Dehydrin CaDHN2 Enhances Drought Tolerance by Affecting Ascorbic Acid Synthesis under Drought in Peppers
Abstract
:1. Introduction
2. Results
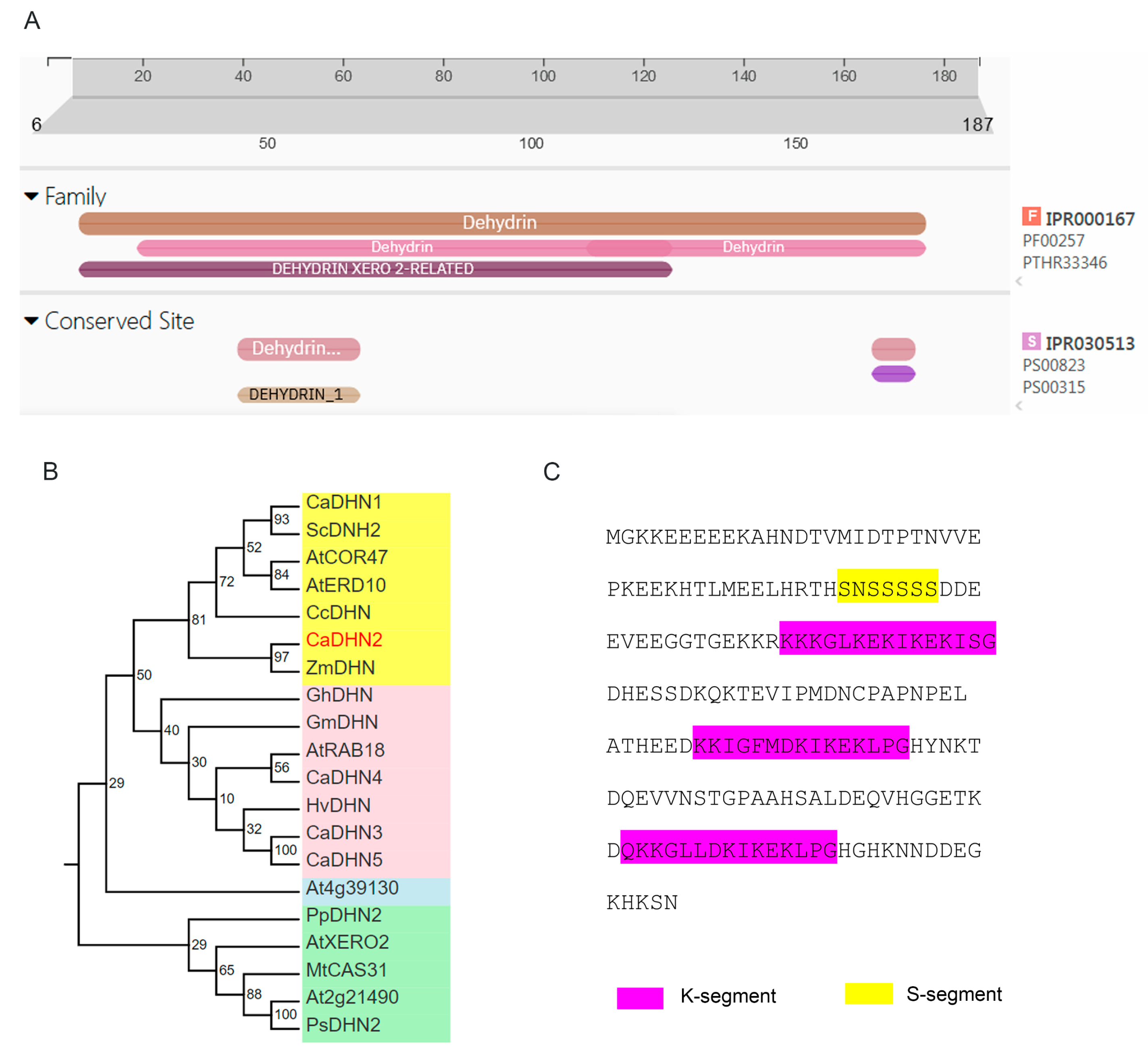
2.1. CaDHN2 Is a SK3-Type Dehydrin
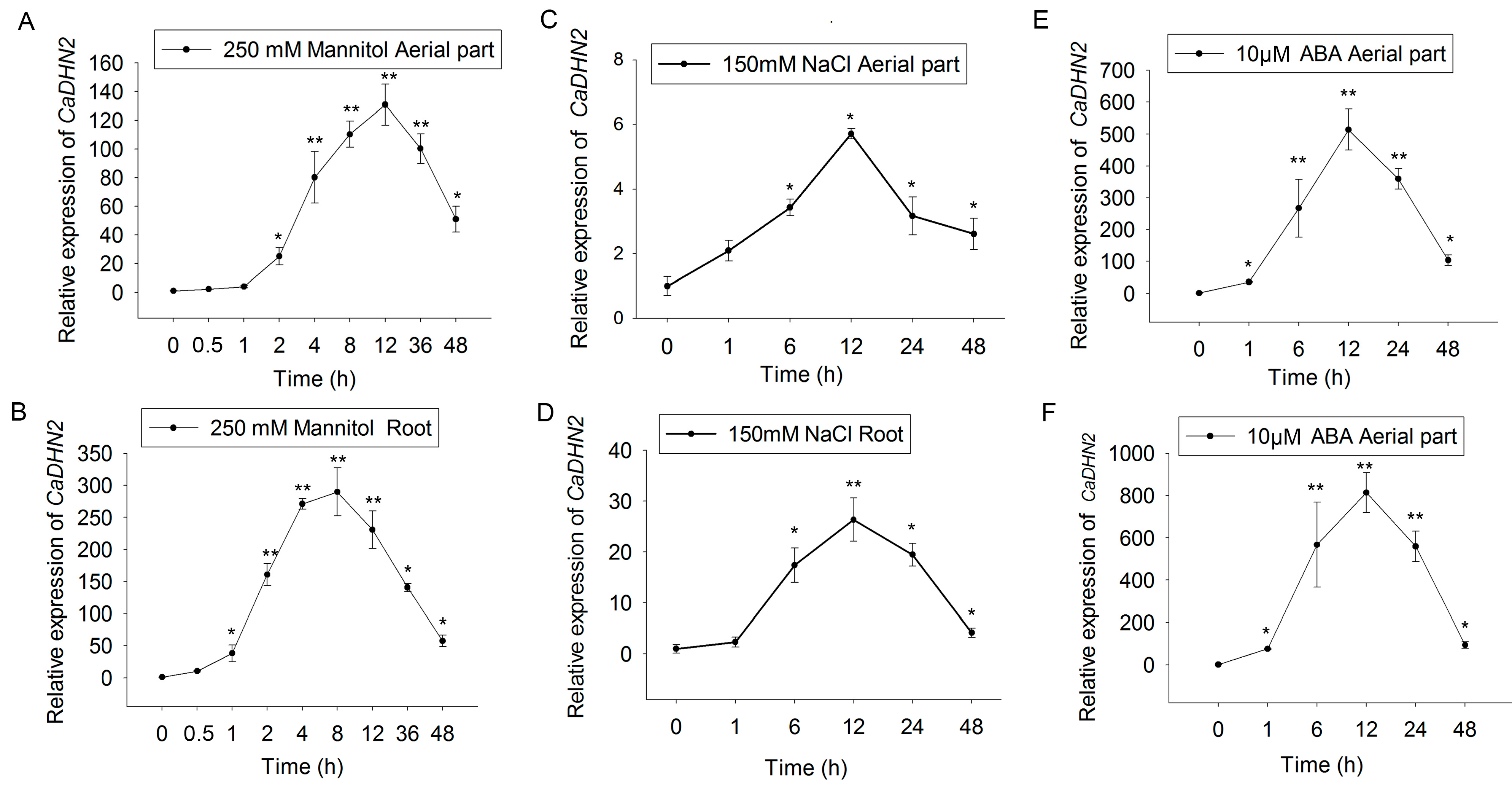
2.2. CaDHN2 Is Induced by Drought, Salt, and ABA
2.3. CaDHN2 Is Localized in the Plasma Membrane, Nucleus, and Cytoplasm
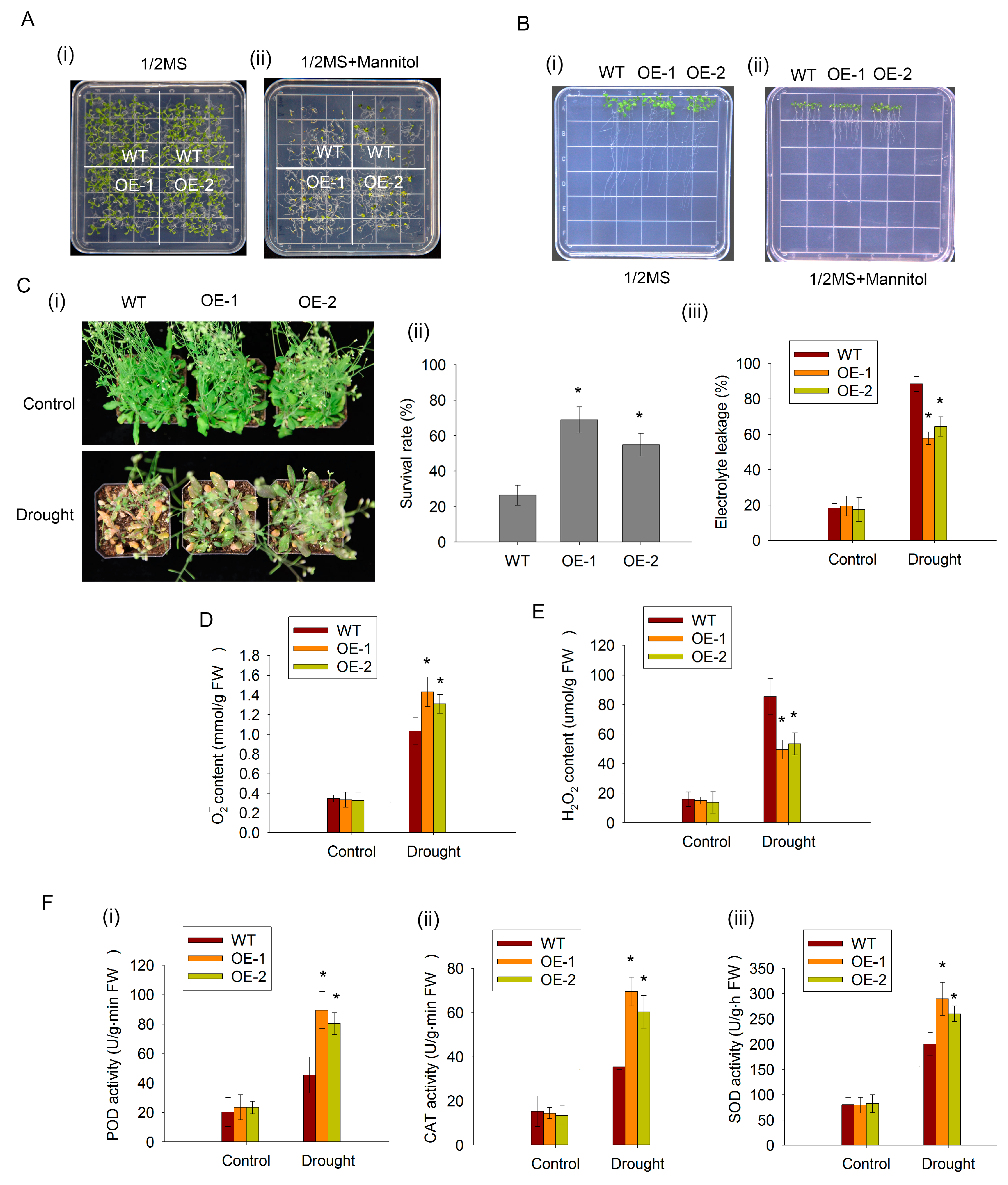
2.4. CaDHN2 Enhances Drought Tolerance in Capsicum annuum L.
2.5. The Overexpression of CaDHN2 in Arabidopsis Enhances Drought Tolerance
2.6. CaDHN2 Interacts with CaGGP1 Directly
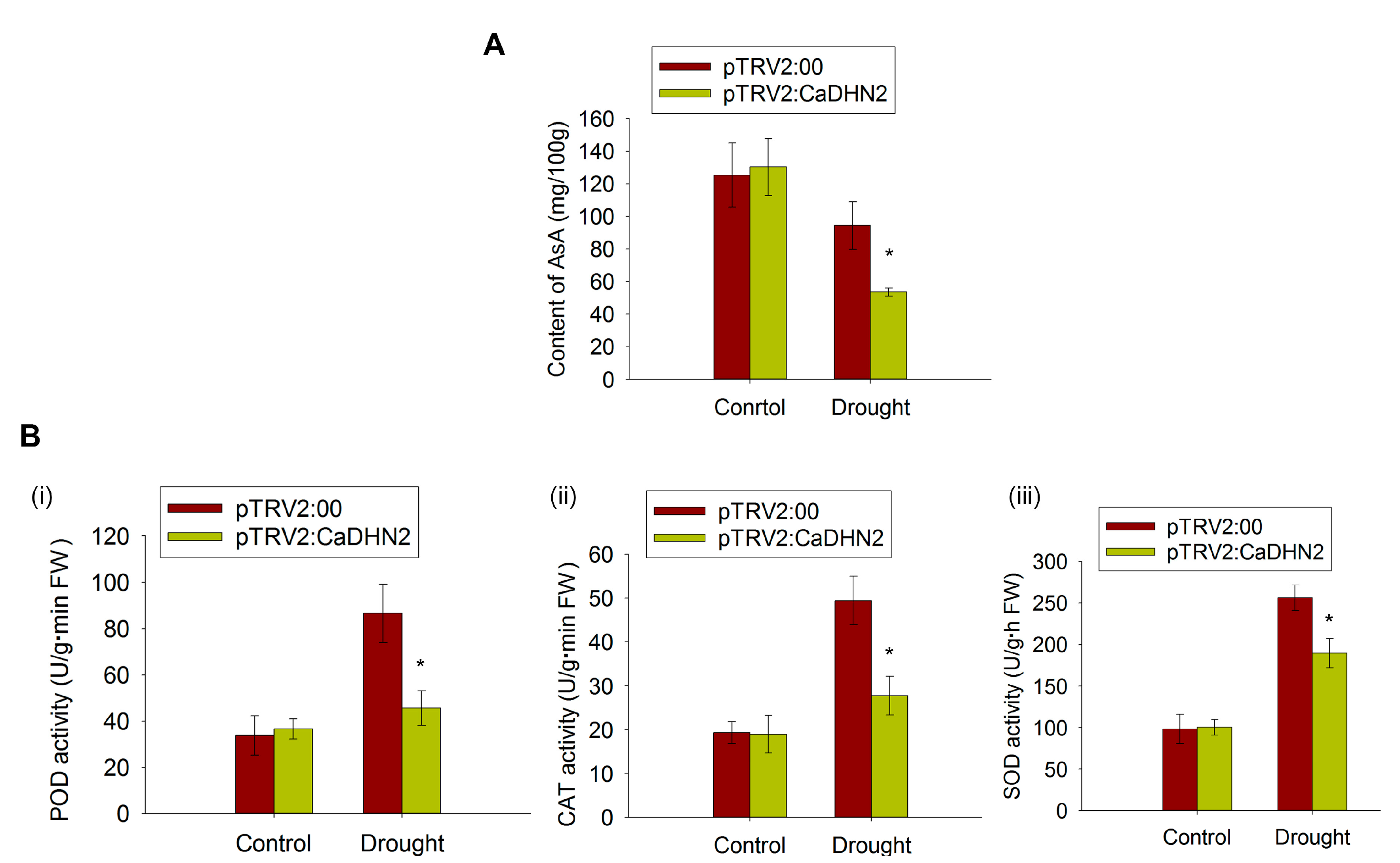
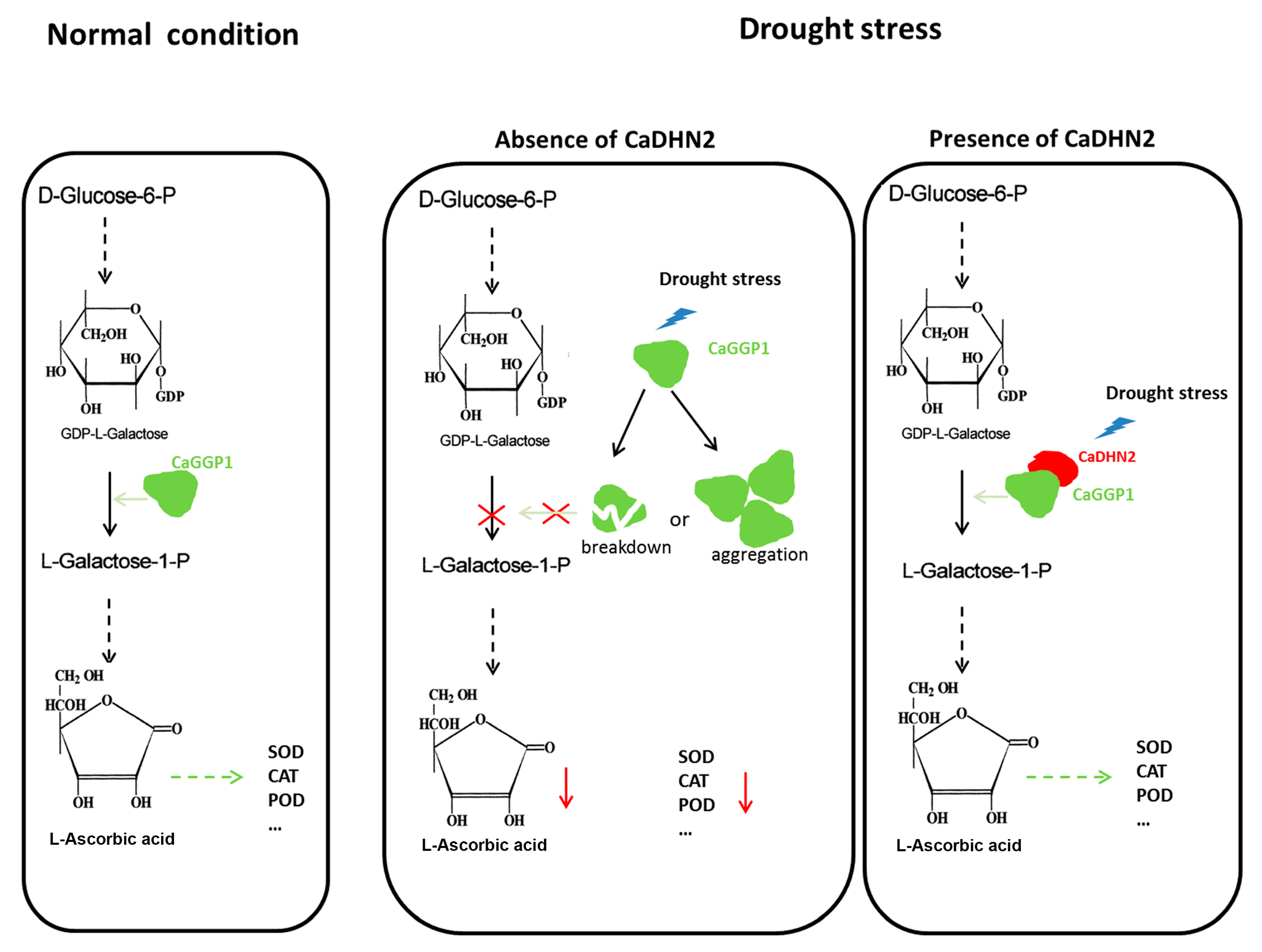
2.7. CaDHN2 Protects CaGGP1 to Maintain AsA Synthesis under Drought Stress
3. Discussion
4. Material and Methods
4.1. Plant Materials
4.2. qRT-PCR
4.3. Stress Treatment
4.4. Electrolyte Leakage
4.5. Enzyme Activity
4.6. NBT and DAB Staining
4.7. Measurement of H2O2 and O2−
4.8. Biomolecular Fluorescence Complementation (BiFC)
4.9. GST Pull-Down
4.10. Yeast Two-Hybrid Assay
4.11. Ascorbic Acid Content Measurement
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Pence, H.E.; Jin, J.B.; Miura, K.; Gosney, M.J.; Hasegawa, P.M.; Mickelbart, M.V. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 2010, 22, 4128–4141. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 156–177. [Google Scholar] [CrossRef]
- Rao, V.; Virupapuram, V. Arabidopsis F-box protein At1g08710 interacts with transcriptional protein ADA2b and imparts drought stress tolerance by negatively regulating seedling growth. Biochem. Biophys. Res. Commun. 2021, 536, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Liang, C.C.; Hou, S.G.; Wang, X.; Chen, D.H.; Shen, J.L.; Zhang, W.; Wang, M. The LRR-RLK Protein HSL3 Regulates Stomatal Closure and the Drought Stress Response by Modulating Hydrogen Peroxide Homeostasis. Front. Plant Sci. 2020, 11, 548034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, Z.; Li, F.; Li, X.; Ji, H.; Yang, S. Retraction Note: The specific MYB binding sites bound by TaMYB in the GAPCp2/3 promoters are involved in the drought stress response in wheat. BMC Plant Biol. 2020, 20, 566. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.; Lim, C.W.; Lee, S.C. Pepper E3 ligase CaAIRE1 promotes ABA sensitivity and drought tolerance by degradation of protein phosphatase CaAITP1. J. Exp. Bot. 2021, 72, 4520–4534. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Choi, J.Y.; Kim, S.J.; Kim, E.Y.; Shin, J.S.; Kim, W.T. Constitutive expression of CaRma1H1, a hot pepper ER-localized RING E3 ubiquitin ligase, increases tolerance to drought and salt stresses in transgenic tomato plants. Plant Cell Rep. 2012, 31, 1659–1665. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Lee, S.C. A pepper RING-type E3 ligase, CaASRF1, plays a positive role in drought tolerance via modulation of CaAIBZ1 stability. Plant J. Cell Mol. Biol. 2019, 98, 5–18. [Google Scholar] [CrossRef]
- Lim, C.W.; Park, C.; Kim, J.H.; Joo, H.; Hong, E.; Lee, S.C. Pepper CaREL1, a ubiquitin E3 ligase, regulates drought tolerance via the ABA-signalling pathway. Sci. Rep. 2017, 7, 477. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Gai, W.X.; Li, C.; Gong, Z.H. The CaCIPK3 gene positively regulates drought tolerance in pepper. Hortic. Res. 2021, 8, 216. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhang, H.X.; Ali, M.; Gai, W.X.; Cheng, G.X.; Yu, Q.H.; Yang, S.B.; Li, X.X.; Gong, Z.H. A small heat shock protein CaHsp25.9 positively regulates heat, salt, and drought stress tolerance in pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 142, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lim, C.W.; Lee, S.C. Pepper Novel Serine-Threonine Kinase CaDIK1 Regulates Drought Tolerance via Modulating ABA Sensitivity. Front. Plant Sci. 2020, 11, 1133. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Lee, S.C. Roles of pepper bZIP transcription factor CaATBZ1 and its interacting partner RING-type E3 ligase CaASRF1 in modulation of ABA signalling and drought tolerance. Plant J. Cell Mol. Biol. 2019, 100, 399–410. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Lee, S.C. Roles of pepper bZIP protein CaDILZ1 and its interacting partner RING-type E3 ligase CaDSR1 in modulation of drought tolerance. Plant J. Cell Mol. Biol. 2018, 96, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.C.; Zhang, H.F.; Pan, X.X.; Chen, N.; Hu, H.F.; Haq, S.U.; Khan, A.; Chen, R.G. CaDHN3, a Pepper (Capsicum annuum L.) Dehydrin Gene Enhances the Tolerance against Salt and Drought Stresses by Reducing ROS Accumulation. Int. J. Mol. Sci. 2021, 22, 3205. [Google Scholar] [CrossRef]
- Hassan, A.; Fasiha Amjad, S.; Hamzah Saleem, M.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Ahmad Joyia, F.; Mobeen; Ahmed, S.; et al. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, C.; Liu, H.; Jiao, Q.; Li, G.; Zhang, J.; Zhang, B.; Jin, W.; Lin, D.; Chen, G.; et al. Exogenous ascorbic acid application alleviates cadmium toxicity in seedlings of two wheat (Triticum aestivum L.) varieties by reducing cadmium uptake and enhancing antioxidative capacity. Environ. Sci. Pollut. Res. Int. 2022, 29, 21739–21750. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Kannan, R.; Jayaseelan, R. Ascorbic Acid Preconditioning Effect on Broccoli Seedling Growth and Photosynthesis under Drought Stress. Plants 2022, 11, 1324. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, H.; Liu, J.X.; Shu, S.; Tan, G.F.; Li, M.Y.; Duan, A.Q.; Liu, H.; Xiong, A.S. AgGMP encoding GDP-D-mannose pyrophosphorylase from celery enhanced the accumulation of ascorbic acid and resistance to drought stress in Arabidopsis. Peerj 2022, 10, e12976. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhai, Z.; Peng, X.; Wang, Y.; Sun, Y.; Li, J.; Shen, X.; Xiao, Y.; Zhu, S.; et al. The Apple microR171i-SCARECROW-LIKE PROTEINS26.1 Module Enhances Drought Stress Tolerance by Integrating Ascorbic Acid Metabolism. Plant Physiol. 2020, 184, 194–211. [Google Scholar] [CrossRef]
- Khazaei, Z.; Esmaielpour, B.; Estaji, A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L) plants. Physiol. Mol. Biol. Plants 2020, 26, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Moghadam, H.T.; Nasri, M.; Kasraie, P.; Larijani, H. The effect of ascorbic acid and bio fertilizers on basil under drought stress. Braz. J. Biol. 2022, 84, e262459. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, X.L.; Niu, J.P. Effects of exogenous ascorbic acid on seed germination and seedling salt-tolerance of alfalfa. PLoS ONE 2021, 16, e0250926. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, A.; Li, Y.; Liu, R.; Shi, L.; Jia, L.; Zhang, J. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J. Exp. Bot. 2012, 63, 1809–1822. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, F.; Cui, R.; Wang, S.; Cui, Y.; Lu, X.; Huang, H.; Ni, K.; Liu, X.; Jiang, T.; et al. GhIMP10D, an inositol monophosphates family gene, enhances ascorbic acid and antioxidant enzyme activities to confer alkaline tolerance in Gossypium hirsutum L. BMC Plant Biol. 2023, 23, 447. [Google Scholar] [CrossRef]
- Kim, A.N.; Lee, K.Y.; Kim, B.G.; Cha, S.W.; Jeong, E.J.; Kerr, W.L.; Choi, S.G. Thermal processing under oxygen-free condition of blueberry puree: Effect on anthocyanin, ascorbic acid, antioxidant activity, and enzyme activities. Food Chem. 2021, 342, 128345. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic Acid Induces the Increase of Secondary Metabolites, Antioxidant Activity, Growth, and Productivity of the Common Bean under Water Stress Conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef]
- Liang, D.; Zhu, T.; Ni, Z.; Lin, L.; Tang, Y.; Wang, Z.; Wang, X.; Wang, J.; Lv, X.; Xia, H. Ascorbic acid metabolism during sweet cherry (Prunus avium) fruit development. PLoS ONE 2017, 12, e0172818. [Google Scholar] [CrossRef]
- Alegre, M.L.; Steelheart, C.; Baldet, P.; Rothan, C.; Just, D.; Okabe, Y.; Ezura, H.; Smirnoff, N.; Gergoff Grozeff, G.E.; Bartoli, C.G. Deficiency of GDP-L-galactose phosphorylase, an enzyme required for ascorbic acid synthesis, reduces tomato fruit yield. Planta 2020, 251, 54. [Google Scholar] [CrossRef]
- Koukounaras, A.; Mellidou, I.; Patelou, E.; Kostas, S.; Shukla, V.; Engineer, C.; Papaefthimiou, D.; Amari, F.; Chatzopoulos, D.; Mattoo, A.K.; et al. Over-expression of GGP1 and GPP genes enhances ascorbate content and nutritional quality of tomato. Plant Physiol. Biochem. 2022, 193, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sanchez, I.E.; Martynowicz, D.M.; Rodriguez-Hernandez, A.A.; Perez-Morales, M.B.; Graether, S.P.; Jimenez-Bremont, J.F. A dehydrin-dehydrin interaction: The case of SK3 from Opuntia streptacantha. Front. Plant Sci 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [PubMed]
- Zolotarov, Y.; Stromvik, M. De Novo Regulatory Motif Discovery Identifies Significant Motifs in Promoters of Five Classes of Plant Dehydrin Genes. PLoS ONE 2015, 10, e0129016. [Google Scholar] [CrossRef]
- Richard Strimbeck, G. Hiding in plain sight: The F segment and other conserved features of seed plant SK(n) dehydrins. Planta 2017, 245, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Graether, S.P. The Disordered Dehydrin and Its Role in Plant Protection: A Biochemical Perspective. Biomolecules 2022, 12, 294. [Google Scholar] [CrossRef]
- Madge, J. The Protection of Membranes from Cold-Stress: A Structural Study of the Intrinsically Disordered Dehydrin Bound to Micelles and Liposomes. Biophys. J. 2014, 106, 426a. [Google Scholar]
- Eriksson, S.; Eremina, N.; Barth, A.; Danielsson, J.; Harryson, P. Membrane-Induced Folding of the Plant Stress Dehydrin Lti30. Plant Physiol. 2016, 171, 932–943. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Feng, H.; Deng, J.; Zhang, R.; Wen, J.; Dong, J.; Wang, T. Dehydrin MtCAS31 promotes autophagic degradation under drought stress. Autophagy 2020, 16, 862–877. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Yu, T.; Chen, S.; Chen, Y.; Lu, C. Heterologous Expression of Three Ammopiptanthus mongolicus Dehydrin Genes Confers Abiotic Stress Tolerance in Arabidopsis thaliana. Plants 2020, 9, 193. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Samarah, N.H.; Tanash, A.A. Effect of drought stress on wheat (Triticum durum) growth and metabolism: Insight from GABA shunt, reactive oxygen species and dehydrin genes expression. Funct. Plant Biol. 2022, 13, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Ohkubo, T.; Kamiya, K.; Hara, M. Cryoprotective activity of Arabidopsis KS-type dehydrin depends on the hydrophobic amino acids of two active segments. Arch. Biochem. Biophys. 2020, 691, 108510. [Google Scholar] [CrossRef] [PubMed]
- Saruhan Guler, N.; Terzi, R.; Demiralay, M.; Ozturk, K.; Kadioglu, A. Increased dehydrin level decreases leaf rolling grade by altering the reactive oxygen species homeostasis and abscisic acid content in maize subjected to osmotic stress. 3 Biotech 2022, 12, 201. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, R.; Qu, Y.; Miao, Z.; Zhang, Y.; Shen, X.; Wang, T.; Dong, J. Overexpression of MtCAS31 enhances drought tolerance in transgenic Arabidopsis by reducing stomatal density. New Phytol. 2012, 195, 124–135. [Google Scholar] [CrossRef]
- Cuevas-Velazquez, C.L.; Rendon-Luna, D.F.; Covarrubias, A.A. Dissecting the cryoprotection mechanisms for dehydrins. Front. Plant Sci. 2014, 5, 583. [Google Scholar] [CrossRef]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef]
- Tompa, P.; Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. Faseb J. 2004, 18, 1169–1175. [Google Scholar] [CrossRef]
- Kim, T.D.; Paik, S.R.; Yang, C.H.; Kim, J. Structural changes in alpha-synuclein affect its chaperone-like activity in vitro. Protein Sci. 2000, 9, 2489–2496. [Google Scholar] [CrossRef]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008, 147, 381–390. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Liu, H.; Zhang, Y.; Bai, Z.; Zhang, L. Effect of K-/S- segments on subcellular localization and dimerization of wheat dehydrin WZY1-2. Plant Signal. Behav. 2020, 15, 1827583. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Allagulova Ch, R.; Bezrukova, M.V.; Gimalov, F.R. Induction of expression of the dehydrin gene TADHN and accumulation of abscisic acid in wheat plants in hypothermia. Doklady. Biochem. Biophys. 2005, 400, 69–71. [Google Scholar] [CrossRef]
- Murvai, N.; Kalmar, L.; Szalaine Agoston, B.; Szabo, B.; Tantos, A.; Csikos, G.; Micsonai, A.; Kardos, J.; Vertommen, D.; Nguyen, P.N.; et al. Interplay of Structural Disorder and Short Binding Elements in the Cellular Chaperone Function of Plant Dehydrin ERD14. Cells 2020, 9, 1856. [Google Scholar] [CrossRef]
- Kimura, Y.; Ohkubo, T.; Shimizu, K.; Magata, Y.; Park, E.Y.; Hara, M. Inhibition of cryoaggregation of phospholipid liposomes by an Arabidopsis intrinsically disordered dehydrin and its K-segment. Colloids Surf. B Biointerfaces 2022, 211, 112286. [Google Scholar] [CrossRef]
- Alsheikh, M.K.; Heyen, B.J.; Randall, S.K. Ion binding properties of the dehydrin ERD14 are dependent upon phosphorylation. J. Biol. Chem. 2003, 278, 40882–40889. [Google Scholar] [CrossRef]
- Saavedra, L.; Svensson, J.; Carballo, V.; Izmendi, D.; Welin, B.; Vidal, S. A dehydrin gene in Physcomitrella patens is required for salt and osmotic stress tolerance. Plant J. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Sun, S.; Han, W.; Irfan, M.; Zhang, X.; Zhang, L.; Chen, L. AnDHN, a Dehydrin Protein From Ammopiptanthus nanus, Mitigates the Negative Effects of Drought Stress in Plants. Front. Plant Sci. 2021, 12, 788938. [Google Scholar] [CrossRef]
- Vazquez-Hernandez, M.; Romero, I.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. Functional characterization of VviDHN2 and VviDHN4 dehydrin isoforms from Vitis vinifera (L.): An in silico and in vitro approach. Plant Physiol. Biochem. 2021, 158, 146–157. [Google Scholar] [CrossRef]
- Li, X.; Feng, H.; Wen, J.; Dong, J.; Wang, T. MtCAS31 Aids Symbiotic Nitrogen Fixation by Protecting the Leghemoglobin MtLb120-1 Under Drought Stress in Medicago truncatula. Front. Plant Sci. 2018, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Kaviraj, M.; Rout, S.; Chakraborty, K.; Swain, P.; Nayak, P.K.; Nayak, A.K. Combined application of ascorbic acid and endophytic N-fixing Azotobacter chroococcum Avi2 modulates photosynthetic efficacy, antioxidants and growth-promotion in rice under moisture deficit stress. Microbiol. Res. 2021, 250, 126808. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Flowerika; Chaturvedi, S.; Tiwari, S. Wheat derived glucuronokinase as a potential target for regulating ascorbic acid and phytic acid content with increased root length under drought and ABA stresses in Arabidopsis thaliana. Plant Sci. 2023, 331, 111671. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D. VIGS vectors for gene silencing: Many targets, many tools. Annu. Rev. Plant Biol. 2004, 55, 495–519. [Google Scholar] [CrossRef]
- Lu, R.; Martin-Hernandez, A.M.; Peart, J.R.; Malcuit, I.; Baulcombe, D.C. Virus-induced gene silencing in plants. Methods 2003, 30, 296–303. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010, 639, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Stengel, F.; Baldwin, A.J.; Painter, A.J.; Jaya, N.; Basha, E.; Kay, L.E.; Vierling, E.; Robinson, C.V.; Benesch, J.L. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc. Natl. Acad. Sci. USA 2010, 107, 2007–2012. [Google Scholar] [CrossRef]
- Gao, J.J.; Zhang, L.; Qin, A.G.; Yu, X.C. Relative expression of SOD and CAT mRNA and activities of SOD and CAT in grafted cucumber leaves under NaCl stress. Ying Yong Sheng Tai Xue Bao 2008, 19, 1754–1758. [Google Scholar] [PubMed]
- Wei, C.; Song, L.; Qin, L. Heterologous expression of HsPstS gene reducing arsenic accumulation and improving As-tolerance in transgenic tobaccos by enhancing CAT activity. J. Plant Physiol. 2023, 282, 153940. [Google Scholar] [CrossRef]
- Able, A.J. Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 2003, 221, 137–143. [Google Scholar] [CrossRef]
- Sekulska-Nalewajko, J.; Goclawski, J.; Chojak-Kozniewska, J.; Kuzniak, E. Automated image analysis for quantification of reactive oxygen species in plant leaves. Methods 2016, 109, 114–122. [Google Scholar] [CrossRef]
- Esfandiari, E.; Shakiba, M.R.; Mahboob, S.A.; Alyari, H.; Shahabivand, S. The effect of water stress on the antioxidant content, protective enzyme activities, proline content and lipid peroxidation in wheat seedling. Pak. J. Biol. Sci. 2008, 11, 1916–1922. [Google Scholar] [CrossRef]
- Ke, D.; Sun, G.; Wang, Z. Effects of superoxide radicals on ACC synthase activity in chilling-stressed etiolated mungbean seedlings. Plant Growth Regul. 2007, 51, 83–91. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Kim, S.Y.; do Hyeon, Y.; Kim, D.H.; Dong, T.; Park, Y.; Jin, J.B.; Joo, S.H.; Kim, S.K.; Hong, J.C.; et al. The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription factors in dehydration and osmotic stress responses. Plant Cell 2013, 25, 4708–4724. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Feng, H.; Liu, S.; Cui, J.; Liu, J.; Shi, M.; Zhao, J.; Wang, L. Dehydrin CaDHN2 Enhances Drought Tolerance by Affecting Ascorbic Acid Synthesis under Drought in Peppers. Plants 2023, 12, 3895. https://doi.org/10.3390/plants12223895
Li X, Feng H, Liu S, Cui J, Liu J, Shi M, Zhao J, Wang L. Dehydrin CaDHN2 Enhances Drought Tolerance by Affecting Ascorbic Acid Synthesis under Drought in Peppers. Plants. 2023; 12(22):3895. https://doi.org/10.3390/plants12223895
Chicago/Turabian StyleLi, Xin, Hao Feng, Sha Liu, Junjun Cui, Jiannan Liu, Mingyu Shi, Jielong Zhao, and Lihu Wang. 2023. "Dehydrin CaDHN2 Enhances Drought Tolerance by Affecting Ascorbic Acid Synthesis under Drought in Peppers" Plants 12, no. 22: 3895. https://doi.org/10.3390/plants12223895
APA StyleLi, X., Feng, H., Liu, S., Cui, J., Liu, J., Shi, M., Zhao, J., & Wang, L. (2023). Dehydrin CaDHN2 Enhances Drought Tolerance by Affecting Ascorbic Acid Synthesis under Drought in Peppers. Plants, 12(22), 3895. https://doi.org/10.3390/plants12223895






