Effects of Exogenous Ethanol Treatment in Nutrient Solution on Growth and Secondary Metabolite Contents of Three Herb Species in an Indoor Vertical Farming System
Abstract
:1. Introduction
2. Results
2.1. The Growth Parameter of Three Plants at Different Ethanol Concentrations
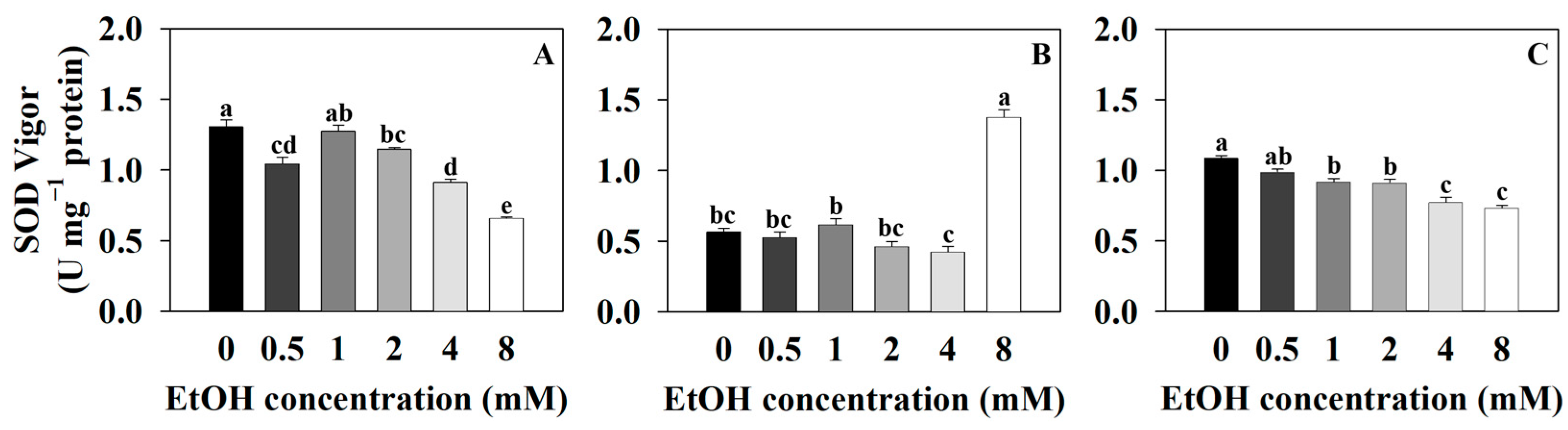
2.2. Antioxidant Capacity Measurements of Three Plants at Different Ethanol Concentrations
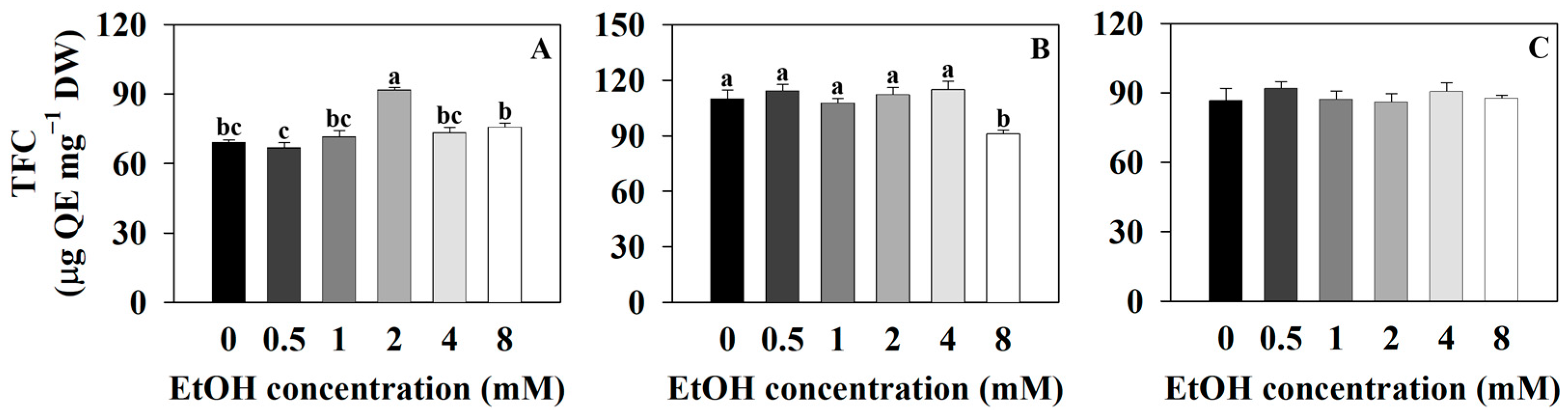
2.3. The Secondary Metabolite Contents of Three Plants at Different Ethanol Concentrations
3. Discussion
3.1. Ethanol Can Either Stimulate or Inhibit Cellular Respiration
3.2. High-Concentration Ethanol Treatment in Plants Induces Oxidative Stress
3.3. Effect of Exogenous Ethanol Treatment in Plants on Secondary Metabolite Contents
4. Materials and Methods
4.1. Plant Materials and Environmental Conditions of the Seedlings
4.2. Environment Conditions and Ethanol Treatments after Transplantation
4.3. Measurements of Plant Growth Parameters
4.4. Preparation of the Extract
4.5. Extraction of Enzymes
4.6. Chlorophyll Pigments and Total Carotenoids
4.7. Total Phenolic Contents
4.8. Total Flavonoid Contents
4.9. DPPH Radical Scavenging Assay
4.10. Total Soluble Protein
4.11. SOD Activity (EC 1.15.1.1)
4.12. POD Activity (EC 1.11.1.7)
4.13. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10. [Google Scholar] [CrossRef]
- Duarte, M.; Duarte, R.; Rodrigues, R.; Rodrigues, M. Essential oils and their characteristics. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 1–19. [Google Scholar] [CrossRef]
- Ahmad, A.; Elisha, I.L.; van Vuuren, S.; Viljoen, A. Volatile phenolics: A comprehensive review of the anti-infective properties of an important class of essential oil constituents. Phytochemistry 2021, 190, 112864. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Speight, J.G. Biomass Processes and Chemicals; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Schlüter, U.; Crawford, R.M. Long-term anoxia tolerance in leaves of Acorus calamus L. and Iris pseudacorus L. J. Exp. Bot. 2001, 52, 2213–2225. [Google Scholar] [CrossRef]
- Tesniere, C.; Torregrosa, L.; Pradal, M.; Souquet, J.-M.; Gilles, C.; Dos Santos, K.; Chatelet, P.; Gunata, Z. Effects of genetic manipulation of alcohol dehydrogenase levels on the response to stress and the synthesis of secondary metabolites in grapevine leaves. J. Exp. Bot. 2006, 57, 91–99. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Diab, A.A.; Kantety, R.; Ozturk, N.; Benscher, D.; Nachit, M.; Sorrells, M. Drought-inducible genes and differentially expressed sequence tags associated with components of drought tolerance in durum wheat. Sci. Res. Essays 2008, 3, 9–26. [Google Scholar]
- Lindlöf, A.; Bräutigam, M.; Chawade, A.; Olsson, B.; Olsson, O. Identification of cold-induced genes in cereal crops and Arabidopsis through comparative analysis of multiple EST sets. In Proceedings of the Bioinformatics Research and Development: First International Conference, BIRD 2007, Berlin, Germany, 12–14 March 2007; pp. 48–65. [Google Scholar]
- Yi, S.Y.; Ku, S.S.; Sim, H.-J.; Kim, S.-K.; Park, J.H.; Lyu, J.I.; So, E.J.; Choi, S.Y.; Kim, J.; Ahn, M.S. An alcohol dehydrogenase gene from Synechocystis sp. confers salt tolerance in transgenic tobacco. Front. Plant Sci. 2017, 8, 1965. [Google Scholar] [CrossRef]
- Das, A.K.; Anik, T.R.; Rahman, M.M.; Keya, S.S.; Islam, M.R.; Rahman, M.A.; Sultana, S.; Ghosh, P.K.; Khan, S.; Ahamed, T. Ethanol treatment enhances physiological and biochemical responses to mitigate saline toxicity in soybean. Plants 2022, 11, 272. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Sako, K.; Matsui, A.; Suzuki, Y.; Mostofa, M.G.; Ha, C.V.; Tanaka, M.; Tran, L.-S.P.; Habu, Y.; Seki, M. Ethanol enhances high-salinity stress tolerance by detoxifying reactive oxygen species in Arabidopsis thaliana and rice. Front. Plant Sci. 2017, 8, 1001. [Google Scholar] [CrossRef]
- Tadege, M.; Kuhlemeier, C. Aerobic fermentation during tobacco pollen development. Plant Mol. Biol. 1997, 35, 343–354. [Google Scholar] [CrossRef]
- Bucher, M.; Brander, K.A.; Sbicego, S.; Mandel, T.; Kuhlemeier, C. Aerobic fermentation in tobacco pollen. Plant Mol. Biol. 1995, 28, 739–750. [Google Scholar] [CrossRef]
- Kar, N.; Gupta, D.; Bellare, J. Ethanol affects fibroblast behavior differentially at low and high doses: A comprehensive, dose-response evaluation. Toxicol. Rep. 2021, 8, 1054–1066. [Google Scholar] [CrossRef]
- Raimundo, N.; Baysal, B.E.; Shadel, G.S. Revisiting the TCA cycle: Signaling to tumor formation. Trends Mol. Med. 2011, 17, 641–649. [Google Scholar] [CrossRef]
- Siedow, J.N.; Umbach, A.L. Plant mitochondrial electron transfer and molecular biology. Plant Cell 1995, 7, 821. [Google Scholar] [CrossRef]
- Schertl, P.; Braun, H.-P. Respiratory electron transfer pathways in plant mitochondria. Front. Plant Sci. 2014, 5, 163. [Google Scholar] [CrossRef]
- Babcock, G.T.; Wikström, M. Oxygen activation and the conservation of energy in cell respiration. Nature 1992, 356, 301–309. [Google Scholar] [CrossRef]
- Estornell, E.; Fato, R.; Pallotti, F.; Lenaz, G. Assay conditions for the mitochondrial NADH: Coenzyme Q oxidoreductase. FEBS Lett. 1993, 332, 127–131. [Google Scholar] [CrossRef]
- Geddes, A.; Paul, C.E.; Hay, S.; Hollmann, F.; Scrutton, N.S. Donor–acceptor distance sampling enhances the performance of “better than nature” nicotinamide coenzyme biomimetics. J. Am. Chem. Soc. 2016, 138, 11089–11092. [Google Scholar] [CrossRef]
- Seelert, H.; Dencher, N.A. ATP synthase superassemblies in animals and plants: Two or more are better. Biochim. Et Biophys. Acta BBA-Bioenerg. 2011, 1807, 1185–1197. [Google Scholar] [CrossRef]
- Boubekeur, S.; Bunoust, O.; Camougrand, N.; Castroviejo, M.; Rigoulet, M.; Guérin, B. A Mitochondrial Pyruvate Dehydrogenase Bypass in the YeastSaccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 21044–21048. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, M.; Oliver, D.J.; Schnable, P.S. The roles of aldehyde dehydrogenases (ALDHs) in the PDH bypass of Arabidopsis. BMC Biochem. 2009, 10, 7. [Google Scholar] [CrossRef]
- Lin, M.; Oliver, D.J. The role of acetyl-coenzyme a synthetase in Arabidopsis. Plant Physiol. 2008, 147, 1822–1829. [Google Scholar] [CrossRef]
- Mellema, S.; Eichenberger, W.; Rawyler, A.; Suter, M.; Tadege, M.; Kuhlemeier, C. The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. Plant J. 2002, 30, 329–336. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Liu, H.; Arbing, M.A.; Bowie, J.U. Expanding the use of ethanol as a feedstock for cell-free synthetic biochemistry by implementing acetyl-CoA and ATP generating pathways. Sci. Rep. 2022, 12, 7700. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Rowe, R.; Farr, D.; Richards, B. Effects of foliar and root applications of methanol or ethanol on the growth of tomato plants (Lycopersicon esculentum Mill). N. Z. J. Crop Hortic. Sci. 1994, 22, 335–337. [Google Scholar] [CrossRef]
- Yavarpanah, Z.; Alizadeh, M.; Seifi, E. Effects of foliar and root applications of hydro-alcoholic solutions on physiological and biochemical attributes and fruit yield and weight of strawberry. J. Plant Physiol. Breed. 2015, 5, 47–54. [Google Scholar]
- Kumar, P.; Dubey, K.K. Citric acid cycle regulation: Back bone for secondary metabolite production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–181. [Google Scholar]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; de la Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxidative Med. Cell. Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Chang, X.; Gou, W.; Zhou, X.; Liu, Z.; Li, Z.; Wu, Y.; Zuo, D. Acetaldehyde induces neurotoxicity in vitro via oxidative stress-and Ca 2+ imbalance-mediated endoplasmic reticulum stress. Oxidative Med. Cell. Longev. 2019, 2019, 2593742. [Google Scholar] [CrossRef]
- Yan, T.; Zhao, Y. Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca2+ levels. Redox Biol. 2020, 28, 101381. [Google Scholar] [CrossRef]
- de Moura Ferreira, M.A.; da Silveira, F.A.; da Silveira, W.B. Ethanol stress responses in Kluyveromyces marxianus: Current knowledge and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1341–1353. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide anion chemistry—Its role at the core of the innate immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Vianello, A.; Macri, F. Generation of superoxide anion and hydrogen peroxide at the surface of plant cells. J. Bioenerg. Biomembr. 1991, 23, 409–423. [Google Scholar] [CrossRef]
- Maurya, R.; Namdeo, M. Superoxide dismutase: A key enzyme for the survival of intracellular pathogens in host. In Reactive Oxygen Species; Ahmad, R., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.; Heazlewood, J.; Herald, V.; Holtzapffel, R.; Day, D.; Leaver, C.; Millar, A. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002, 32, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Li, S.-H. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 2006, 48, 137–144. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Deeba, F.; Pandey, V. Effects of reactive oxygen species on crop productivity: An overview. In Reactive Oxygen Species in Plants: Boon or Bane—Revisiting the Role of ROS; Wiley: Hoboken, NJ, USA, 2017; pp. 117–136. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Zhang, D.; Li, C.; Li, M.; Ye, J.; Zhang, Y.; Wang, T.; Ouyang, B.; Hong, Z. Tomato methionine sulfoxide reductase B2 functions in drought tolerance by promoting ROS scavenging and chlorophyll accumulation through interaction with Catalase 2 and RBCS3B. Plant Sci. 2022, 318, 111206. [Google Scholar] [CrossRef]
- Wan, W.-L.; Tian, B.; Lin, Y.-J.; Korupalli, C.; Lu, M.-Y.; Cui, Q.; Wan, D.; Chang, Y.; Sung, H.-W. Photosynthesis-inspired H2 generation using a chlorophyll-loaded liposomal nanoplatform to detect and scavenge excess ROS. Nat. Commun. 2020, 11, 534. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Springer: Cham, Switzerland, 2016; pp. 111–139. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Bashir, K.; Todaka, D.; Rasheed, S.; Matsui, A.; Ahmad, Z.; Sako, K.; Utsumi, Y.; Vu, A.T.; Tanaka, M.; Takahashi, S. Ethanol-mediated novel survival strategy against drought stress in plants. Plant Cell Physiol. 2022, 63, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Deng, L.; Ruan, C.; Yi, L.; Zeng, K. Pichia galeiformis induces resistance in postharvest citrus by activating the phenylpropanoid biosynthesis pathway. J. Agric. Food Chem. 2021, 69, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Muro-Villanueva, F.; Mao, X.; Chapple, C. Linking phenylpropanoid metabolism, lignin deposition, and plant growth inhibition. Curr. Opin. Biotechnol. 2019, 56, 202–208. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Dar, F.A.; Tahir, I.; Hakeem, K.R.; Rehman, R.U. Silicon application enhances the photosynthetic pigments and phenolic/flavonoid content by modulating the phenylpropanoid pathway in common buckwheat under aluminium stress. Silicon 2022, 14, 323–334. [Google Scholar] [CrossRef]
- Rezaie, R.; Abdollahi Mandoulakani, B.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 5290. [Google Scholar] [CrossRef]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 8. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P.R. A review on macroscale and microscale cell lysis methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Severo, J.; Tiecher, A.; Chaves, F.C.; Silva, J.A.; Rombaldi, C.V. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chem. 2011, 126, 995–1000. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]

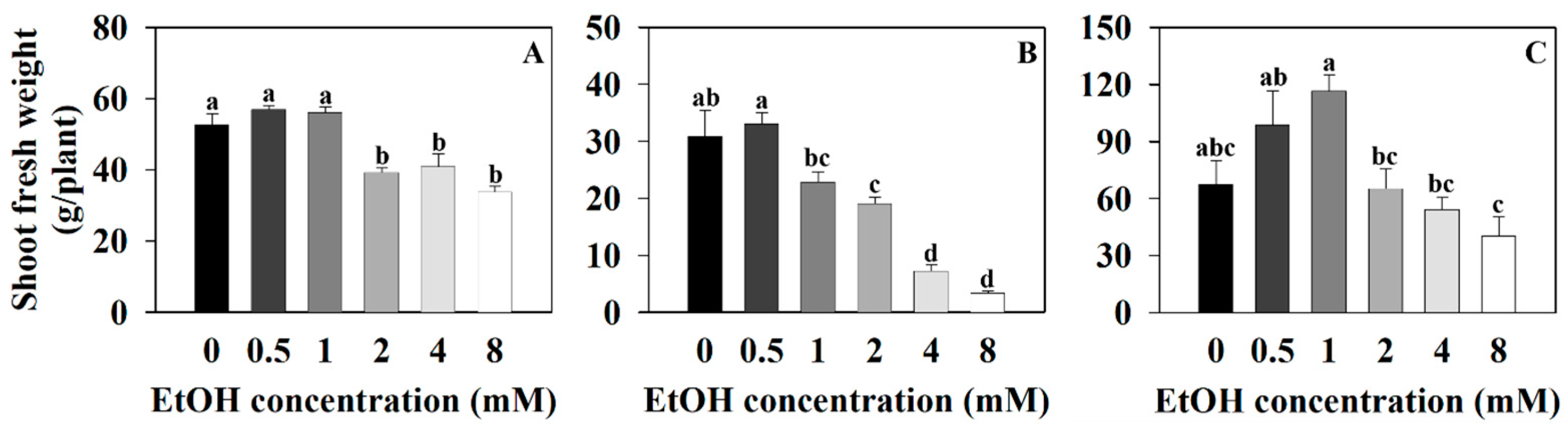
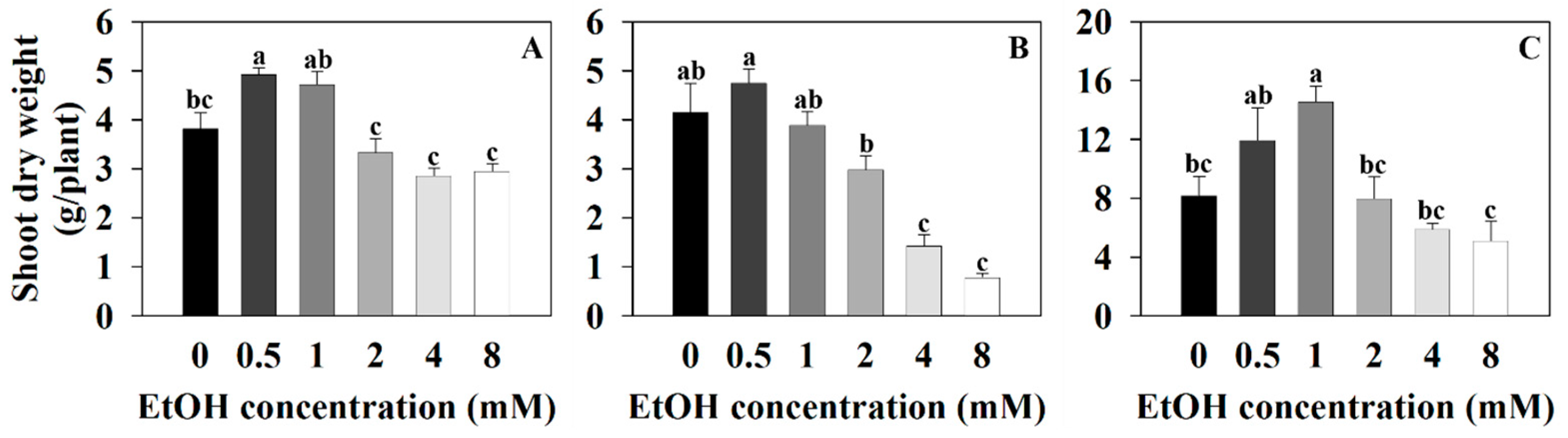
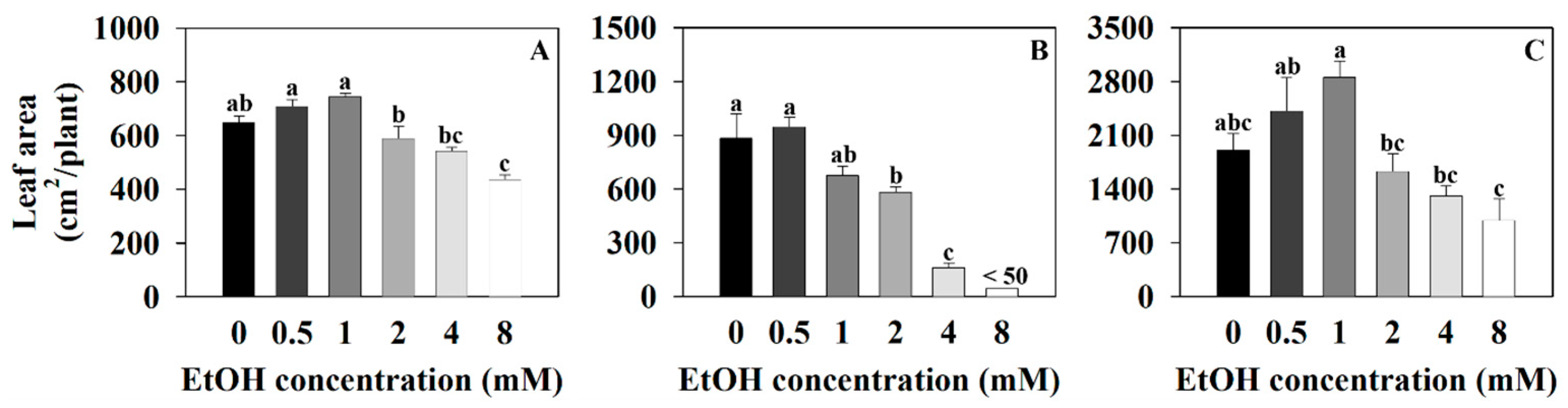
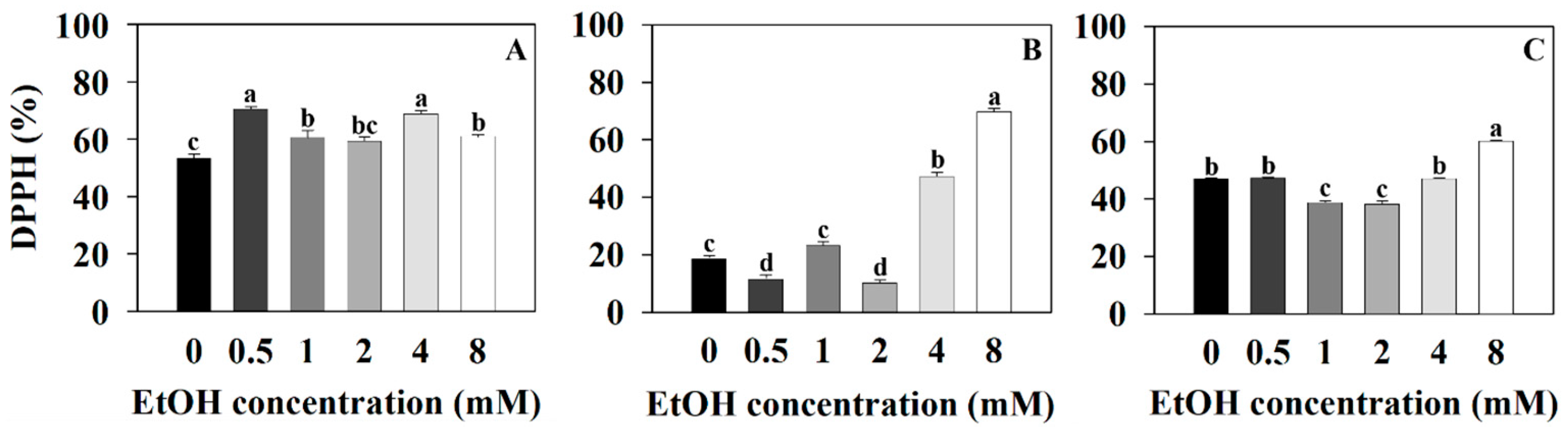


| Plant Name | Treatment (mM) | Photosynthetic Pigments (μg·mg−1 DW) | |||
|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Chlorophyll a + b | Carotenoids | ||
| Sweet basil | 0 | 5.12 0.16 a | 2.81 0.08 b | 7.93 0.24 a | 0.46 0.02 ab |
| 0.5 | 4.39 0.10 b | 2.48 0.04 c | 6.87 0.14 b | 0.38 0.01 b | |
| 1 | 4.76 0.12 ab | 2.75 0.04 b | 7.51 0.15 ab | 0.41 0.02 ab | |
| 2 | 5.12 0.13 a | 3.14 0.05 a | 8.27 0.18 a | 0.38 0.02 b | |
| 4 | 4.88 0.07 ab | 2.68 0.04 bc | 7.56 0.10 ab | 0.47 0.01 a | |
| 8 | 4.99 0.14 a | 2.70 0.05 bc | 7.68 0.20 ab | 0.49 0.02 a | |
| Korean mint | 0 | 4.68 0.10 a | 2.36 0.03 a | 7.03 0.13 a | 0.65 0.01 a |
| 0.5 | 4.34 0.04 b | 2.19 0.01 b | 6.53 0.05 b | 0.60 0.01 ab | |
| 1 | 3.49 0.09 c | 1.90 0.03 c | 5.39 0.12 c | 0.47 0.02 c | |
| 2 | 4.24 0.06 b | 2.17 0.03 b | 6.41 0.09 b | 0.58 0.01 b | |
| 4 | 2.86 0.00 d | 1.44 0.01 d | 4.30 0.01 d | 0.43 0.00 c | |
| 8 | 2.08 0.06 e | 1.21 0.02 e | 3.29 0.06 e | 0.28 0.01 d | |
| Sweet wormwood | 0 | 4.58 0.08 a | 2.77 0.04 b | 7.36 0.12 b | 0.55 0.01 ab |
| 0.5 | 5.00 0.18 a | 2.87 0.07 b | 7.86 0.25 ab | 0.60 0.03 a | |
| 1 | 4.93 0.16 a | 2.89 0.05 ab | 7.83 0.21 ab | 0.56 0.01 ab | |
| 2 | 5.11 0.03 a | 3.07 0.01 a | 8.19 0.04 a | 0.65 0.01 a | |
| 4 | 5.13 0.18 a | 2.92 0.04 ab | 8.05 0.22 ab | 0.64 0.03 a | |
| 8 | 3.30 0.07 b | 1.84 0.03 c | 5.14 0.10 c | 0.48 0.01 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Lee, Y.; Hahm, S.; Lee, K.; Park, J. Effects of Exogenous Ethanol Treatment in Nutrient Solution on Growth and Secondary Metabolite Contents of Three Herb Species in an Indoor Vertical Farming System. Plants 2023, 12, 3842. https://doi.org/10.3390/plants12223842
Shin J, Lee Y, Hahm S, Lee K, Park J. Effects of Exogenous Ethanol Treatment in Nutrient Solution on Growth and Secondary Metabolite Contents of Three Herb Species in an Indoor Vertical Farming System. Plants. 2023; 12(22):3842. https://doi.org/10.3390/plants12223842
Chicago/Turabian StyleShin, Juhyung, YongJae Lee, Seungyong Hahm, Kwangya Lee, and Jongseok Park. 2023. "Effects of Exogenous Ethanol Treatment in Nutrient Solution on Growth and Secondary Metabolite Contents of Three Herb Species in an Indoor Vertical Farming System" Plants 12, no. 22: 3842. https://doi.org/10.3390/plants12223842
APA StyleShin, J., Lee, Y., Hahm, S., Lee, K., & Park, J. (2023). Effects of Exogenous Ethanol Treatment in Nutrient Solution on Growth and Secondary Metabolite Contents of Three Herb Species in an Indoor Vertical Farming System. Plants, 12(22), 3842. https://doi.org/10.3390/plants12223842






