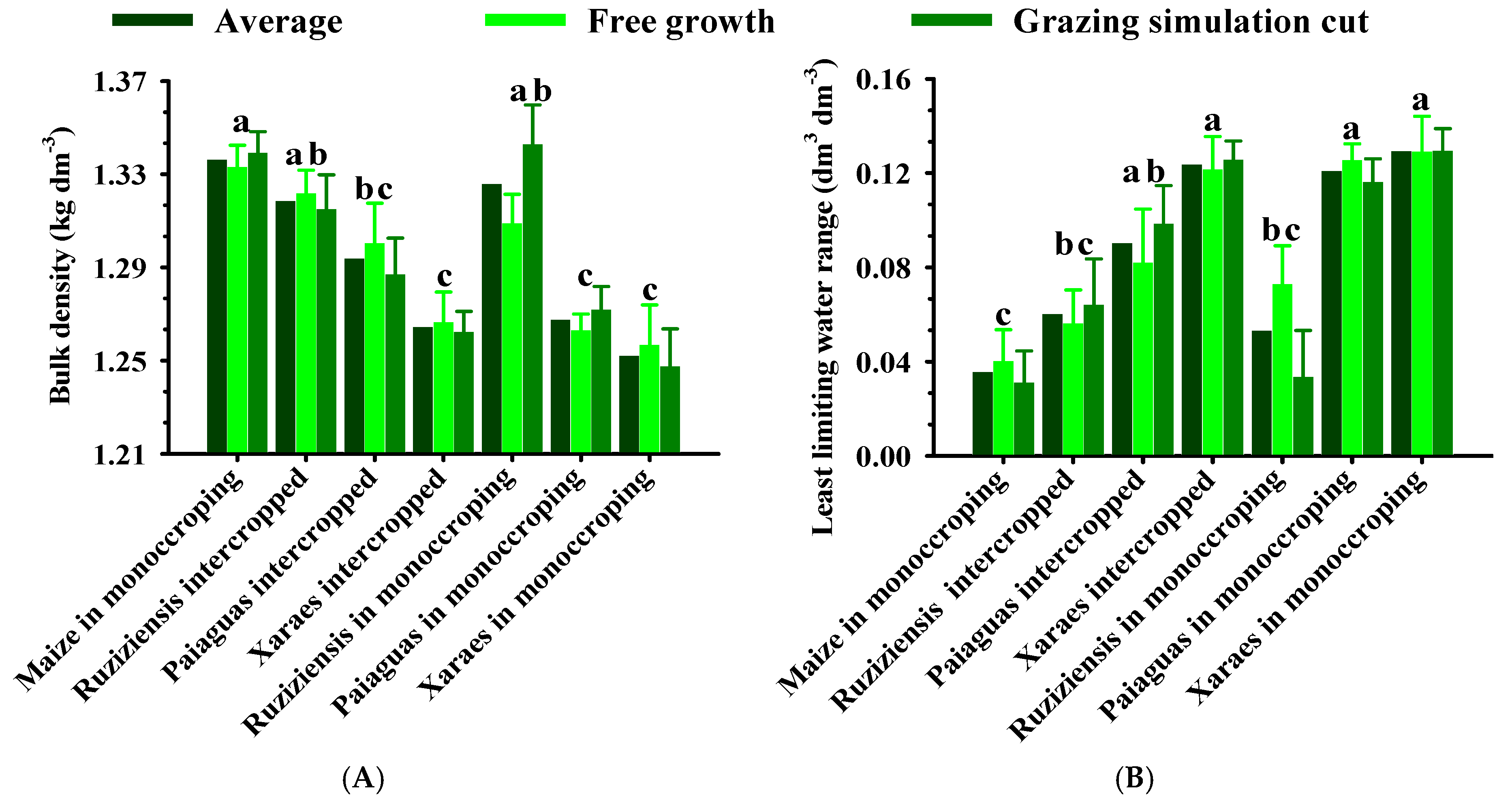
2.1. Physical Quality of the Soil
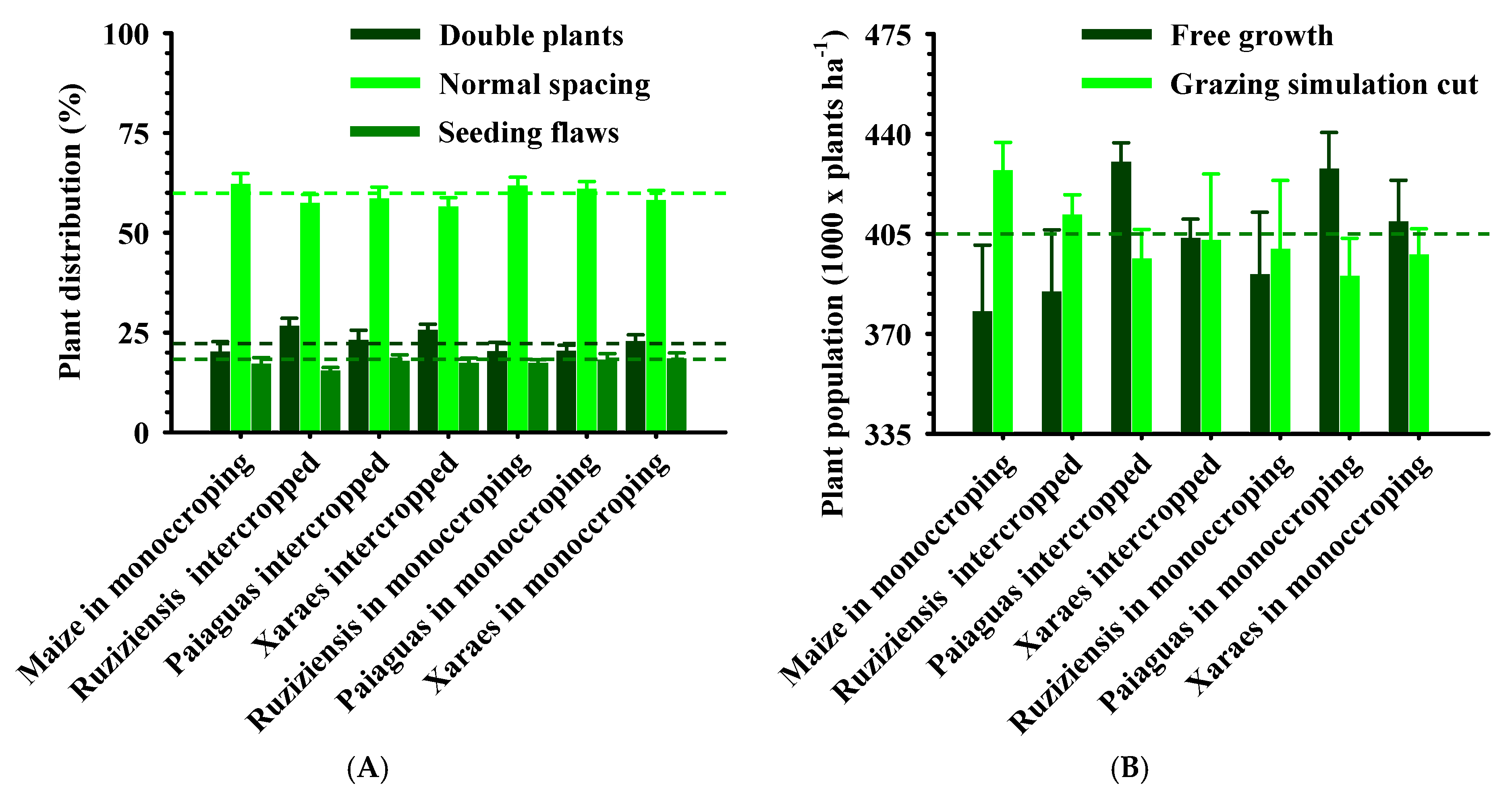
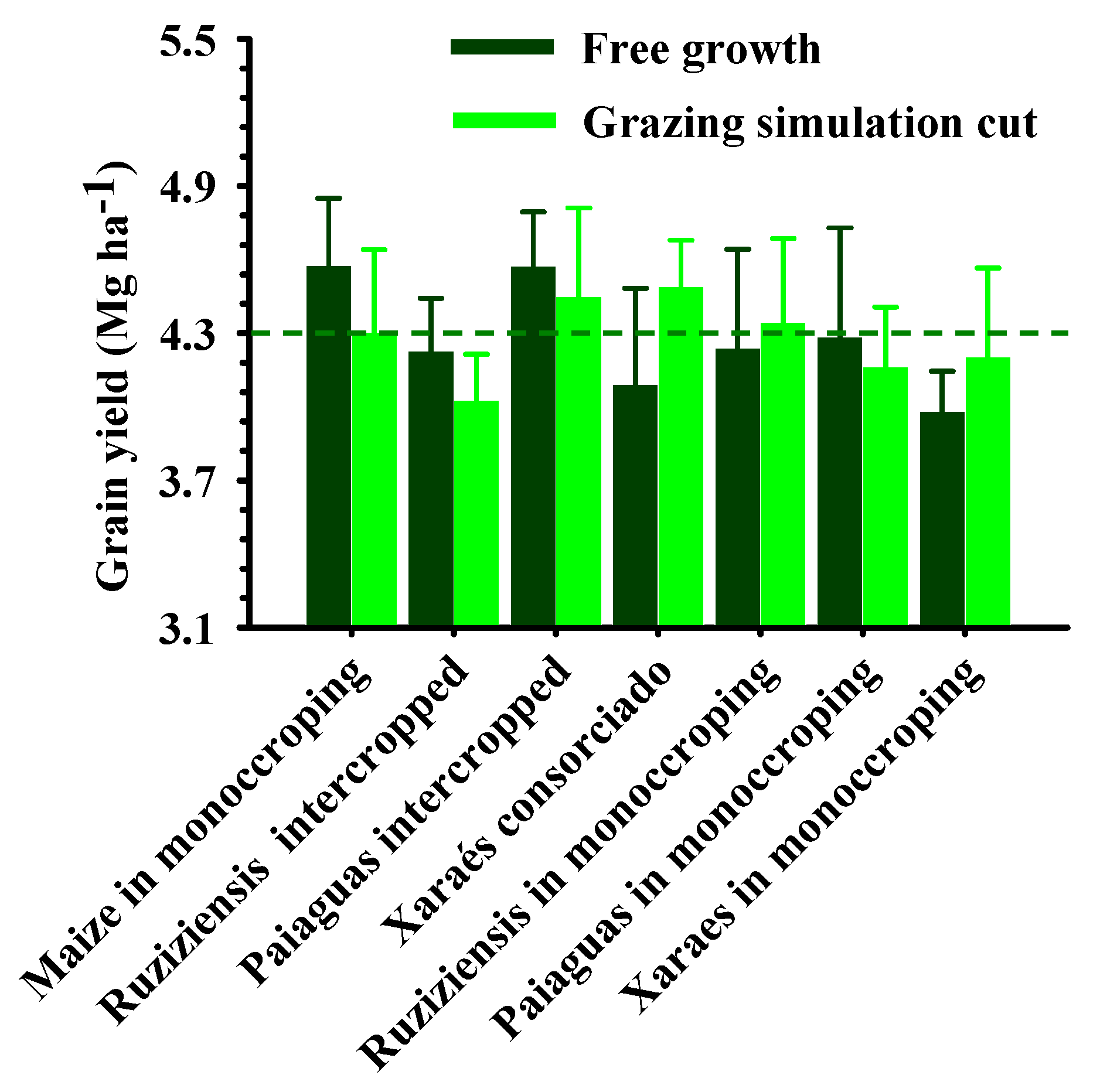
2.2. Agronomic Aspects for Soybean Crops in Succession
3. Materials and Methods
3.1. Study Site and Experimental Arrangement
3.2. Soil Sampling and Physical Analysis
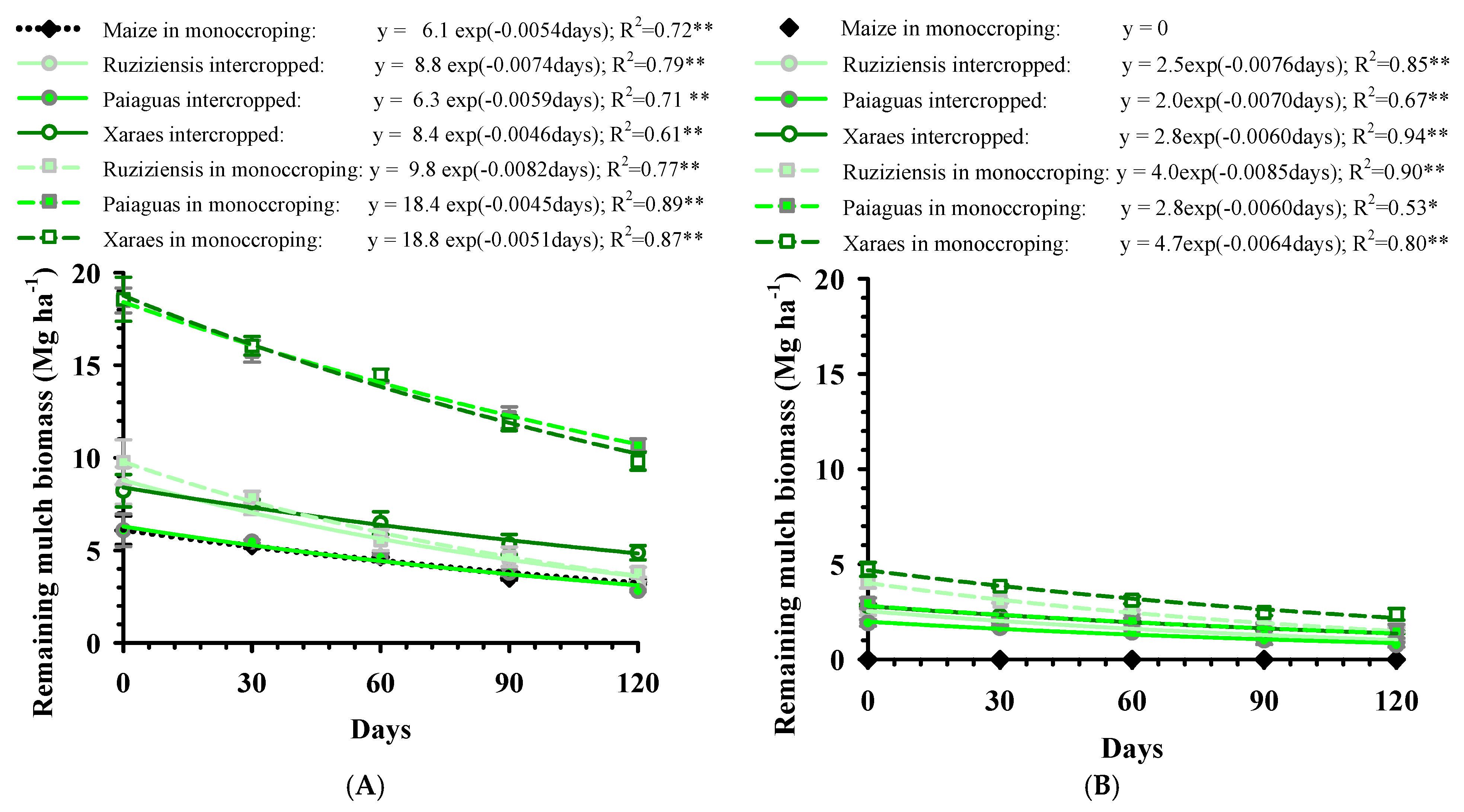
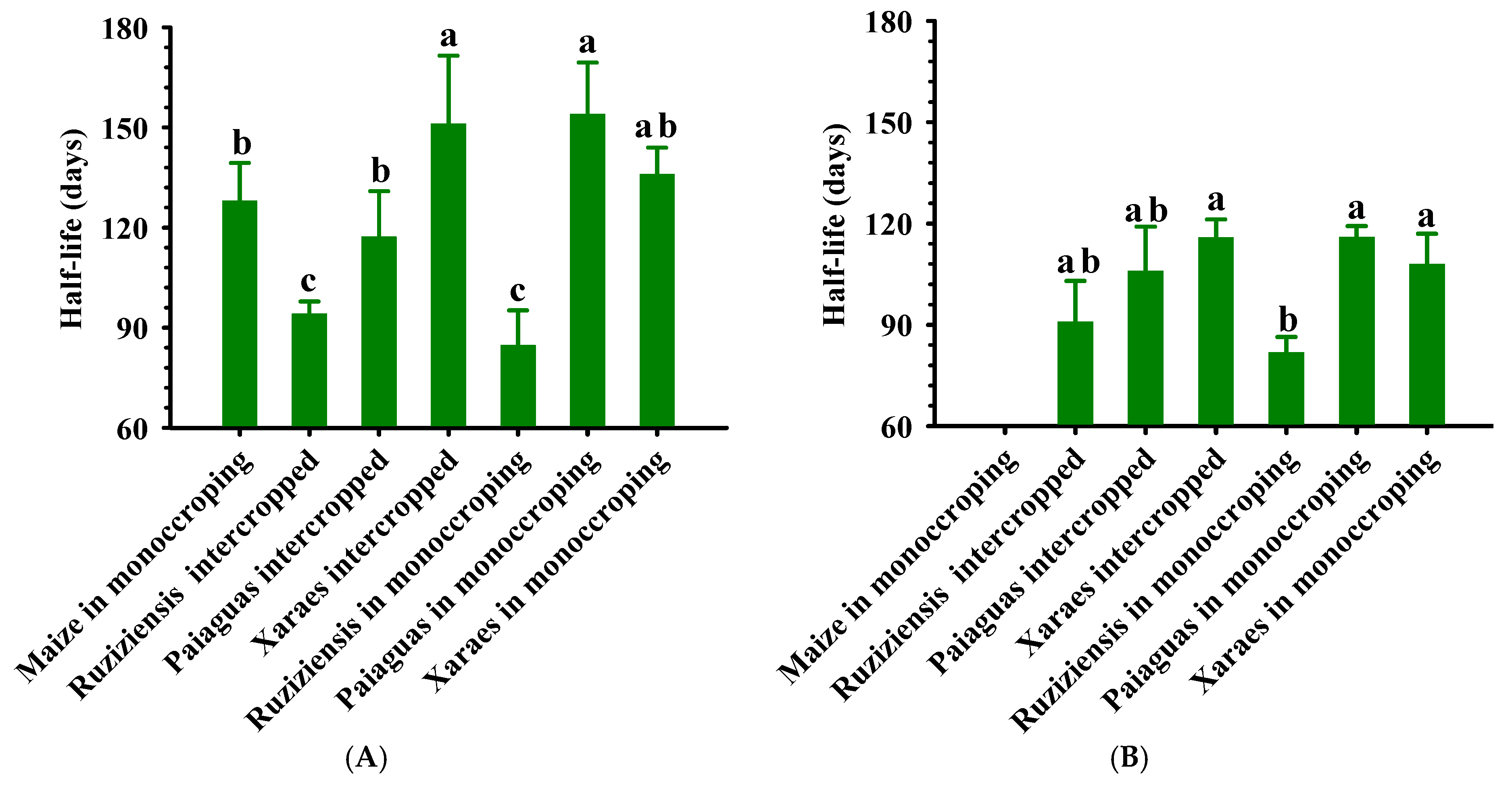
3.3. Sampling and Decomposition of Straw
3.4. Evaluation of Plantability, Plant Health Treatments and Soybean Productivity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rada, N.; Helfand, S.; Magalhães, M. Agricultural productivity growth in Brazil: Large and small farms excel. Food Policy 2019, 84, 176–185. [Google Scholar] [CrossRef]
- Souza, G.S.; Gomes, E.G.; Andrade Alves, E.R.; Gasques, J.G. Technological progress in the Brazilian agriculture. Socio-Econ. Plan Sci. 2020, 72, 100879. [Google Scholar] [CrossRef]
- Reis, J.C.; Rodrigues, G.S.; Barros, I.; Rodrigues, R.D.A.R.; Garrett, R.D.; Valentim, J.F.; Kamoi, M.Y.T.; Michetti, M.; Wruck, F.J.; Rodrigues-Filho, S.; et al. Integrated crop-livestock systems: A sustainable land-use alternative for food production in the Brazilian Cerrado and Amazon. J. Clean. Prod. 2021, 283, 124580. [Google Scholar] [CrossRef]
- Silva, J.A.G.; Costa, K.A.P.; Silva, L.M.; Severiano, E.C.; Silva, F.G.; Habermann, E.; Martinez, C.A.; Vilela, L.; Silva, A.G.; Costa, A.C.; et al. Integrated systems improve the sustainability of soybean cultivation in the tropical region. Front. Sustain. Food Syst. 2023, 7, 1224530. [Google Scholar] [CrossRef]
- Paula, A.D.M.; Lima, D.T.; Torres, J.L.R.; Rodrigues, G.I.; Lemes, E.M. Crop-livestock integration under no-tillage: Genuine Brazilian technology with economic and environmental sustainability: A review. Aust. J. Crop Sci. 2017, 11, 1161–1167. [Google Scholar] [CrossRef]
- Dias, M.B.C.; Costa, K.A.P.; Severiano, E.C.; Bilego, U.O.; Vilela, L.; Souza, W.F.; Oliveira, I.P.; Silva, A.C.G. Cattle performance with Brachiaria and Panicum maximum forages in an integrated crop-livestock system. Afr. J. Range Forage Sci. 2021, 39, 230–243. [Google Scholar] [CrossRef]
- Strassburg, B.B.N.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; Oliveira Filho, F.J.B.; Scaramuzza, C.A.M.; Scarano, F.R.; et al. Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 2017, 1, 0099. [Google Scholar] [CrossRef]
- Yue, Q.; Guo, P.; Wu, H.; Wang, Y.; Zhang, C. Towards sustainable circular agriculture: An integrated optimization framework for crop-livestock-biogas-crop recycling system management under uncertainty. Agric. Syst. 2022, 196, 103347. [Google Scholar] [CrossRef]
- Alghamdi, R.S.; Cihacek, L. Do post-harvest crop residues in no-till systems provide for nitrogen needs of following crops? Agron. J. 2022, 114, 835–852. [Google Scholar] [CrossRef]
- Bansal, S.; Chakraborty, P.; Kumar, S. Crop–livestock integration enhanced soil aggregate-associated carbon and nitrogen, and phospholipid fatty acid. Sci. Rep. 2022, 12, 2781. [Google Scholar] [CrossRef]
- Muhandiram, N.P.; Humphreys, M.W.; Fychan, R.; Davies, J.W.; Sanderson, R.; Marley, C.L. Do agricultural grasses bred for improved root systems provide resilience to machinery-derived soil compaction? Food Energy Secur. 2020, 9, e227. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Babu, S.; Shekhawat, K.; Singh, R.; Yadav, S.K.; Singh, V.K.; Singh, C. Designing energy cum carbon-efficient environmentally clean production system for achieving green economy in agriculture. Sustain. Energy Technol. Assess. 2022, 52, 102190. [Google Scholar] [CrossRef]
- Severiano, E.C.; Oliveira, G.C.; Dias Junior, M.S.; Curi, N.; Costa, K.A.P.; Carducci, C.E. Preconsolidation pressure, soil water retention characteristics, and texture of Latosols in the Brazilian Cerrado. Soil Res. 2013, 51, 193–202. [Google Scholar] [CrossRef]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A review on the effect of soil compaction and its management for sustainable crop production. J. Biosyst. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Keller, T.; Sandin, M.; Colombi, T.; Horn, R.; Or, D. Historical increase in agricultural machinery weights enhanced soil stress levels and adversely affected soil functioning. Soil Tillage Res. 2019, 194, 104293. [Google Scholar] [CrossRef]
- Nascimento Junior, L.F.; Torino, A.B.; Silva, L.M.; Costa, K.A.P.; Bilego, U.O.; Menezes, C.C.E.; Severiano, E.C. Modeling and quantification of soil compaction promoted by animal trampling in an integrated crop?livestock system. Semin. Cienc. Agrar. 2023, 44, 1179–1196. [Google Scholar] [CrossRef]
- Singh, K.; Choudhary, O.P.; Singh, H.P.; Singh, A.; Mishra, S.K. Sub-soiling improves productivity and economic returns of cotton-wheat cropping system. Soil Tillage Res. 2019, 189, 131–139. [Google Scholar] [CrossRef]
- Ferreira, C.J.B.; Tormena, C.A.; Severiano, E.C.; Nunes, M.R.; Menezes, C.C.E.; Antille, D.L.; Preto, V.R.O. Effectiveness of narrow tyne and double-discs openers to overcome shallow compaction and improve soybean yield in long-term no-tillage soil. Soil Tillage Res. 2023, 227, 105622. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, K.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Brasilia, Brazil, 2018; p. 355. [Google Scholar]
- Anghinoni, G.; Anghinoni, F.B.G.; Tormena, C.A.; Braccini, A.L.; Carvalho Mendes, I.; Zancanaro, L.; Lal, R. Conservation agriculture strengthen sustainability of Brazilian grain production and food security. Land Use Policy 2021, 108, 105591. [Google Scholar] [CrossRef]
- Flávio Neto, J.; Severiano, E.C.; Costa, K.A.P.; Guimarães Junnyor, W.S.; Gonçalves, W.G.; Andrade, R. Biological soil loosening by grasses from genus Brachiaria in croplivestock integration. Acta Sci. Agron. 2015, 37, 375–383. [Google Scholar] [CrossRef]
- Cavalieri-Polizeli, K.M.V.; Marcolino, F.C.; Tormena, C.A.; Keller, T.; Moraes, A.D. Soil structural quality and relationships with root properties in single and integrated farming systems. Front. Environ. Sci. 2022, 10, 901302. [Google Scholar] [CrossRef]
- Dias, M.B.C.; Costa, K.A.P.; Severiano, E.C.; Bilego, U.O.; Furtini Neto, A.E.; Almeida, D.P.; Brand, S.C.; Lourival, V. Brachiaria and Panicum maximum in an integrated crop-livestock system and a second-crop corn system in succession with soybean. J. Agric. Sci. 2020, 158, 206–217. [Google Scholar] [CrossRef]
- Guimarães Júnnyor, W.S.; Maria, I.C.; Araujo-Junior, C.F.; Diserens, E.; Severiano, E.C.; Farhate, C.V.V.; Souza, Z.M. Conservation systems change soil resistance to compaction caused by mechanised harvesting. Ind. Crops Prod. 2022, 177, 114532. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. No-tillage and soil physical environment. Geoderma 2018, 326, 164–200. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Medeiros, A.S.; Santos, T.C.; Lyra, G.B.; Lal, R.; Assad, E.D.; Cerri, C.E.P. Potential of no-till agriculture as a nature-based solution for climate-change mitigation in Brazil. Soil Tillage Res. 2022, 220, 105368. [Google Scholar] [CrossRef]
- Laroca, J.V.S.; Souza, J.M.A.; Pires, G.C.; Pires, G.J.C.; Pacheco, L.P.; Silva, F.D.; Wruck, F.J.; Carneiro, M.A.C.; Silva, L.S.; Souza, E.D. Soil quality and soybean productivity in crop-livestock integrated system in no-tillage. Pesqui. Agropecu. Bras. 2018, 53, 1248–1258. [Google Scholar] [CrossRef]
- Simões, V.J.L.P.; Souza, E.S.; Martins, A.P.; Tiecher, T.; Bremm, C.; Ramos, J.S.; Farias, G.D.; Carvalho, P.C.F. Structural soil quality and system fertilization efficiency in integrated crop-livestock system. Agric. Ecosyst. Environ. 2023, 349, 108453. [Google Scholar] [CrossRef]
- Severiano, E.C.; Oliveira, G.C.; Dias Júnior, M.S.; Costa, K.A.P.; Silva, F.G.; Ferreira Filho, S.M. Structural changes in Latosols of the Cerrado Region: I—Relationships between soil physical properties and Least Limiting Water Range. Rev. Bras. Cienc. Solo 2011, 35, 773–782. [Google Scholar] [CrossRef]
- Barbosa, S.; Oliveira, G.C.; Carducci, C.E.; Montoani, S.B. Potencialidade de uso de zeólitas na atenuação do déficit hídrico em Latossolo do Cerrado. Semin. Cienc. Agrar. 2014, 35, 2357–2368. [Google Scholar] [CrossRef]
- Silva, A.P.; Kay, B.D.; Perfect, E. Characterization of the least limiting water range of soils. Soil Sci. Soc. Am. J. 1994, 58, 1775–1781. [Google Scholar] [CrossRef]
- Torino, A.B.; Nascimento, L.F.; Brito, M.F.; Lima, J.D.P.; Goncalves, W.G.; Costa, K.A.P.; Severiano, E.C. Agronomic performance of maize and Brachiaria grasses cultivated at monocropping and intercropping in a compacted latossolo. Aust. J. Crop Sci. 2020, 14, 1533–1540. [Google Scholar] [CrossRef]
- Pezzopane, C.G.; Santos, P.M.; Cruz, P.G.; Altoé, J.; Ribeiro, F.A.; Valle, C.B. Estresse por deficiência em genótipos de Brachiaria brizantha. Ciênc. Rural 2015, 45, 871–876. [Google Scholar] [CrossRef][Green Version]
- Chioderoli, C.A.; Mello, L.M.M.; Grigolli, P.J.; Furlani, C.E.A.; Silva, J.O.R.; Cesarin, A.L. Atributos físicos do solo e produtividade de soja em sistema de consórcio milho e braquiária. Rev. Bras. Eng. Agric. Ambient. 2012, 16, 37–43. [Google Scholar] [CrossRef]
- Calonego, J.C.; Borghi, E.; Crusciol, C.A.C. Intervalo hídrico ótimo e compactação do solo com o cultivo de milho e braquiária. Rev. Bras. Cienc. Solo 2011, 35, 2183–2190. [Google Scholar] [CrossRef]
- Ferreira, R.V.; Tavares, R.L.M.; Fillho, S.V.P.; Marasca, I.; Silva, A.G. Off-season strategies to improve soil physical attributes in a no-till system in the Brazilian Cerrado. Rev. Bras. Milho Sorgo 2020, 19, e1183. [Google Scholar] [CrossRef]
- Moraes, A.E.L.; Rossato, F.G.; Susaeta, A.; Binotto, E.; Malafaia, G.C.; Azevedo, D.B. Environmental impacts in integrated production systems: An overview. J. Clean. Prod. 2023, 420, 138400. [Google Scholar] [CrossRef]
- Ceballos, G.A.; Fabian, A.J.; Silva, J.C.O.; Torino, A.B.; Bernardes, G.F. Production and speed of decomposition of species of soil coverage in direct sowing system. Rev. Ciênc. Agrar. Amaz. J. Agric. Environ. Sci. 2018, 61, 1–6. [Google Scholar] [CrossRef]
- Meo-Filho, P.; Berndt, A.; Pezzopane, J.R.M.; Pedroso, A.F.; Bernardi, A.C.C.; Rodrigues, P.H.M.; Bueno, I.C.S.; Corte, R.R.; Oliveira, P.P.A. Can intensified pasture systems reduce enteric methane emissions from beef cattle in the atlantic forest biome? Agronomy 2022, 12, 2738. [Google Scholar] [CrossRef]
- Piao, R.S.; Silva, V.L.; Aguila, I.N.; Burgos Jiménez, J. Green growth and agriculture in Brazil. Sustainability 2021, 13, 1162. [Google Scholar] [CrossRef]
- Sulc, R.M.; Franzluebbers, A.J. Exploring integrated crop–livestock systems in different ecoregions of the United States. Eur. J. Agron. 2014, 57, 21–30. [Google Scholar] [CrossRef]
- Lal, R. Why Carbon Sequestration in Agricultural Soils. In Agricultural Practices and Policies for Carbon Sequestration in Soil, 1st ed.; Kimble, J.M., Lal, R., Follett, R.F., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 21–31. [Google Scholar]
- Medina, G.; Santos, A.P.S. Curbing enthusiasm for Brazilian agribusiness: The use of actorspecific assessments to transform sustainable development on the ground. Appl. Geogr. 2017, 85, 101–112. [Google Scholar] [CrossRef]
- Sá, J.C.M.; Lal, R.; Cerri, C.C.; Lorenz, K.; Hungria, M.; Carvalho, P.C.F. Low-carbon agriculture in South America to mitigate global climate change and advance food security. Environ. Int. 2017, 98, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.P.S.; Paludo, V.; Souza, S.F.G.; Baio, T.P.; Silva, P.R.A. Distribuição de sementes de soja com tenologia Rampflow no disco horizontal. In Proceedings of the 4a Jornada Científica e Tecnológica da Faculdade de Tecnologia do Estado de São Paulo, Botucatu, SP, Brasil, 7–9 October 2015. [Google Scholar]
- Xu, C.; Li, R.; Song, W.; Wu, T.; Sun, S.; Han, T.; Wu, C. High density and uniform plant distribution improve soybean yield by regulating population uniformity and canopy light interception. Agronomy 2021, 11, 1880. [Google Scholar] [CrossRef]
- Masino, A.; Rugeroni, P.; Borrás, L.; Rotundo, J.L. Spatial and temporal plant-to-plant variability effects on soybean yield. Eur. J. Agron. 2018, 98, 14–24. [Google Scholar] [CrossRef]
- Pereyra, V.M.; Bastos, L.M.; Borja Reis, A.F.; Melchiori, R.J.; Maltese, N.E.; Appelhans, S.C.; Prasad, P.V.V.; Wright, Y.; Brokesh, E.; Sharda, A.; et al. Early-season plant-to-plant spatial uniformity can affect soybean yields. Sci. Rep. 2022, 12, 17128. [Google Scholar] [CrossRef]
- Carmo, E.L.; Braz, G.B.P.; Simon, G.A.; Silva, A.G.; Rocha, A.G.C. Desempenho agronômico da soja cultivada em diferentes épocas e distribuição de plantas. Rev. Ciênc. Agrovet. 2018, 17, 61–69. [Google Scholar] [CrossRef]
- Franchini, J.C.; Junior Balbinot, A.A.; Debiasi, H.; Conte, O. Desempenho da soja em consequência de manejo de pastagem, época de dessecação e adubação nitrogenada. Pesqui. Agropecu. Bras. 2015, 50, 1131–1138. [Google Scholar] [CrossRef]
- Aratani, R.G.; Freddi, O.S.; Centurion, J.F.; Andrioli, I. Qualidade física de um Latossolo Vermelho Acriférrico sob diferentes sistemas de uso e manejo. Rev. Bras. Cienc. Solo 2009, 33, 677–687. [Google Scholar] [CrossRef]
- Brighenti, A.M.; Sobrinho, S.F.; Rocha, W.S.D.; Martins, C.E.; Demartini, D.; Costa, T.R. Suscetibilidade diferencial de espécies de braquiária ao herbicida glifosato. Pesqui. Agropecu. Bras. 2011, 46, 1241–1246. [Google Scholar] [CrossRef]
- Machado, L.A.Z.; Comunello, É.; Cecato, U.; Concenço, G. Susceptibility of perennial tropical forage plants to glyphosate herbicide in integrated crop-livestock farming systems. Planta Daninha 2018, 36, e018160687. [Google Scholar] [CrossRef]
- Machado, L.A.; Valle, C.B. Desempenho agronômico de genótipos de capim-braquiária em sucessão à soja. Pesqui. Agropecu. Bras. 2011, 46, 1454–1462. [Google Scholar] [CrossRef]
- Constantin, J.; Oliveira Júnior, R.S.; Inoue, M.H.; Cavalieri, S.D.; Arantes, J.G. Sistemas de manejo de plantas daninhas no desenvolvimento e na produtividade da soja. Bragantia 2009, 68, 125–135. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária. Árvore do conhecimento: Solos. In Agência Embrapa de Informações Técnológica; Parque Estação Biológica: Brasília, Brazil, 2018. [Google Scholar]
- Bonetti, J.A.; Paulino, H.B.; Souza, E.D.; Carneiro, M.A.C.; Silva, G.N. Influência do sistema integrado de produção agropecuária no solo e na produtividade de soja e braquiária. Pesqui. Agropecu. Trop. 2015, 45, 104–112. [Google Scholar] [CrossRef]
- Cecagno, D.; Costa, S.E.V.G.A.; Anghinoni, I.; Kunrath, T.R.; Martins, A.P.; Reichert, J.M.; Gubiani, P.I.; Balerini, F.; Fink, J.R.; Carvalho, P.C.F. Least limiting water range and soybean yield in a long-term, no-till, integrated crop-livestock system under different grazing intensities. Soil Tillage Res. 2016, 156, 54–62. [Google Scholar] [CrossRef]
- Balbinot Junior, A.A.; Procópio, S.O.; Debiase, H.; Frnchini, J.C.; Panison, F. Semeadura cruzada em cultivares de soja com tipo de crescimento determinado. Semin. Cienc. Agrar. 2015, 36, 1215–1226. [Google Scholar] [CrossRef]
- Marasca, I.; Oliveira, C.A.; Guimaraes, E.C.; Cunha, J.P.A.R.; Assis, R.L.; Perin, A.; Menezes, L.A.S. Variabilidade espacial da resistência do solo à penetração e teor de água em sistema de plantio direto, na cultura da soja. Biosci. J. 2011, 27, 239–246. [Google Scholar]
- USDA, United States Department of Agriculture. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 410.
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Revista e Ampliada; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Souza, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação, 2nd ed.; Embrapa Cerrados: Brasília, Brazil, 2004; p. 416. [Google Scholar]
- Guimarães Junnyor, W.S.; Severiano, E.C.; Silva, A.G.; Gonçalves, W.G.; Andrade, R.; Martins, B.R.R.; Costodio, G.D. Sweet sorghum performance affected by soil compaction and sowing time as a second crop in the brazilian cerrado. Rev. Bras. Cienc. Solo 2015, 39, 1744–1754. [Google Scholar] [CrossRef][Green Version]
- Blake, G.R.; Hartge, K.H. Particle density. In Methods of Soil Analysis Part 1, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 377–382. [Google Scholar]
- Busscher, W.J. Adjustment of flat-tipped penetrometer resistance data to a common water content. Am. Soc. Agric. Eng. 1990, 33, 519–524. [Google Scholar] [CrossRef]
- Silva, A.P.; Kay, B.D.; Tormena, C.A.; Imhoff, S. Least limiting water range of soils. In Encyclopedia of Soil Science; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 1, pp. 1026–1029. [Google Scholar]
- Grable, A.R.; Siemer, E.G. Effects of bulk density, aggregate size, and soil water suction on oxygen diffusion, redox potentials, and elongation of corn roots. Soil Sci. Soc. Am. J. 1968, 32, 180–186. [Google Scholar] [CrossRef]
- Richards, L.A.; Weaver, L.R. Fitten-atmosphere percentage as related to the permanent wilting percentage. Soil Sci. 1943, 56, 331–339. [Google Scholar] [CrossRef]
- Wieder, R.K.; Lang, G.E. A critique of the analytical methods used examining decomposition data obtained from litter bags. Ecology 1982, 63, 1636–1642. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1989; p. 254. [Google Scholar]
- Nidera Sementes. Catalogo de Produtos: Soja. Available online: http://www.niderasementes.com.br/soja/ (accessed on 14 May 2018).
- Rauta, E.A.P.; Semensati, E.R.; Guimarães, A.M. Estudo da influência da velocidade no tratamento de Imagens de distribuição de sementes. In Proceedings of the XIX Encontro Anual de Iniciação Científica Universidade, Guarapuava, Brazil, 28–30 October 2010. [Google Scholar]
- Associação Brasileira de Normas Técnicas. Projeto de Norma 04:015.06-004—Semeadoras de Precisão; Ensaio de Laboratório—Método de Ensaio; Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 1994; p. 26. [Google Scholar]


| Layer | Pd (1) | Bd (2) | Texture (2) | Oxides in Sulfuric Attack | Ki | Kr | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | SiO2 | Al2O3 | Fe2O3 | |||||
| (m) | (kg dm−3) | --------------------------- (g kg−1) --------------------- | ||||||||
| 0–0.20 | 2.80 | 1.34 | 350 | 200 | 450 | 46 | 198 | 224 | 0.39 | 0.23 |
| Ca | Mg | Al | Al + H | P | K | S | Zn | B | Cu | Mn | Mo | V (1) | m(2) | O.M. (3) | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ----- cmolc dm−3 ----- | --------------- mg dm−3 -------------- | --- % --- | g kg−1 | ||||||||||||
| 1.83 | 0.75 | 0.01 | 5.15 | 2.8 | 34 | 10.55 | 0.62 | 0.17 | 3.82 | 25.95 | 25.71 | 34.25 | 0.37 | 25.17 | 4.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, J.D.P.; Torino, A.B.; Silva, L.M.d.; Nascimento Júnior, L.F.d.; Brito, M.F.d.; Costa, K.A.d.P.; Silva, B.M.; Severiano, E.d.C. Crop-Livestock Integration Improves Physical Soil, Agronomic and Environmental Aspects in Soybean Cultivation. Plants 2023, 12, 3746. https://doi.org/10.3390/plants12213746
Lima JDP, Torino AB, Silva LMd, Nascimento Júnior LFd, Brito MFd, Costa KAdP, Silva BM, Severiano EdC. Crop-Livestock Integration Improves Physical Soil, Agronomic and Environmental Aspects in Soybean Cultivation. Plants. 2023; 12(21):3746. https://doi.org/10.3390/plants12213746
Chicago/Turabian StyleLima, Jordaanny Danyelly Pereira, Aline Borges Torino, Luciana Maria da Silva, Lucas Freitas do Nascimento Júnior, Marlete Ferreira de Brito, Kátia Aparecida de Pinho Costa, Bruno Montoani Silva, and Eduardo da Costa Severiano. 2023. "Crop-Livestock Integration Improves Physical Soil, Agronomic and Environmental Aspects in Soybean Cultivation" Plants 12, no. 21: 3746. https://doi.org/10.3390/plants12213746
APA StyleLima, J. D. P., Torino, A. B., Silva, L. M. d., Nascimento Júnior, L. F. d., Brito, M. F. d., Costa, K. A. d. P., Silva, B. M., & Severiano, E. d. C. (2023). Crop-Livestock Integration Improves Physical Soil, Agronomic and Environmental Aspects in Soybean Cultivation. Plants, 12(21), 3746. https://doi.org/10.3390/plants12213746






