Arabidopsis MYB21 Negatively Regulates KTN1 to Fine-Tune the Filament Elongation
Abstract
:1. Introduction
2. Results
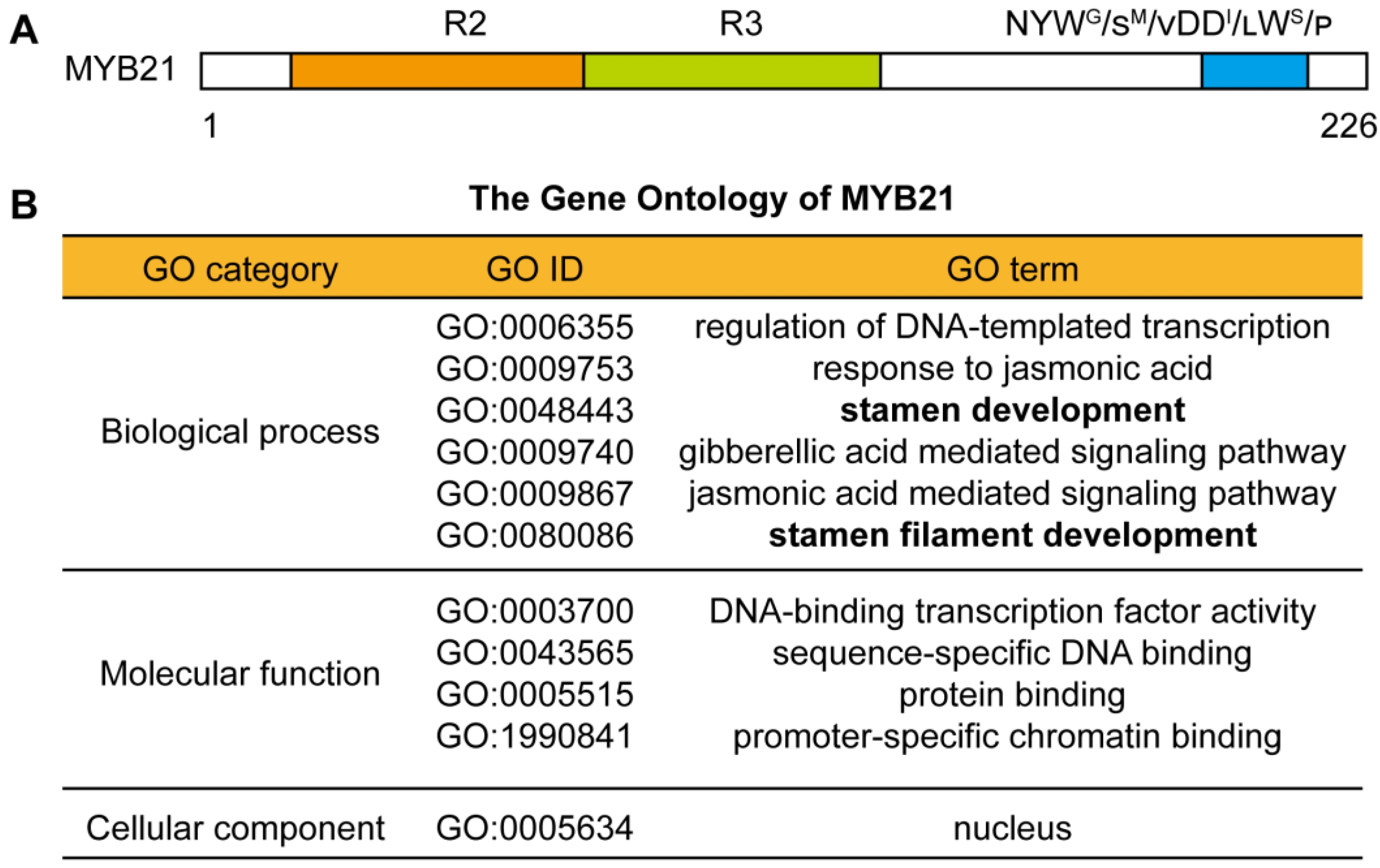
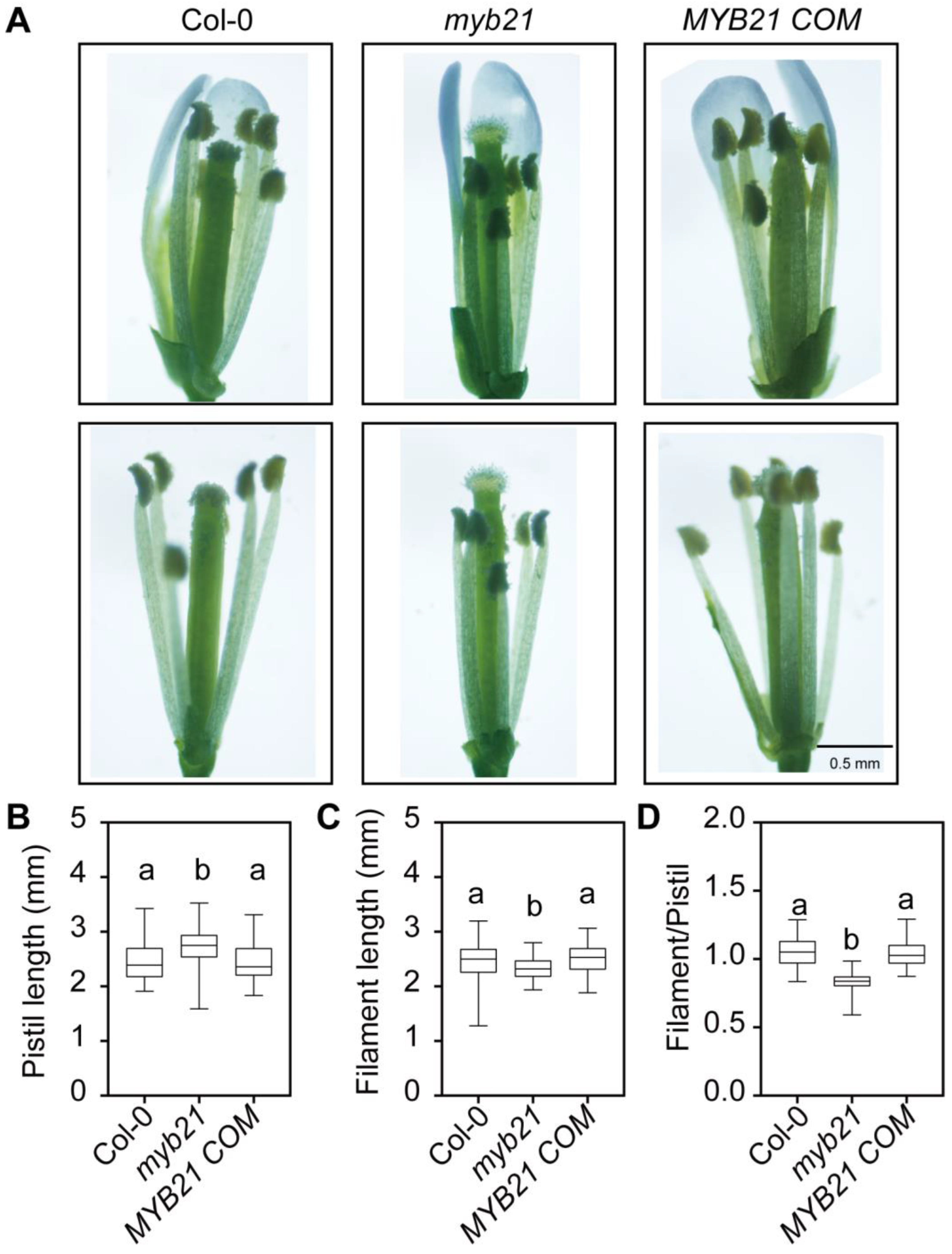
2.1. MYB21 Positively Regulates the Stamen Filament Elongation in Arabidopsis
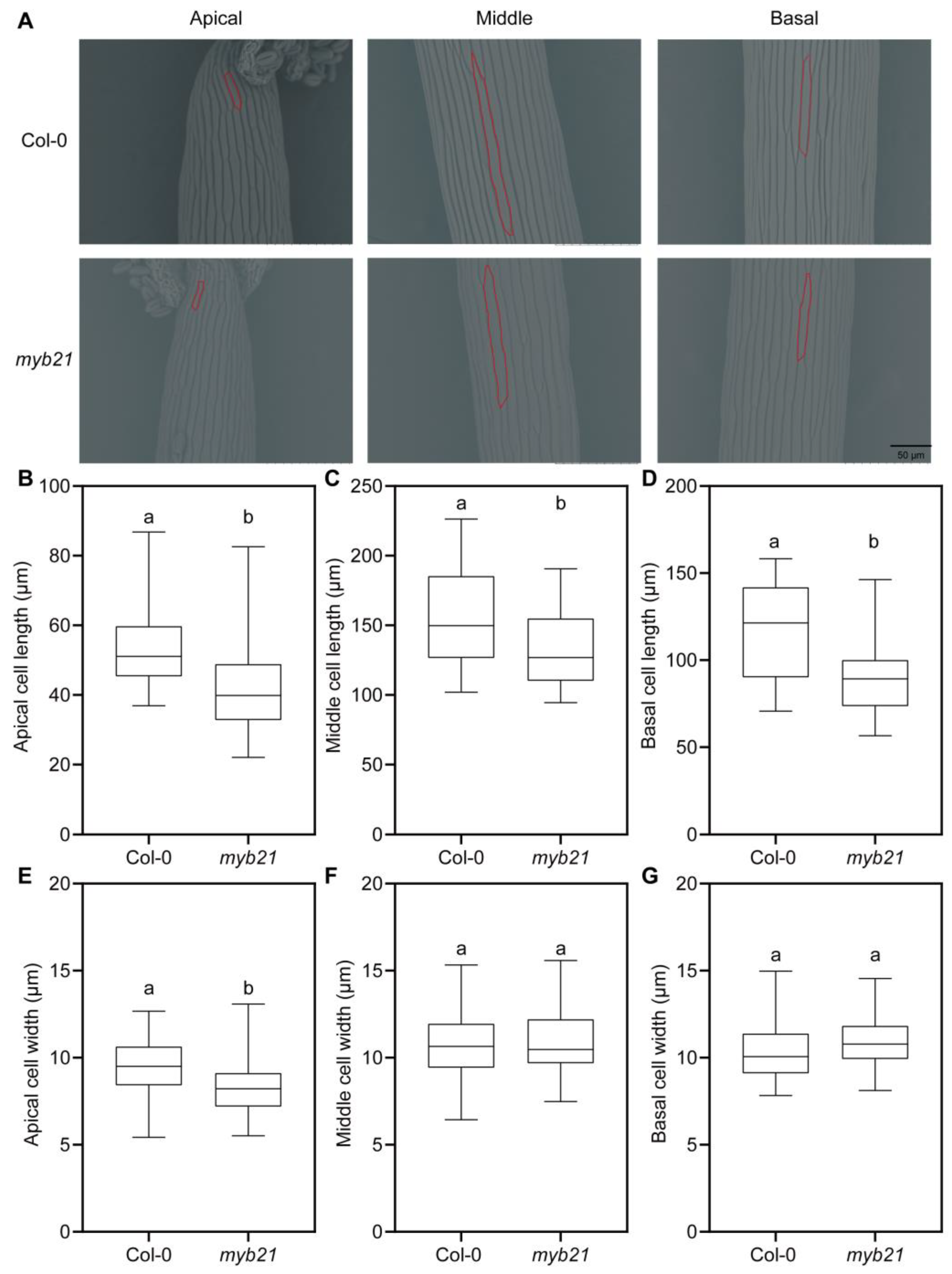
2.2. MYB21 Positively Regulates Filament Cell Elongation in Arabidopsis
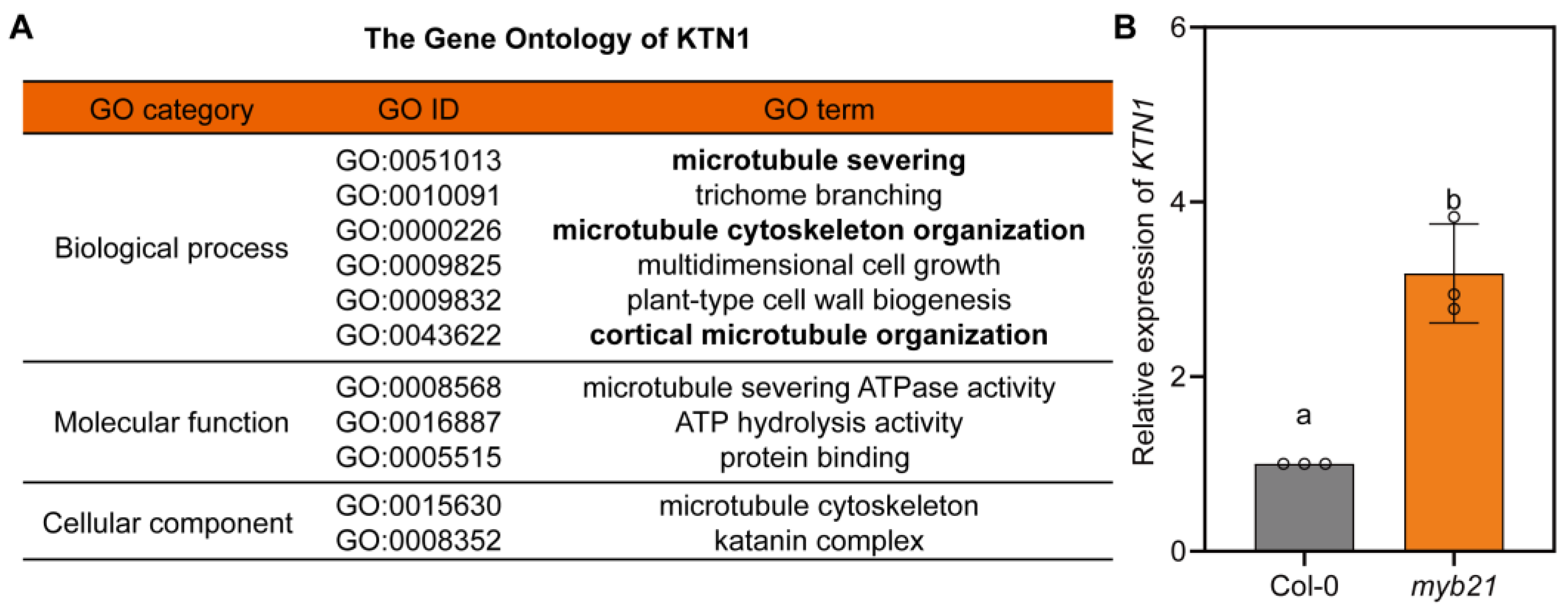
2.3. MYB21 Negatively Regulates the Expression of KTN1
2.4. MYB21 May Directly Bind to the Promoter of KTN1
2.5. MYB21 Represses the Activity of the KTN1 Promoter
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Plasmid Construction and Generation of Transgenic Arabidopsis Plants
4.3. Yeast-One-Hybrid (Y1H) Assay
4.4. Reverse-Transcription Quantitative PCR (RT-qPCR) Analysis
4.5. Light Microscopy and Scanning Electron Microscopy
4.6. Transient Expression Assays in Nicotiana benthamiana
4.7. Bioinformatic Analysis
4.8. Alexander Staining Assay
4.9. Resin-Embedded Filament Cross-Section Assay
4.10. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.-C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant Cell 2004, 16, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Przybyl, M. Jasmonate signaling during Arabidopsis stamen maturation. Plant Cell Physiol. 2019, 60, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; Cecchetti, V. Auxin polar transport in stamen formation and development: How many actors? Front. Plant Sci. 2014, 5, 333. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Cheng, H.; Song, S.; Xiao, L.; Soo, H.M.; Cheng, Z.; Xie, D.; Peng, J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 2009, 5, e1000440. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Huang, H.; Ren, Q.; Wu, D.; Chang, C.; Peng, W.; Liu, Y.; Peng, J.; Xie, D. The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell. 2011, 23, 1000–1013. [Google Scholar] [CrossRef]
- Huang, H.; Gao, H.; Liu, B.; Qi, T.; Tong, J.; Xiao, L.; Xie, D.; Song, S. Arabidopsis MYB24 regulates jasmonate-mediated stamen development. Front. Plant Sci. 2017, 8, 1525. [Google Scholar] [CrossRef]
- Mandaokar, A.; Thines, B.; Shin, B.; Lange, B.M.; Choi, G.; Koo, Y.J.; Yoo, Y.J.; Choi, Y.D.; Choi, G.; Browse, J. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006, 46, 984–1008. [Google Scholar] [CrossRef]
- Huang, H.; Gong, Y.; Liu, B.; Wu, D.; Zhang, M.; Xie, D.; Song, S. The DELLA proteins interact with MYB21 and MYB24 to regulate filament elongation in Arabidopsis. BMC Plant Biol. 2020, 20, 64. [Google Scholar] [CrossRef]
- Marciniak, K.; Przedniczek, K. Comprehensive insight into gibberellin- and jasmonate-mediated stamen development. Genes 2019, 10, 811. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Liu, W.; Yang, H.; Wang, C.; Chen, W.; Zhang, X.; Tian, J.; Yu, Y.; Li, J.; et al. Brassinosteroid signals cooperate with katanin-mediated microtubule severing to control stamen filament elongation. EMBO J. 2023, 42, e111883. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Wang, L.; Huang, S.; Ali, K.; Li, G.; Ren, H.; Zheng, B. The receptor-like kinase EMS1 and BRI1 coordinately regulate stamen elongation via the transcription factors BES1/BZR1 in Arabidopsis. Plant Sci. 2023, 331, 111673. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, J.; Xia, Y.; Zhang, L.; Du, H. Evolution and functional diversification of R2R3-MYB transcription factors in plants. Hortic. Res. 2022, 9, uhac058. [Google Scholar] [CrossRef]
- Haga, N.; Kato, K.; Murase, M.; Araki, S.; Kubo, M.; Demura, T.; Suzuki, K.; Muller, I.; Voss, U.; Jurgens, G.; et al. R1R2R3-Myb proteins positively regulate cytokinesis through activation of KNOLLE transcription in Arabidopsis thaliana. Development 2007, 134, 1101–1110. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Q.; Zeng, D.; Xu, J.; Zhou, H.; Wang, F.; Ma, N.; Li, Y. RhMYB108, an R2R3-MYB transcription factor, is involved in ethylene- and JA-induced petal senescence in rose plants. Hortic. Res. 2019, 6, 131. [Google Scholar] [CrossRef]
- Li, C.; Shi, L.; Li, X.; Wang, Y.; Bi, Y.; Li, W.; Ma, H.; Chen, B.; Zhu, L.; Fu, Y. ECAP is a key negative regulator mediating different pathways to modulate salt stress-induced anthocyanin biosynthesis in Arabidopsis. New Phytol. 2022, 233, 2216–2231. [Google Scholar] [CrossRef]
- Schubert, R.; Dobritzsch, S.; Gruber, C.; Hause, G.; Athmer, B.; Schreiber, T.; Marillonnet, S.; Okabe, Y.; Ezura, H.; Acosta, I.F.; et al. Tomato MYB21 acts in ovules to mediate jasmonate-regulated fertility. Plant Cell. 2019, 31, 1043–1062. [Google Scholar] [CrossRef]
- Stintzi, A.; Browse, J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10625–10630. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, W.; Wang, G.; Li, J.; Dong, L.; Han, L.; Wang, Q.; Tian, J.; Yu, Y.; Gao, C.; et al. KTN80 confers precision to microtubule severing by specific targeting of katanin complexes in plant cells. EMBO J. 2017, 36, 3435–3447. [Google Scholar] [CrossRef] [PubMed]
- Bichet, A.; Desnos, T.; Turner, S.; Grandjean, O.; Höfte, H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001, 25, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Cao, L.; Zhou, Z.; Zhu, L.; Ehrhardt, D.; Yang, Z.; Fu, Y. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr. Biol. 2013, 23, 290–297. [Google Scholar] [CrossRef]
- Miao, R.; Siao, W.; Zhang, N.; Lei, Z.; Lin, D.; Bhalerao, R.P.; Lu, C.; Xu, W. Katanin-dependent microtubule ordering in association with ABA is important for root hydrotropism. Int. J. Mol. Sci. 2022, 23, 3846. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- He, Y.; He, X.; Wang, X.; Hao, M.; Gao, J.; Wang, Y.; Yang, Z.N.; Meng, X. An EPFL peptide signaling pathway promotes stamen elongation via enhancing filament cell proliferation to ensure successful self-pollination in Arabidopsis thaliana. New Phytol. 2023, 238, 1045–1058. [Google Scholar] [CrossRef]
- Ma, H.; Xu, L.; Fu, Y.; Zhu, L. Arabidopsis QWRF1 and QWRF2 redundantly modulate cortical microtubule arrangement in floral organ growth and fertility. Front. Cell Dev. Biol. 2021, 9, 634218. [Google Scholar] [CrossRef]
- Ma, D.; Constabel, C.P. MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019, 24, 275–289. [Google Scholar] [CrossRef]
- Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004, 40, 979–995. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, H.; Bae, W.; Hwang, I. Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nat. Commun. 2014, 5, 4138. [Google Scholar] [CrossRef]
- Kagale, S.; Rozwadowski, K. EAR motif-mediated transcriptional repression in plants: An underlying mechanism for epigenetic regulation of gene expression. Epigenetics 2011, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, L.; Wang, Y.; Li, W.; Chen, B.; Zhu, L.; Fu, Y. Arabidopsis ECAP is a new adaptor protein that connects JAZ repressors with the TPR2 co-repressor to suppress jasmonate-responsive anthocyanin accumulation. Mol. Plant 2020, 13, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Chopy, M.; Binaghi, M.; Cannarozzi, G.; Halitschke, R.; Boachon, B.; Heutink, R.; Bomzan, D.P.; Jäggi, L.; van Geest, G.; Verdonk, J.C.; et al. A single MYB transcription factor with multiple functions during flower development. New Phytol. 2023, 239, 2007–2025. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Zhao, S.; Fu, Y.; Zhu, L. HOMEOBOX PROTEIN 24 mediates the conversion of indole-3-butyric acid to indole-3-acetic acid to promote root hair elongation. New Phytol. 2021, 232, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Qin, X.; Peng, J.; Han, R.; Lv, Y.; Li, C.; Qi, L.; Qu, G.P.; Yang, L.; et al. Reconstitution of phytochrome A-mediated light modulation of the ABA signaling pathways in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2302901120. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.R.; Bowman, J.L.; Meyerowitz, E.M. Early flower development in Arabidopsis. Plant Cell. 1990, 2, 755–767. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, L.Q.; Xu, Q.; Kong, Y.H.; Wang, H.; Wu, W.H. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell. 2009, 21, 3554–3566. [Google Scholar] [CrossRef]
- Chow, C.N.; Lee, T.Y.; Hung, Y.C.; Li, G.Z.; Tseng, K.C.; Liu, Y.H.; Kuo, P.L.; Zheng, H.Q.; Chang, W.C. PlantPAN3.0: A new and updated resource for reconstructing transcriptional regulatory networks from ChIP-seq experiments in plants. Nucleic Acids Res. 2019, 47, D1155–D1163. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, L.; Feng, W.; Fu, Y.; Li, C. Arabidopsis MYB21 Negatively Regulates KTN1 to Fine-Tune the Filament Elongation. Plants 2023, 12, 3884. https://doi.org/10.3390/plants12223884
Wang Y, Shi L, Feng W, Fu Y, Li C. Arabidopsis MYB21 Negatively Regulates KTN1 to Fine-Tune the Filament Elongation. Plants. 2023; 12(22):3884. https://doi.org/10.3390/plants12223884
Chicago/Turabian StyleWang, Yanan, Lei Shi, Wutao Feng, Ying Fu, and Changjiang Li. 2023. "Arabidopsis MYB21 Negatively Regulates KTN1 to Fine-Tune the Filament Elongation" Plants 12, no. 22: 3884. https://doi.org/10.3390/plants12223884





