Anti-Lymphangiogenic Terpenoids from the Heartwood of Taiwan Juniper, Juniperus chinensis var. tsukusiensis
Abstract
1. Introduction
2. Results
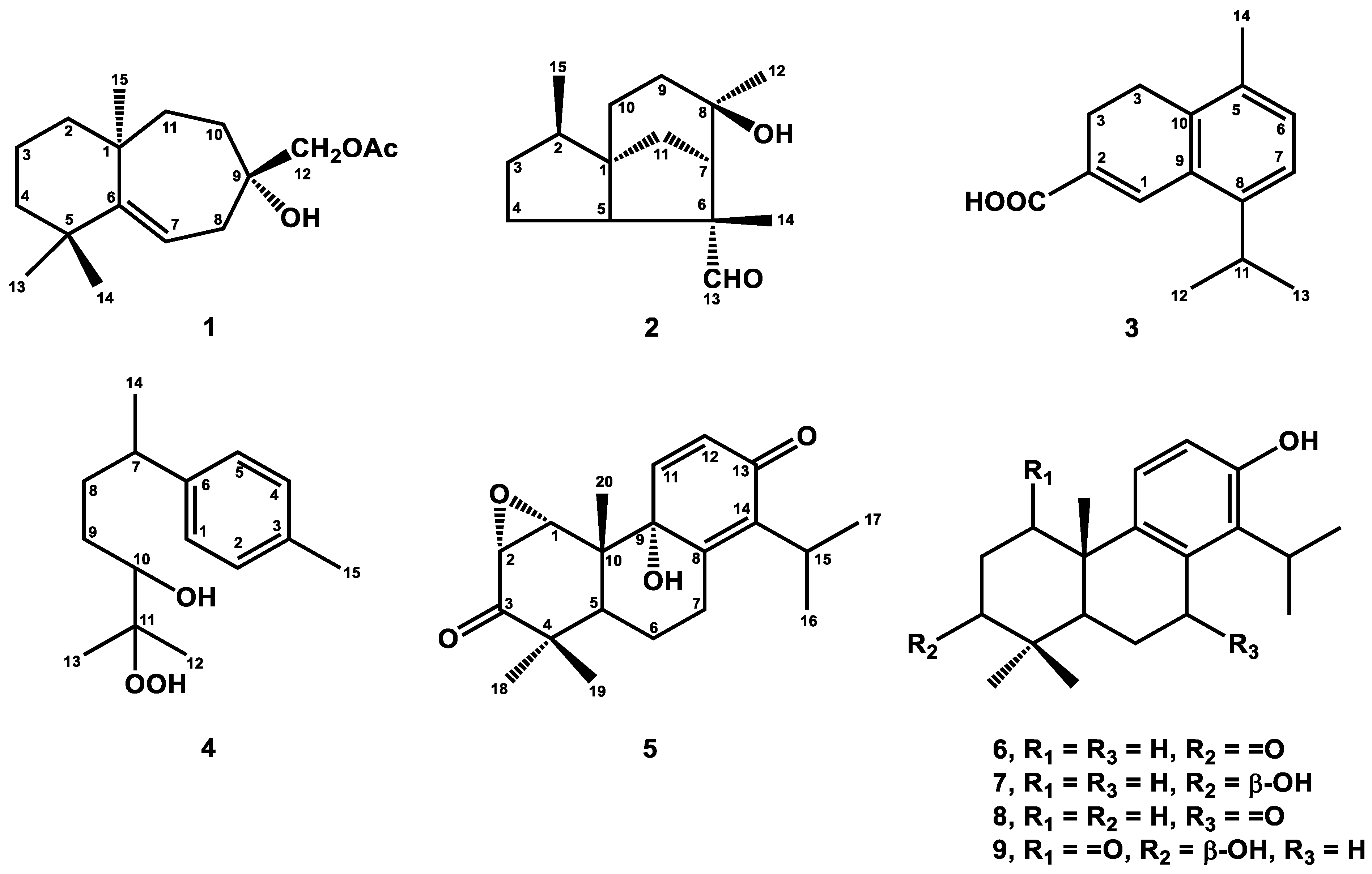
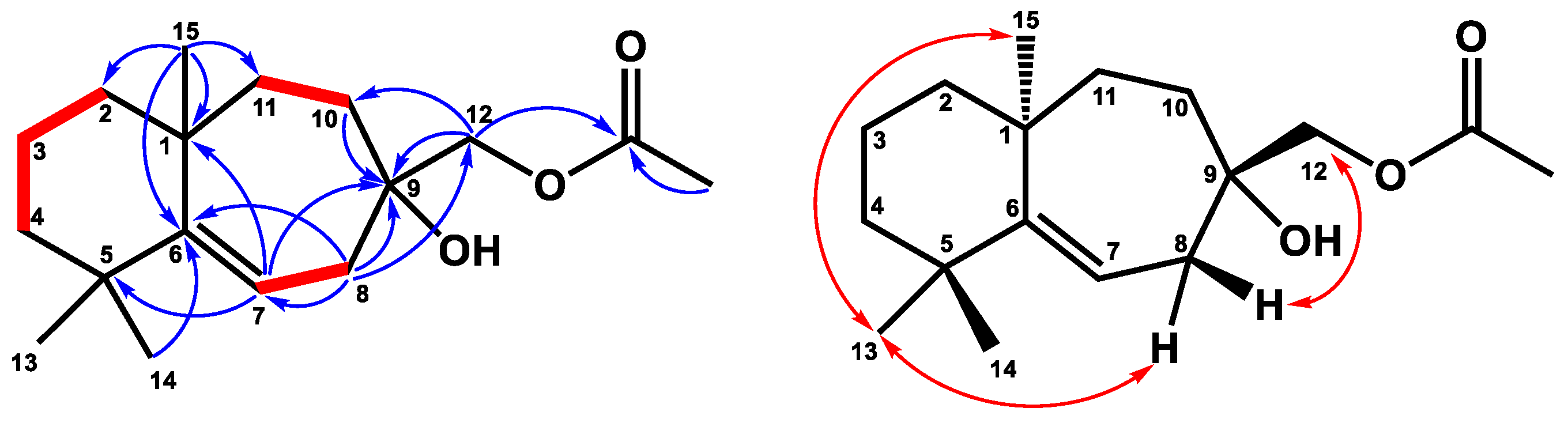
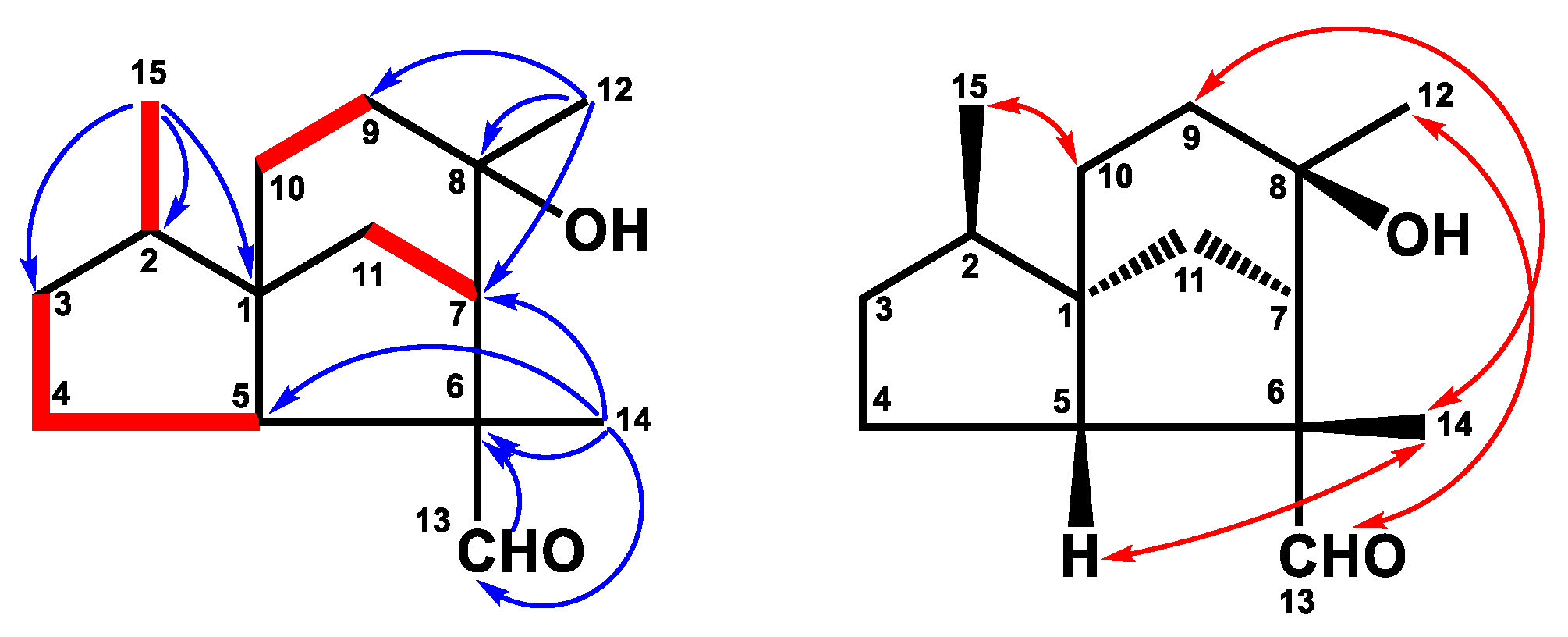
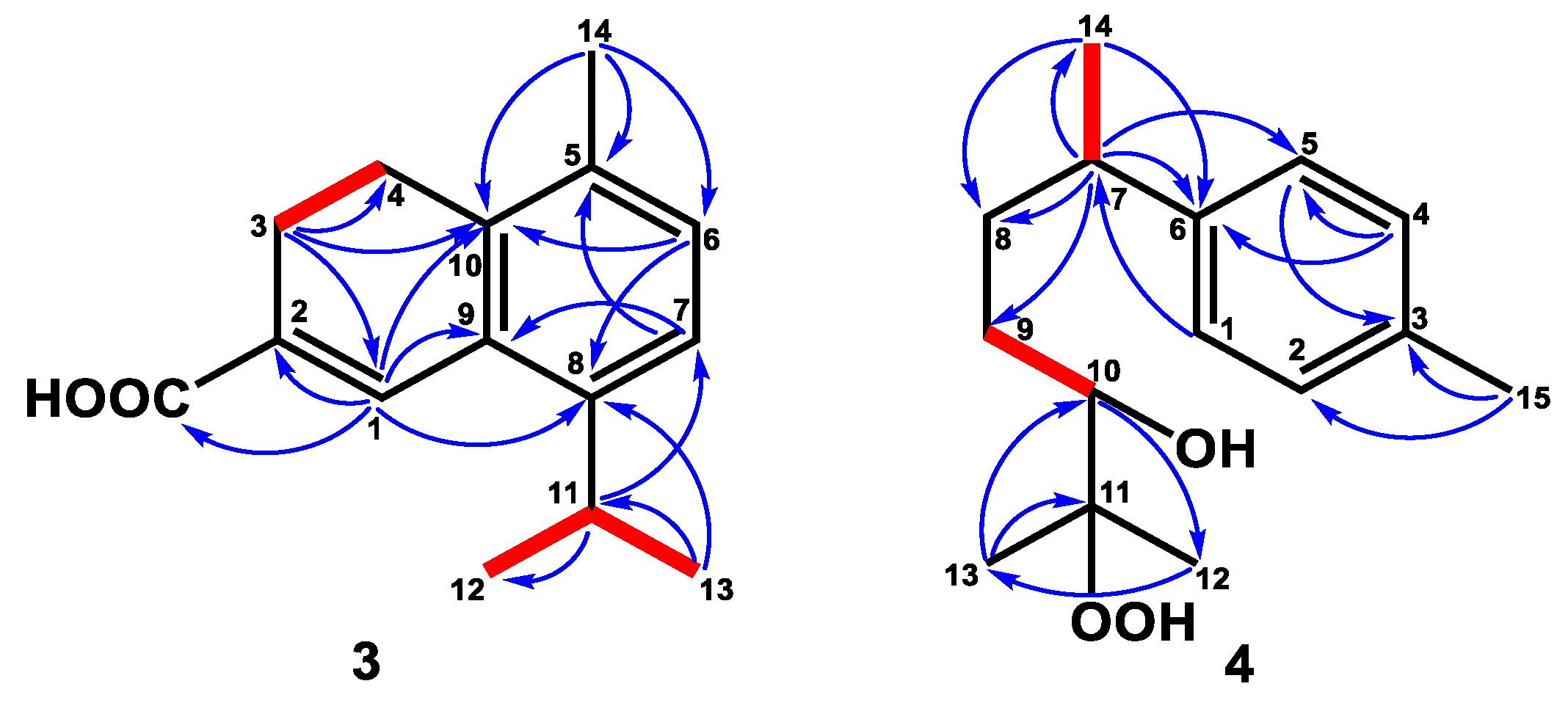
2.1. Structure Elucidation
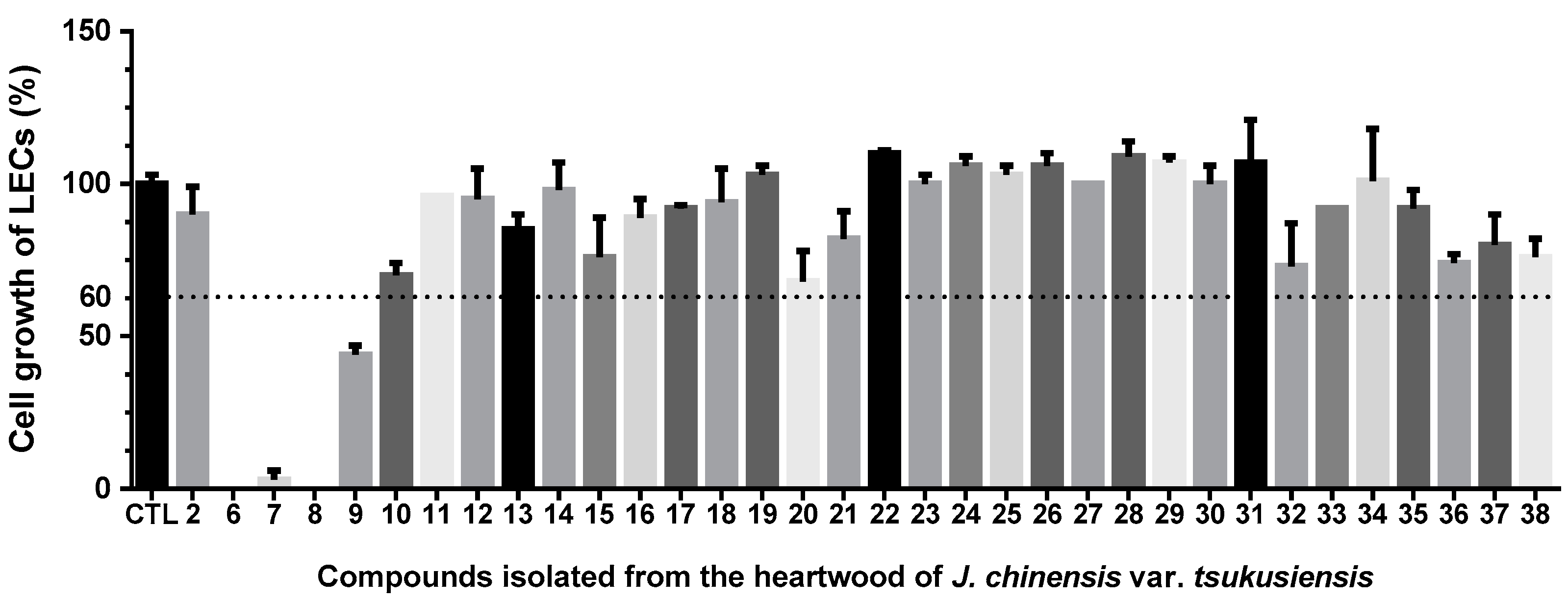
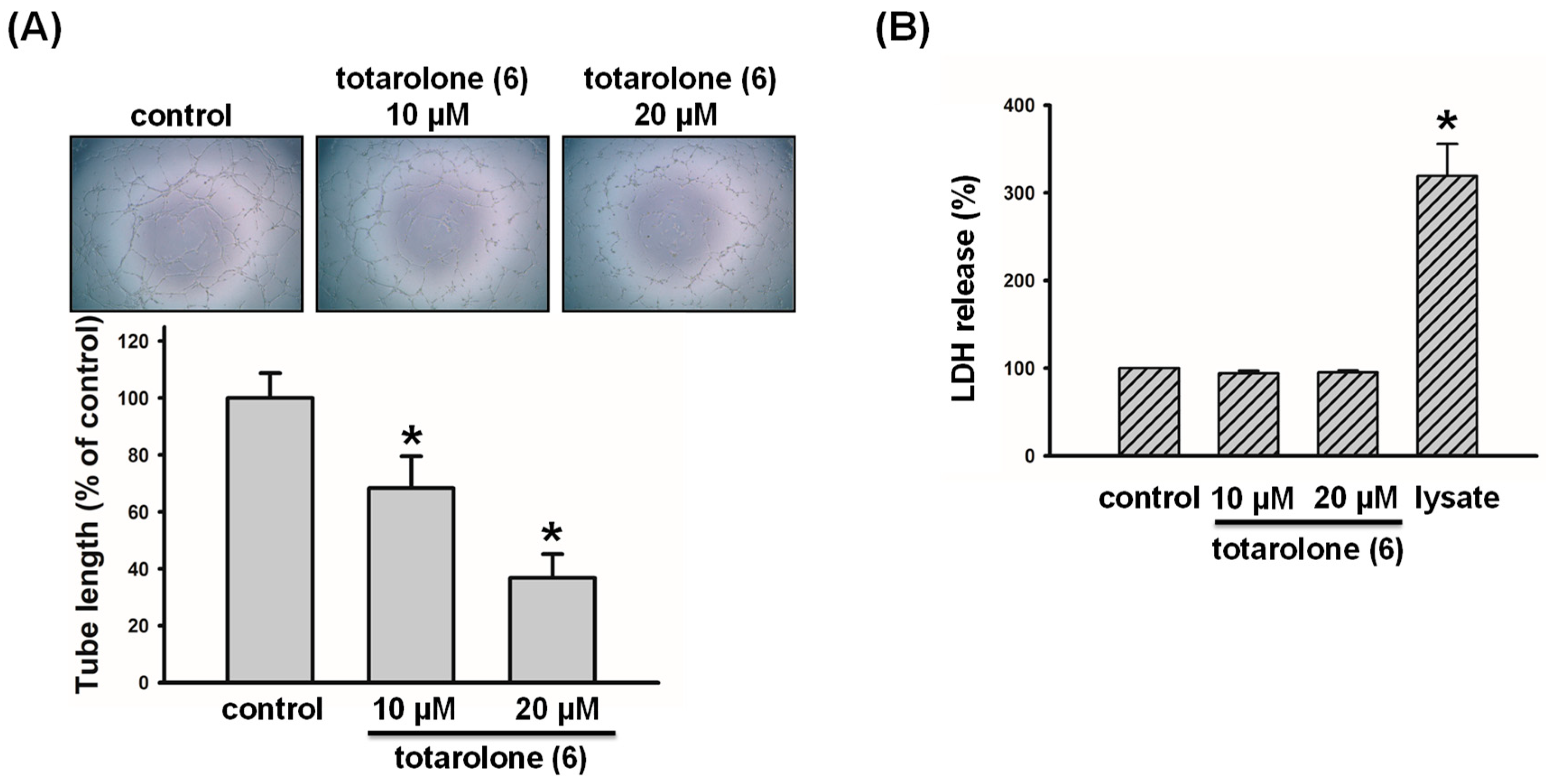
2.2. Anti-Lymphangiogenic Effects of Compounds Isolated from J. chinensis var. tsukusiensis
3. Discussion
4. Material and Methods
4.1. General Experiment Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectroscopic Data of Compounds
4.4.1. 12-Acetoxywiddrol (1)
4.4.2. Cedrol-13-al (2)
4.4.3. α-Corocalen-15-oic Acid (3)
4.4.4. 1,3,5-Bisaoltrien-10-hydroperoxy-11-ol (4)
4.4.5. 1β,2β-Epoxy-9α-hydroxy-8(14),11-totaradiene-3,13-dione (5)
4.5. Anti-Lymphangiogenic Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L. as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; He, F.; Fu, W.; Tang, B.; Bin, Y.; Fang, M.; Wu, Z.; Qiu, Y. Anti-inflammatory sesquiterpenoids from the heartwood of Juniperus formosana Hayata. Fitoterapia 2022, 157, 105105. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Hernández-Galán, R. The antifungal activity of widdrol and its biotransformation by Colletotrichum gloeosporioides (penz.) Penz. & Sacc. and Botrytis cinerea Pers.: Fr. J. Agric. Food Chem. 2006, 54, 7517–7521. [Google Scholar] [CrossRef]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Hernández-Galán, R. Sesquiterpenes from the wood of Juniperus lucayana. Phytochemistry 2007, 68, 2409–2414. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quílez del Moral, J.F.; Lara, A.; Herrador, M.M. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera wood. Planta Med. 2005, 71, 67–71. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Silva, A.M.S. The chemical composition of the Juniperus genus (1970–2004). In Recent Progress in Medicinal Plants; Studium Press LLC: Houston, TX, USA, 2007; Volume 16, pp. 401–522. [Google Scholar] [CrossRef]
- De Marino, S.; Cattaneo, F.; Festa, C.; Zollo, F.; Iaccio, A.; Ammendola, R.; Incollingo, F.; Iorizzi, M. Imbricatolic acid from Juniperus communis L. prevents cell cycle progression in CaLu-6 cells. Planta Med. 2011, 77, 1822–1828. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, J.Y.; Fu, W.H.; Liu, S.Z.; Xie, S.Z.; Wang, Z.; Qiu, Y.K. Two new troponoides with anti-inflammatory activity from the stems of Juniperus formosana Hayata. Nat. Prod. Res. 2021, 35, 4901–4906. [Google Scholar] [CrossRef]
- Al Groshi, A.; Jasim, H.A.; Evans, A.R.; Ismail, F.M.D.; Dempster, N.M.; Nahar, L.; Sarker, S.D. Growth inhibitory activity of biflavonoids and diterpenoids from the leaves of the Libyan Juniperus phoenicea against human cancer cells. Phytother. Res. 2019, 33, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Quílez del Moral, J.F.; Herrador, M.M.; Akssira, M.; Bennamara, A.; Akkad, S.; Aitigri, M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry 2004, 65, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Janar, J.; Nugroho, A.E.; Wong, C.P.; Hirasawa, Y.; Kaneda, T.; Shirota, O.; Morita, H. Sabiperones A-F, new diterpenoids from Juniperus sabina. Chem. Pharm. Bull. 2012, 60, 154–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comte, G.; Allais, D.P.; Chulia, A.J.; Vercauteren, J.; Pinaud, N. Three phenylpropanoids from Juniperus phœnicea. Phytochemistry 1997, 44, 1169–1173. [Google Scholar] [CrossRef]
- Comte, G.; Chulia, A.J.; Vercauteren, J.; Allais, D.P. Phenylpropane glycosides from Juniperus phœnicea. Planta Med. 1996, 62, 88–89. [Google Scholar] [CrossRef]
- Inatomi, Y.; Murata, H.; Inada, A.; Nakanishi, T.; Lang, F.A.; Murata, J.; Iinuma, M. New glycosides of acetophenone derivatives and phenylpropanoids from Juniperus occidentalis. J. Nat. Med. 2013, 67, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ved, A.; Gupta, A.; Rawat, A.K. Antioxidant and hepatoprotective potential of phenol-rich fraction of Juniperus communis Linn. leaves. Pharmacogn. Mag. 2017, 13, 108–113. [Google Scholar]
- Bais, S.; Gill, N.S.; Kumar, N. Neuroprotective effect of Juniperus communis on chlorpromazine induced parkinson disease in animal model. Chin. J. Biol. 2015, 2015, 542542. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupre, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagege, D.; Lamblin, F.; Laine, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12 other Juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Flores-Félix, J.D.; Coutinho, P.; Alves, G.; Silva, L.R. Zimbro (Juniperus communis L.) as a promising source of bioactive compounds and biomedical activities: A review on recent trends. Int. J. Mol. Sci. 2022, 23, 3197. [Google Scholar] [CrossRef]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A phytopharmacological review on a medicinal plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef] [PubMed]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazević, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Cimpeanu, C.; Georgescu, M.I.; Fierascu, R.C.; Ortan, A.; Sutan, A.N.; Anuta, V.; Zanfirescu, A.; et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement. Altern. Med. 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Marei, G.I.K. Antifungal and chemical composition of essential oils of Juniperus communis L. and Thymus vulgaris L. against two phytopathogenic fungi. J. Appl. Sci. Res. 2013, 9, 4584–4588. [Google Scholar]
- Garg, G.P. Screening and evaluation of pharmacognostic, phytochemical and hepatoprotective activity of J. communis L. stems. Int. J. Pharma Bio Sci. 2010, 1, 17–23. [Google Scholar]
- Sánchez de Medina, F.; Gámez, M.J.; Jiménez, I.; Jiménez, J.; Osuna, J.I.; Zarzuelo, A. Hypoglycemic activity of juniper “berries”. Planta Med. 1994, 60, 197–200. [Google Scholar] [CrossRef]
- Bais, S.; Patel, N.J. In vitro anti diabetic and anti obesity effect of J. communis extract on 3T3L1 mouse adipocytes: A possible role of MAPK/ERK activation. Obes. Med. 2020, 18, 100219. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsiao, C.Y.; Lee, S.C.; Huang, X.F.; Chang, K.F.; Lee, M.S.; Hsieh, M.C.; Tsai, N.M. Suppression of oral cancer by induction of cell cycle arrest and apoptosis using Juniperus communis extract. Biosci. Rep. 2020, 40, BSR20202083. [Google Scholar] [CrossRef]
- Li, C.Y.; Lee, S.C.; Lai, W.L.; Chang, K.F.; Huang, X.F.; Hung, P.Y.; Lee, C.P.; Hsieh, M.C.; Tsai, N.M. Cell cycle arrest and apoptosis induction by Juniperus communis extract in esophageal squamous cell carcinoma through activation of p53-induced apoptosis pathway. Food Sci. Nutr. 2021, 9, 1088–1098. [Google Scholar] [CrossRef]
- Sahin Yaglioglu, A.; Eser, F. Screening of some Juniperus extracts for the phenolic compounds and their antiproliferative activities. S. Afr. J. Bot. 2017, 113, 29–33. [Google Scholar] [CrossRef]
- van Slambrouck, S.; Daniels, A.L.; Hooten, C.J.; Brock, S.L.; Jenkins, A.R.; Ogasawara, M.A.; Baker, J.M.; Adkins, G.; Elias, E.M.; Agustin, V.J.; et al. Effects of crude aqueous medicinal plant extracts on growth and invasion of breast cancer cells. Oncol. Rep. 2007, 17, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Huang, R.L.; Huang, X.F.; Chang, K.F.; Lee, C.J.; Hsiao, C.Y.; Lee, S.C.; Tsai, N.M. Evaluation of anticancer effects of Juniperus communis extract on hepatocellular carcinoma cells in vitro and in vivo. Biosci. Rep. 2021, 41, BSR20211143. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Shiu, L.L. Two new sesquiterpenes, 12-hydroxy-α-longipinene (I) and 15- hydroxyacora-4(14),8-diene (II), from the heartwood of Juniperus chinensis Linn. var. tsukusiensis Masam. Chem. Pharm. Bull. 1996, 44, 1758–1760. [Google Scholar] [CrossRef][Green Version]
- Shiu, L.L.; Chen, W.C.; Kuo, Y.H. Five new cis-himachalane-type sesquiterpenes from the heartwood of Juniperus chinensis var. tsukusiensis. Chem. Pharm. Bull. 1999, 47, 557–560. [Google Scholar] [CrossRef][Green Version]
- Chang, F.R.; Wang, S.W.; Chen, S.R.; Lee, C.Y.; Sheu, J.H.; Cheng, Y.B. Aleuritin, a novel dinor-diterpenoid from the twigs of Aleurites moluccanus with an anti-lymphangiogenic effect. Org. Biomol. Chem. 2020, 18, 7892–7898. [Google Scholar] [CrossRef]
- Jeong, D.; Watari, K.; Shirouzu, T.; Ono, M.; Koizumi, K.; Saiki, I.; Kim, Y.C.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Studies on lymphangiogenesis inhibitors from Korean and Japanese crude drugs. Biol. Pharm. Bull. 2013, 36, 152–157. [Google Scholar] [CrossRef]
- Lee, T.H.; Hsieh, C.L.; Wu, H.C.; Wang, S.W.; Yu, C.L.; Hsiao, G.; Cheng, M.J.; Hsieh, W.T.; Kuo, Y.H. Anti-lymphangiogenic diterpenes from the bark of Calocedrus macrolepis var. formosana. J. Food Drug Anal. 2021, 29, 606–621. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Yang, I.C.; Chen, C.S.; Lin, Y.T. Five new sesquiterpenes from the heartwood of Juniperus Squamata Lamb. J. Chin. Chem. Soc. 1987, 34, 125–134. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, W.C. Three new diterpenes, 1,3-dioxototarol, isototarolenone, and 1-oxo-3β-hydroxytotarol, from the roots of Juniperus chinensis Linn. Chem. Pharm. Bull. 1994, 42, 1774–1776. [Google Scholar] [CrossRef][Green Version]
- Lin, T.C.; Fang, J.M.; Cheng, Y.S. Terpenes and lignans from leaves of Chamaecyparis formosensis. Phytochemistry 1999, 51, 793–801. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Ming Tsang, Y. Dehydroabietane diterpenes from Juniperus formosana hay. var. concolor hay. Phytochemistry 1996, 42, 779–781. [Google Scholar] [CrossRef]
- Feliciano, A.S.; López, J.L.; Medarde, M.; del Corral, J.M.M.; de Pascual-Teresa, B.; Puebla, P. Thuriferic acid. A novel lignan type from Juniperus thurifera L. Tetrahedron 1988, 44, 7255–7260. [Google Scholar] [CrossRef]
- Huang, T.; Ying, S.H.; Li, J.Y.; Chen, H.W.; Zang, Y.; Wang, W.X.; Li, J.; Xiong, J.; Hu, J.F. Phytochemical and biological studies on rare and endangered plants endemic to China. Part XV. Structurally diverse diterpenoids and sesquiterpenoids from the vulnerable conifer Pseudotsuga sinensis. Phytochemistry 2020, 169, 112184. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Fang, J.M.; Chen, Y.C.; Wang, B.W.; Cheng, Y.S. Terpenes from heartwood of Juniperus chinensis. Phytochemistry 1996, 41, 1361–1365. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Pu, F.S.; Lin, Y.T. The structure of epicedranediol. J. Chin. Chem. Soc. 1977, 24, 141–142. [Google Scholar] [CrossRef]
- Chetty, G.L.; Dev, S. Mayurone, A C14-sesquiterpene ketone. Tetrahedron Lett. 1965, 6, 3773–3776. [Google Scholar] [CrossRef]
- Cool, L.G.; Jiang, K. Thujopsene- and cis-muurolane-related sesquiterpenoids from Cupressus bakeri. Phytochemistry 1995, 40, 177–181. [Google Scholar] [CrossRef]
- Nagahama, S.; Tazaki, M. Terpenoids. IX. Permanganate oxidation of Thujopsene. neutral products. Bull. Chem. Soc. Jpn. 1987, 60, 4175–4177. [Google Scholar] [CrossRef]
- Schneider, I.; Gibbons, S.; Bucar, F. Inhibitory activity of Juniperus communis on 12(S)-HETE production in human platelets. Planta Med. 2004, 70, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Itô, S.; Endo, K.; Honma, H.; Ota, K. New constituents of Thujopsis dolabrata. Tetrahedron Lett. 1965, 6, 3777–3781. [Google Scholar] [CrossRef]
- Tai, H.C.; Lee, T.H.; Tang, C.H.; Chen, L.P.; Chen, W.C.; Lee, M.S.; Chen, P.C.; Lin, C.Y.; Chi, C.W.; Chen, Y.J.; et al. Phomaketide A inhibits lymphangiogenesis in human lymphatic endothelial cells. Mar. Drugs 2019, 17, 215. [Google Scholar] [CrossRef] [PubMed]
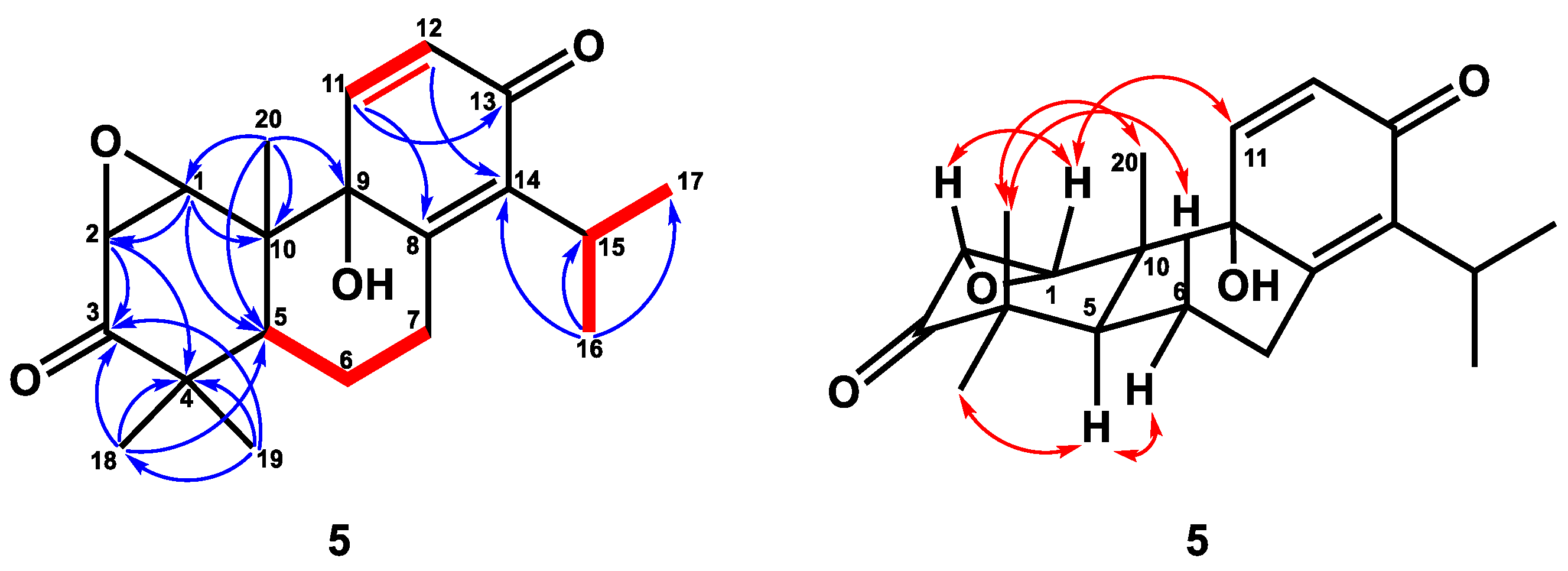
| Position | 1 a | 2 a | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| δH (mult, J in Hz) | δH, mult (J in Hz) | δH, mult (J in Hz) | δH, mult (J in Hz) | δH, mult (J in Hz) | |
| 1 | 8.03 (s) | 7.05 (d, 7.8) | 3.64 (d, 4.6) | ||
| 2 | 1.23 (m) 1.49 (m) | 1.62 (m) | 7.06 (d, 7.8) | 3.38 (d, 4.6) | |
| 3 | 1.48 (m) 1.72 (m) | 1.23 (m) 1.88 (m) | 2.55 (t, 8.0) | ||
| 4 | 1.32 (m) 1.79 (m) | 1.24 (m) 1.80 (m) | 2.79 (t, 8.0) | 7.06 (d, 7.8) | |
| 5 | 1.9 (t, 7.6) (β) | 7.05 (d, 7.8) | |||
| 6 | 7.07 (d, 8.0) | 1.38 (dd, 13.3, 4.0) (β) 1.81 (m) (α) | |||
| 7 | 5.41 (dd, 8.8, 6.0) | 2.26 (d, 4.4) | 7.12 (d, 8.0) | 2.63 (sex, 7.2) | 2.58 (m) (β) 2.94 (m) (α) |
| 8 | 2.06 (dd, 14.0, 8.8) (β) 2.46 (dd, 14.0, 6.0) (α) | 1.58 (m) 1.84 (m) | |||
| 9 | 1.71 (dt, 5.6, 1.6) (β) 1.82 (m) (α) | 1.28 (m) 1.32 (m) | |||
| 10 | 1.33 (m) 1.62 (m) | 1.35 (m) (β) 1.44 (m) (α) | 3.26 (dd, 10.0, 2.4) | ||
| 11 | 1.24 (m) 1.41 (m) | 1.42 (m) 1.76 (m) | 3.33 (sept, 6.8) | 7.01 (d, 10.0) | |
| 12 | 3.99 (s) | 1.31 (s) | 1.23 (d, 6.8) | 1.08 (s) | 6.28 (d, 10.0) |
| 13 | 1.07 (s) | 9.42 (s) | 1.23 (d, 6.8) | 1.11 (s) | |
| 14 | 1.04 (s) | 1.41 (s) | 2.26 (s) | 1.21 (d, 7.2) | |
| 15 | 1.20 (s) | 0.84 (d, 7.2) | 2.29 (s) | 3.22 (sept, 7.2) | |
| 16 | 1.23 (d, 7.2) | ||||
| 17 | 2.10 (s) | 1.21 (d, 7.2) | |||
| 18 | 1.20 (s) | ||||
| 19 | 0.98 (s) | ||||
| 20 | 0.71 (s) |
| Position | 1 a | 2 a | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| δC | δC | δC | δC | δC | |
| 1 | 39.8 | 53.7 | 135.3 | 126.8 | 60.9 |
| 2 | 41.4 | 41.0 | 127.7 | 129.1 | 54.5 |
| 3 | 18.6 | 37.8 | 21.0 | 135.3 | 211.0 |
| 4 | 38.1 | 25.9 | 24.6 | 129.1 | 44.8 |
| 5 | 36.9 | 58.0 | 132.6 | 126.8 | 38.6 |
| 6 | 155.2 | 57.6 | 122.9 | 144.4 | 24.0 |
| 7 | 116.3 | 53.3 | 132.0 | 39.7 | 26.6 |
| 8 | 34.4 | 73.8 | 144.6 | 35.5 | 153.7 |
| 9 | 73.8 | 34.6 | 129.1 | 29.9 | 74.2 |
| 10 | 32.7 | 42.6 | 136.0 | 78.8 | 46.7 |
| 11 | 40.5 | 30.8 | 28.5 | 73.1 | 144.9 |
| 12 | 70.2 | 30.2 | 23.8 | 23.1 | 131.3 |
| 13 | 31.7 | 205.6 | 23.8 | 26.5 | 184.6 |
| 14 | 32.9 | 19.3 | 19.4 | 22.4 | 140.0 |
| 15 | 27.5 | 15.4 | 172.3 | 20.9 | 26.6 |
| 16 | 171.3 | 21.5 | |||
| 17 | 20.9 | 21.4 | |||
| 18 | 28.4 | ||||
| 19 | 20.8 | ||||
| 20 | 13.9 |
| Compound | IC50 (µM) |
|---|---|
| totarolone (6) | 6 ± 1 |
| 3β-hydroxytotarol (7) | 29 ± 1 |
| 7-oxototarol (8) | 28 ± 1 |
| 1-oxo-3β-hydroxytotarol (9) | 45 ± 2 |
| Rapamycin # | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-C.; Shiu, L.-L.; Wang, S.-W.; Huang, C.-Y.; Lee, T.-H.; Sung, P.-J.; Kuo, Y.-H. Anti-Lymphangiogenic Terpenoids from the Heartwood of Taiwan Juniper, Juniperus chinensis var. tsukusiensis. Plants 2023, 12, 3828. https://doi.org/10.3390/plants12223828
Wu H-C, Shiu L-L, Wang S-W, Huang C-Y, Lee T-H, Sung P-J, Kuo Y-H. Anti-Lymphangiogenic Terpenoids from the Heartwood of Taiwan Juniper, Juniperus chinensis var. tsukusiensis. Plants. 2023; 12(22):3828. https://doi.org/10.3390/plants12223828
Chicago/Turabian StyleWu, Ho-Cheng, Lung-Lin Shiu, Shih-Wei Wang, Chia-Ying Huang, Tzong-Huei Lee, Ping-Jyun Sung, and Yueh-Hsiung Kuo. 2023. "Anti-Lymphangiogenic Terpenoids from the Heartwood of Taiwan Juniper, Juniperus chinensis var. tsukusiensis" Plants 12, no. 22: 3828. https://doi.org/10.3390/plants12223828
APA StyleWu, H.-C., Shiu, L.-L., Wang, S.-W., Huang, C.-Y., Lee, T.-H., Sung, P.-J., & Kuo, Y.-H. (2023). Anti-Lymphangiogenic Terpenoids from the Heartwood of Taiwan Juniper, Juniperus chinensis var. tsukusiensis. Plants, 12(22), 3828. https://doi.org/10.3390/plants12223828








