Enhancing Soybean Yield: The Synergy of Sulfur and Rhizobia Inoculation
Abstract
:1. Introduction
2. Results
2.1. Screening of Rhizobia
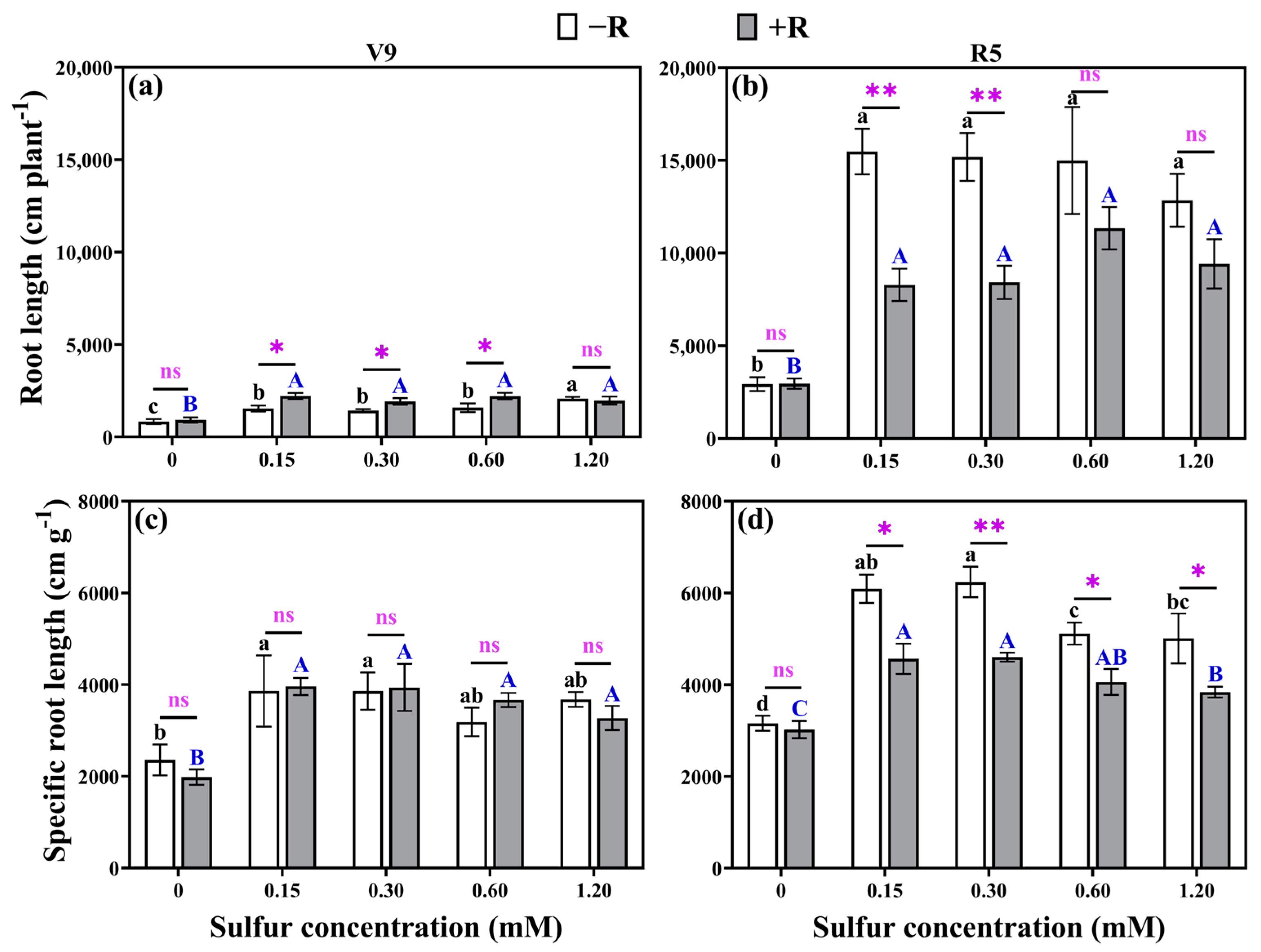
2.2. Root Lengths and Specific Root Lengths
2.3. Number and Dry Weight of Nodules
2.4. Nitrogenase Activity
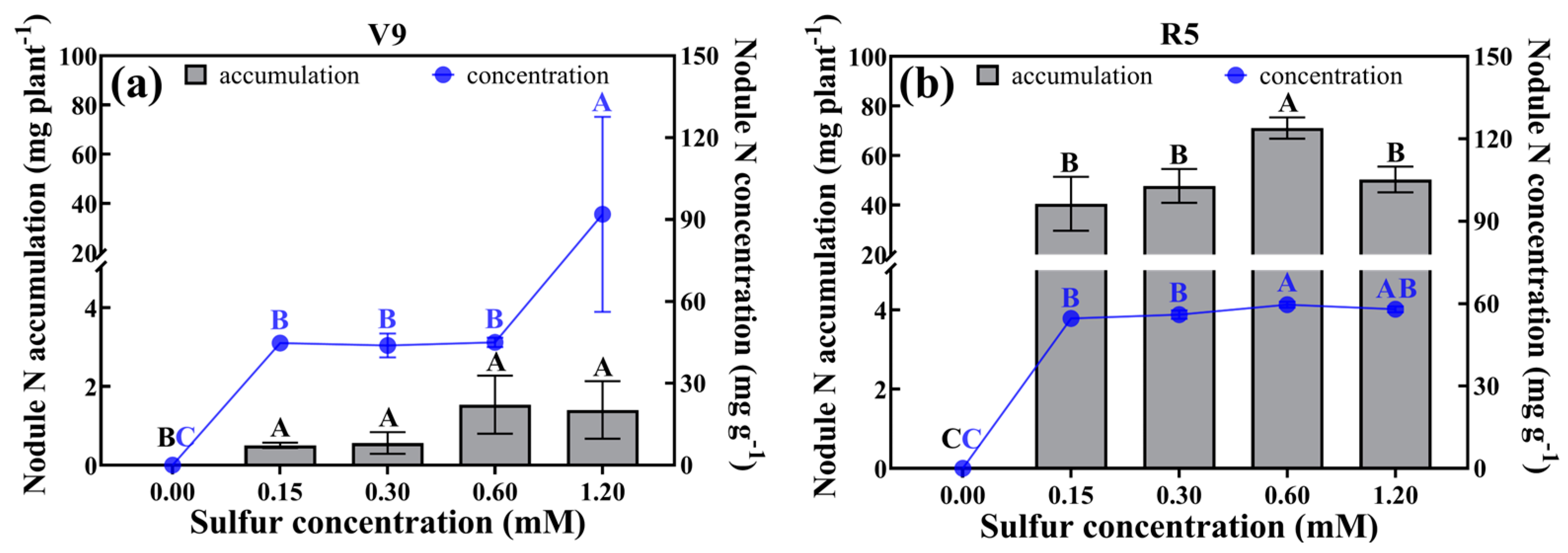
2.5. Nodule Nitrogen Concentration and Accumulation
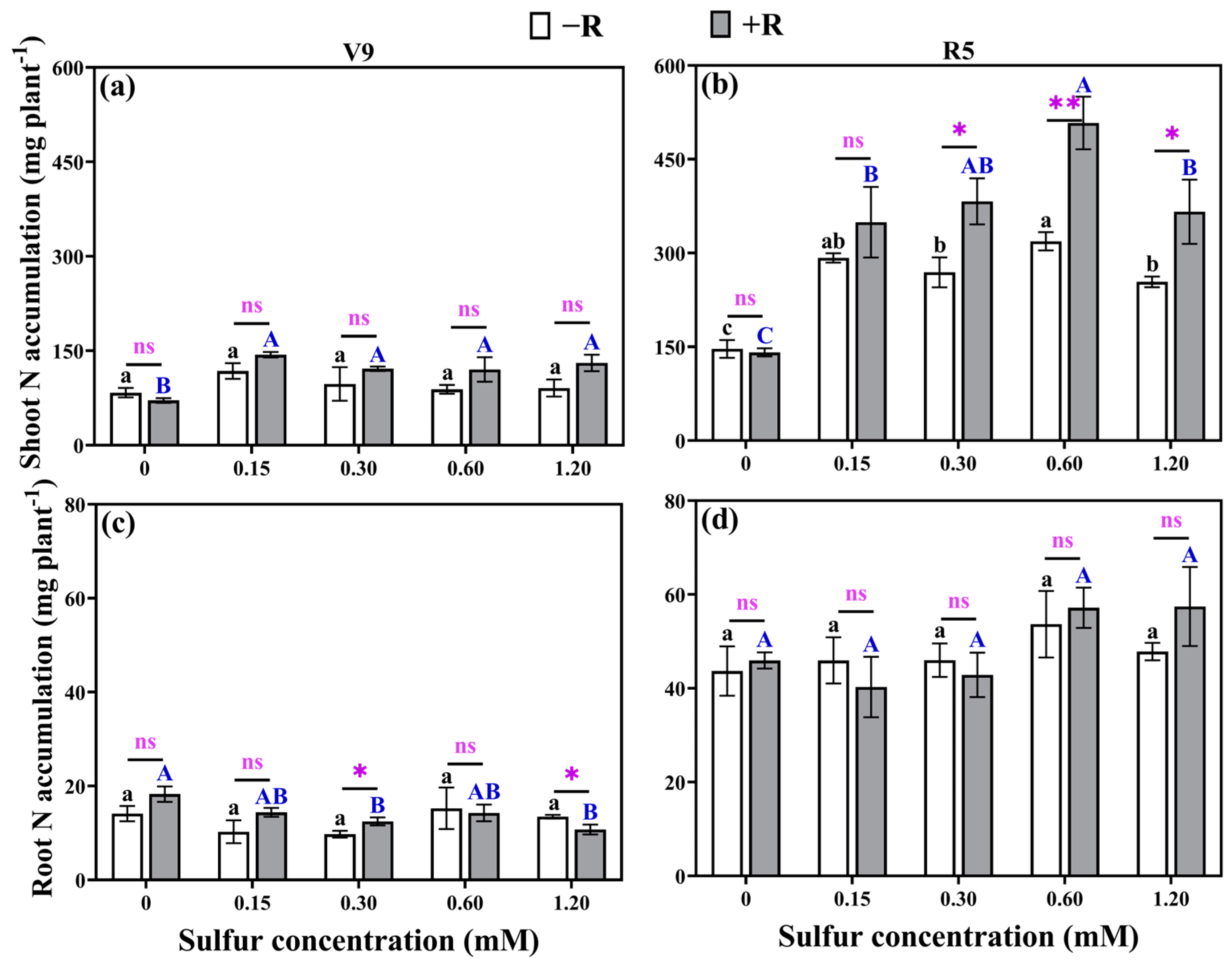
2.6. Nitrogen Accumulation
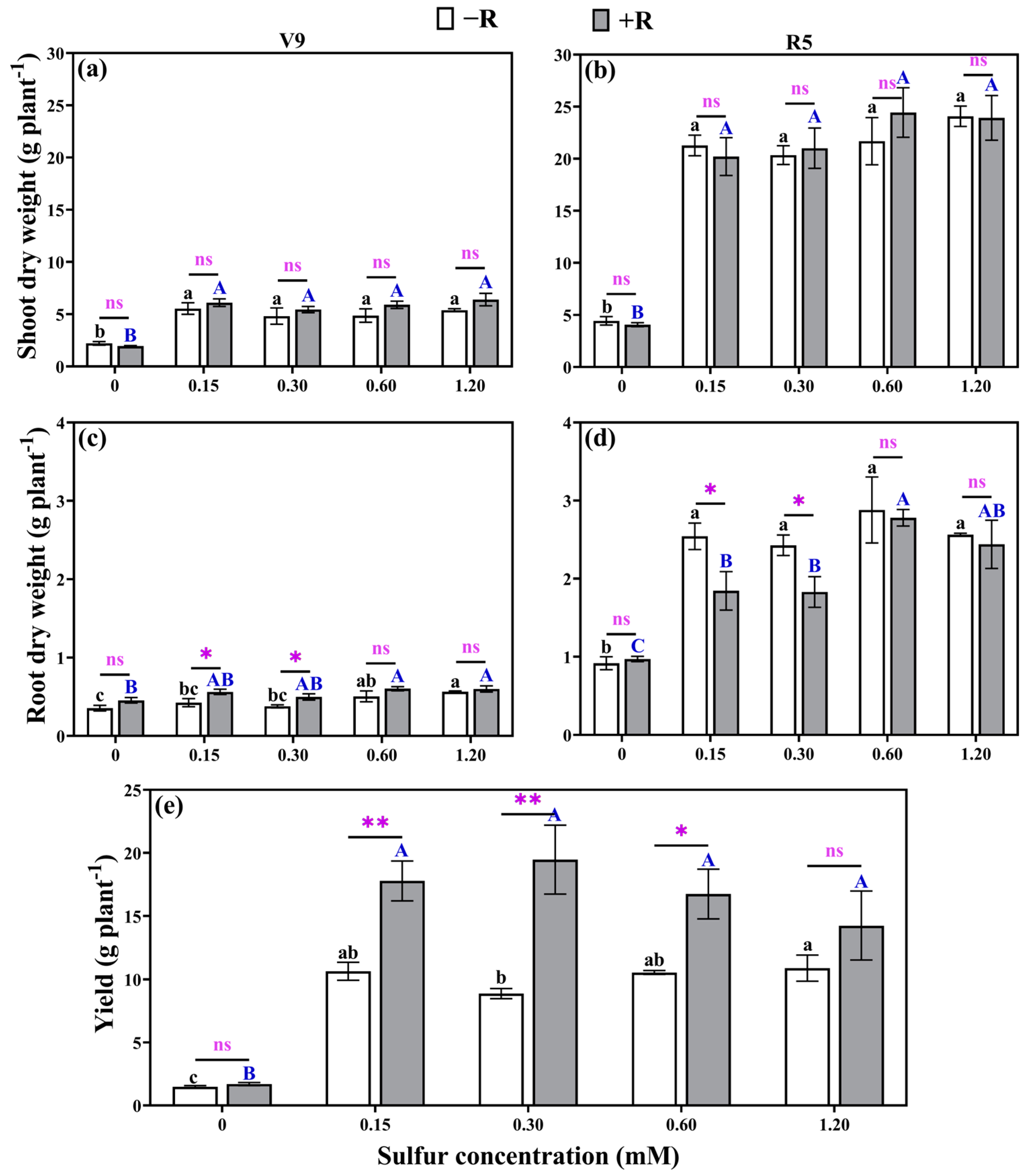
2.7. Dry Weight and Yield
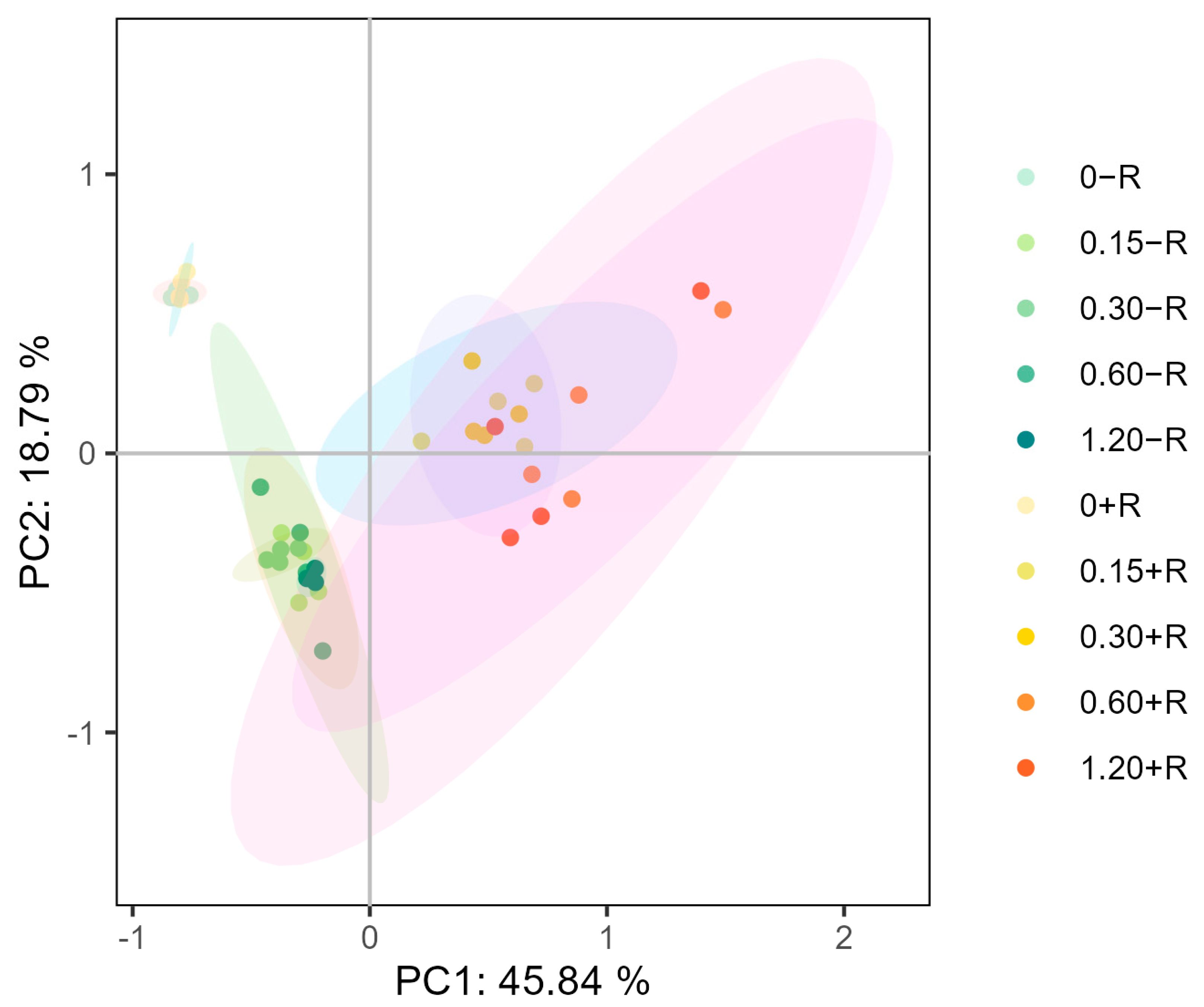
2.8. Principal Component Analysis (PCA)
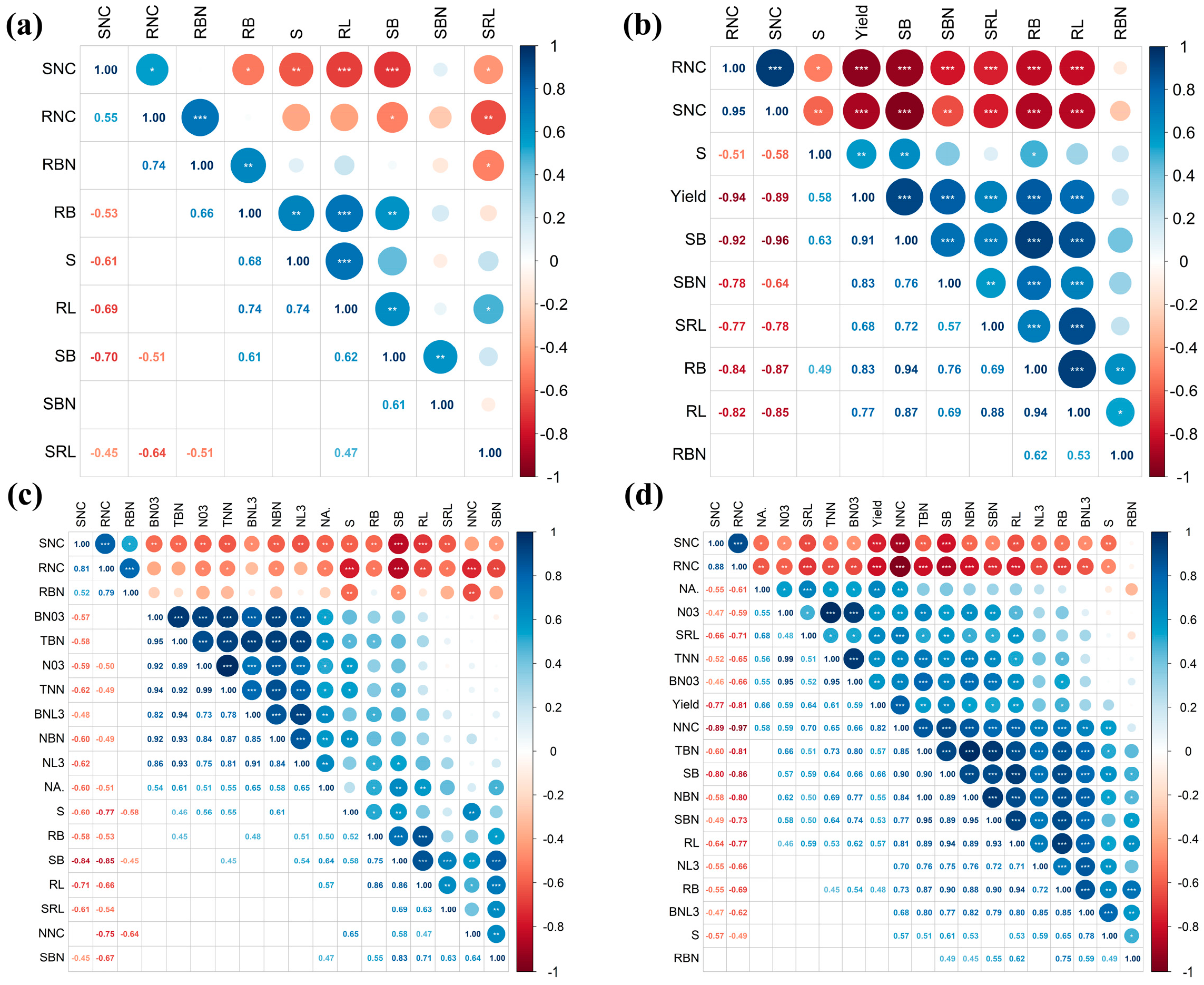
2.9. Pearson Correlation Coefficients
3. Discussion
3.1. The Impact of Sulfur on Soybean Growth
3.2. The Influence of Rhizobia on Soybean Growth
3.3. Sulfur and Rhizobia Combined Impact
3.4. Roots and Nodules in Soybean
4. Materials and Methods
4.1. Rhizobia Culture Experiment
4.2. Soybean Hydroponic Experiment
4.2.1. Strain Preparation
4.2.2. Soybean Transplant
4.2.3. Plant Sampling
4.2.4. Sample Determination
4.2.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mangena, P. Impact of Polyploidy Induction for Salinity Stress Mitigation in Soybean (Glycine max L. Merrill). Plants 2023, 12, 1356. [Google Scholar] [CrossRef]
- Engelbrecht, G.; Claassens, S.; Mienie, C.M.S.; Fourie, H. South Africa: An Important Soybean Producer in Sub-Saharan Africa and the Quest for Managing Nematode Pests of the Crop. Agriculture 2020, 10, 242. [Google Scholar] [CrossRef]
- Asif, M.; Acharya, M. Phytochemicals and Nutritional Health Benefits of Soy Plant. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 64. [Google Scholar] [CrossRef]
- Pointke, M.; Ohlau, M.; Risius, A.; Pawelzik, E. Plant-Based Only: Investigating Consumers’ Sensory Perception, Motivation, and Knowledge of Different Plant-Based Alternative Products on the Market. Foods 2022, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Corgneau, M.; Gaiani, C.; Petit, J.; Nikolova, Y.; Banon, S.; Ritié-Pertusa, L.; Le, D.T.L.; Scher, J. Nutritional Quality Evaluation of Commercial Protein Supplements. Int. J. Food Sci. Technol. 2019, 54, 2586–2594. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Online Database, Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/zh/#data/QCL (accessed on 31 August 2023).
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Bensmihen, S. Hormonal Control of Lateral Root and Nodule Development in Legumes. Plants 2015, 4, 523–547. [Google Scholar] [CrossRef]
- Pessi, G.; Ahrens, C.H.; Rehrauer, H.; Lindemann, A.; Hauser, F.; Fischer, H.-M.; Hennecke, H. Genome-Wide Transcript Analysis of Bradyrhizobium Japonicum Bacteroids in Soybean Root Nodules. Mol. Plant-Microbe Interact. 2007, 20, 1353–1363. [Google Scholar] [CrossRef]
- Farssi, O.; Mouradi, M.; Ghoulam, C.; Berrougui, H.; Farissi, M. Genes and Molecular Signals Involved in Legumes- Rhizobia Symbiosis. Anna. West Univ. Timis. Ser. Biol. 2018, 21, 193. [Google Scholar]
- Lardi, M.; Murset, V.; Fischer, H.-M.; Mesa, S.; Ahrens, C.H.; Zamboni, N.; Pessi, G. Metabolomic Profiling of Bradyrhizobium Diazoefficiens-Induced Root Nodules Reveals Both Host Plant-Specific and Developmental Signatures. Int. J. Mol. Sci. 2016, 17, 815. [Google Scholar] [CrossRef] [PubMed]
- Mortland, M.M. Ammonia Interactions with Soil Minerals. In Agricultural Anhydrous Ammonia Technology and Use; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1966; pp. 188–197. ISBN 978-0-89118-887-2. [Google Scholar]
- Leigh, G.J. Nitrogen Fixation at the Millennium; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 978-0-08-053757-3. [Google Scholar]
- Lindström, K.; Murwira, M.; Willems, A.; Altier, N. The Biodiversity of Beneficial Microbe-Host Mutualism: The Case of Rhizobia. Res. Microbiol. 2010, 161, 453–463. [Google Scholar] [CrossRef]
- Hanoon, M.B.; Haran, M.S.; Sahi, M.K. Effect of Rhizobium Inoculation and Different Levels of Organic and Nitrogen Fertilizers on Growth and Production of Broad Bean (Vicia faba L.) and Nitrogen Readiness in Soil. Int. J. Agric. Stat. Sci. 2020, 16, 229–236. [Google Scholar]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V. Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.). Agronomy 2020, 10, 826. [Google Scholar] [CrossRef]
- Maitra, S.; Praharaj, S.; Brestic, M.; Sahoo, R.K.; Sagar, L.; Shankar, T.; Palai, J.B.; Sahoo, U.; Sairam, M.; Pramanick, B.; et al. Rhizobium as Biotechnological Tools for Green Solutions: An Environment-Friendly Approach for Sustainable Crop Production in the Modern Era of Climate Change. Curr. Microbiol. 2023, 80, 219. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Mushtaq, N.U.; Rasool, A.; Shah, W.H.; Tahir, I.; Rehman, R.U. Chapter Two—Plant Nutrition and Soil Fertility: Physiological and Molecular Avenues for Crop Improvement. In Sustainable Plant Nutrition; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 23–49. ISBN 978-0-443-18675-2. [Google Scholar]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic Concepts of Iron-Sulfur Protein Biogenesis in Biology. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef]
- Mohammadi, K.; Rokhzadi, A. An Integrated Fertilization System of Canola (Brassica Napus L.) Production under Different Crop Rotations. Ind. Crops Prod. 2012, 37, 264–269. [Google Scholar] [CrossRef]
- Rashmi, I.; Mina, B.L.; Kuldeep, K.; Ali, S.; Kumar, A.; Kala, S.; Singh, R.K. Gypsum—An Inexpensive, Effective Sulphur Source with Multitude Impact on Oilseed Production and Soil Quality—A Review. Agric. Rev. 2018, 39, 218–225. [Google Scholar]
- Franzen, D.; Grant, C.A. Sulfur Response Based on Crop, Source, and Landscape Position. In Agronomy Monographs; Jez, J., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 105–116. ISBN 978-0-89118-186-6. [Google Scholar]
- Miyatake, M.; Ohyama, T.; Yokoyama, T.; Sugihara, S.; Motobayashi, T.; Kamiya, T.; Fujiwara, T.; Yuan, K.; Bellingrath-Kimura, S.D.; Ohkama-Ohtsu, N. Effects of Deep Placement of Controlled-Release Nitrogen Fertilizer on Soybean Growth and Yield under Sulfur Deficiency. Soil Sci. Plant Nutr. 2019, 65, 259–266. [Google Scholar] [CrossRef]
- Ibañez, T.B.; de Melo Santos, L.F.; de Marcos Lapaz, A.; Ribeiro, I.V.; Ribeiro, F.V.; dos Reis, A.R.; Moreira, A.; Heinrichs, R. Sulfur Modulates Yield and Storage Proteins in Soybean Grains. Sci. Agric. (Piracicaba Braz.) 2021, 78, e20190020. [Google Scholar] [CrossRef]
- Ahmed, M.; Hasanuzzaman, M.; Raza, M.A.; Malik, A.; Ahmad, S. Plant Nutrients for Crop Growth, Development and Stress Tolerance. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 43–92. ISBN 978-3-030-45669-6. [Google Scholar]
- Głowacka, A.; Jariene, E.; Flis-Olszewska, E.; Kiełtyka-Dadasiewicz, A. The Effect of Nitrogen and Sulphur Application on Soybean Productivity Traits in Temperate Climates Conditions. Agronomy 2023, 13, 780. [Google Scholar] [CrossRef]
- Duke, S.H.; Reisenauer, H.M. Roles and Requirements of Sulfur in Plant Nutrition. In Agronomy Monographs; Tabatabai, M.A., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 123–168. ISBN 978-0-89118-220-7. [Google Scholar]
- Varin, S.; Cliquet, J.-B.; Personeni, E.; Avice, J.-C.; Lemauviel-Lavenant, S. How Does Sulphur Availability Modify N Acquisition of White Clover (Trifolium Repens L.)? J. Exp. Bot. 2010, 61, 225–234. [Google Scholar] [CrossRef]
- Schoenau, J.J.; Malhi, S.S. Sulfur Forms and Cycling Processes in Soil and Their Relationship to Sulfur Fertility. In Agronomy Monographs; Jez, J., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 1–10. ISBN 978-0-89118-186-6. [Google Scholar]
- Messick, D.L.; Fan, M.X.; De Brey, C. Global Sulfur Requirement and Sulfur Fertilizers. FAL—Agric Res 2005, 283, 97–104. [Google Scholar]
- Mascagni, H.J.; Harrison, S.A.; Padgett, G.B. Influence of Sulfur Fertility on Wheat Yield Performance on Alluvial and Upland Soils. Commun. Soil Sci. Plant Anal. 2008, 39, 2133–2145. [Google Scholar] [CrossRef]
- Sexton, P.J.; Paek, N.C.; Shibles, R. Soybean Sulfur and Nitrogen Balance under Varying Levels of Available Sulfur. Crop Sci. 1998, 38, 975–982. [Google Scholar] [CrossRef]
- Almeida, L.F.A.; Correndo, A.; Ross, J.; Licht, M.; Casteel, S.; Singh, M.; Naeve, S.; Vann, R.; Bais, J.; Kandel, H.; et al. Soybean Yield Response to Nitrogen and Sulfur Fertilization in the United States: Contribution of Soil N and N Fixation Processes. Eur. J. Agron. 2023, 145, 126791. [Google Scholar] [CrossRef]
- Brooks, K.; Mourtzinis, S.; Conley, S.P.; Reiter, M.S.; Gaska, J.; Holshouser, D.L.; Irby, T.; Kleinjan, J.; Knott, C.; Lee, C.; et al. Soybean Yield Response to Sulfur and Nitrogen Additions across Diverse U.S. Environments. Agron. J. 2023, 115, 370–383. [Google Scholar] [CrossRef]
- Sexton, P.J.; Batchelor, W.D.; Shibles, R. Sulfur Availability, Rubisco Content, and Photosynthetic Rate of Soybean. Crop Sci. 1997, 37, 1801–1806. [Google Scholar] [CrossRef]
- Gutierrez Boem, F.H.; Prystupa, P.; Ferraris, G. Seed Number and Yield Determination in Sulfur Deficient Soybean Crops. J. Plant Nutr. 2007, 30, 93–104. [Google Scholar] [CrossRef]
- Rushovich, D.; Weil, R. Sulfur Fertility Management to Enhance Methionine and Cysteine in Soybeans. J. Sci. Food Agric. 2021, 101, 6595–6601. [Google Scholar] [CrossRef]
- Sugiura, H.; Sugihara, S.; Kamiya, T.; Artigas Ramirez, M.D.; Miyatake, M.; Fujiwara, T.; Takuji, O.; Motobayashi, T.; Yokoyama, T.; Bellingrath-Kimura, S.D.; et al. Sulfur Application Enhances Secretion of Organic Acids by Soybean Roots and Solubilization of Phosphorus in Rhizosphere. Soil Sci. Plant Nutr. 2021, 67, 400–407. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Moretti, L.G.; Aquino, G.S. Phosphorus, Potassium and Sulfur Interactions in Soybean Plants on a Typic Hapludox. Commun. Soil Sci. Plant Anal. 2018, 49, 405–415. [Google Scholar] [CrossRef]
- Chandra, N.; Pandey, N. Role of Sulfur Nutrition in Plant and Seed Metabolism of Glycine max L. J. Plant Nutr. 2016, 39, 1103–1111. [Google Scholar] [CrossRef]
- Zhou, X.J.; Liang, Y.; Chen, H.; Shen, S.H.; Jing, Y.X. Effects of Rhizobia Inoculation and Nitrogen Fertilization on Photosynthetic Physiology of Soybean. Photosynt. 2006, 44, 530–535. [Google Scholar] [CrossRef]
- Sogut, T. Rhizobium Inoculation Improves Yield and Nitrogen Accumulation in Soybean (Glycine max) Cultivars Better than Fertiliser. N. Z. J. Crop Hortic. Sci. 2006, 34, 115–120. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Temprano, F.J. Soybean Inoculation: Dose, N Fertilizer Supplementation and Rhizobia Persistence in Soil. Field Crops Res. 2009, 113, 352–356. [Google Scholar] [CrossRef]
- Qin, L.; Jiang, H.; Tian, J.; Zhao, J.; Liao, H. Rhizobia Enhance Acquisition of Phosphorus from Different Sources by Soybean Plants. Plant Soil 2011, 349, 25–36. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Q.; Chen, F.; Yan, X.; Liao, H. Effects of Co-Inoculation with Arbuscular Mycorrhizal Fungi and Rhizobia on Soybean Growth as Related to Root Architecture and Availability of N and P. Mycorrhiza 2011, 21, 173–181. [Google Scholar] [CrossRef]
- Alam, F.; Bhuiyan, M.; Alam, S.S.; Waghmode, T.R.; Kim, P.J.; Lee, Y.B. Effect of Rhizobium Sp. BARIRGm901 Inoculation on Nodulation, Nitrogen Fixation and Yield of Soybean (Glycine max) Genotypes in Gray Terrace Soil. Biosci. Biotechnol. Biochem. 2015, 79, 1660–1668. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, X.; Bi, D.; Hu, F. Effects of Sulfur Fertilization on Soybean Root and Leaf Traits, and Soil Microbial Activity. J. Plant Nutr. 2008, 31, 473–483. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C. Soybean Nodule Size and Relationship to Nitrogen Fixation Response to Water Deficit. Crop Sci. 2001, 41, 1099–1107. [Google Scholar] [CrossRef]
- Becana, M.; Wienkoop, S.; Matamoros, M.A. Sulfur Transport and Metabolism in Legume Root Nodules. Front. Plant Sci. 2018, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.J.; Paulsen, G.M.; Murphy, L.S. Response of Nodulated and Nonnodulated Soybean Seedlings to Sulfur Nutrition. Agron. J. 1970, 62, 277–280. [Google Scholar] [CrossRef]
- Santachiara, G.; Salvagiotti, F.; Rotundo, J.L. Nutritional and Environmental Effects on Biological Nitrogen Fixation in Soybean: A Meta-Analysis. Field Crops Res. 2019, 240, 106–115. [Google Scholar] [CrossRef]
- Fabre, F.; Planchon, C. Nitrogen Nutrition, Yield and Protein Content in Soybean. Plant Sci. 2000, 152, 51–58. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. A Meta-Analysis of the Effectiveness of Diverse Rhizobia Inoculants on Soybean Traits under Field Conditions. Soil Biol. Biochem. 2017, 105, 177–196. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Q.; Chen, Y.; Tian, B.; Xu, L.; Bai, Y.; Chen, W.; Li, X. Variation in Rhizosphere Microbial Communities and Its Association with the Symbiotic Efficiency of Rhizobia in Soybean. ISME J. 2020, 14, 1915–1928. [Google Scholar] [CrossRef]
- Nakei, M.D.; Venkataramana, P.B.; Ndakidemi, P.A. Soybean-Nodulating Rhizobia: Ecology, Characterization, Diversity, and Growth Promoting Functions. Front. Sustain. Food Syst. 2022, 6, 824444. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ono, Y.; Ohtake, N.; Sueyoshi, K.; Tanabata, S.; Ohyama, T. Transcriptome and Metabolome Analyses Reveal That Nitrate Strongly Promotes Nitrogen and Carbon Metabolism in Soybean Roots, but Tends to Repress It in Nodules. Plants 2018, 7, 32. [Google Scholar] [CrossRef]
- Hardarson, G.; Golbs, M.; Danso, S. Nitrogen Fixation in Soybean (Glycine max L. Merrill) as Affected by Nodulation Patterns. Soil Biol. Biochem. 1989, 21, 783–787. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, G.; Wang, X.; Zhao, J.; Yan, X.; Liao, H. Isolation and Application of Effective Nitrogen Fixation Rhizobial Strains on Low-Phosphorus Acid Soils in South China. Sci. Bull. 2009, 54, 412–420. [Google Scholar] [CrossRef]
- Mathis, J.N.; McMillin, D.E.; Champion, R.A.; Hunt, P.G. Genetic Variation in Two Cultures of Bradyrhizobium Japonicum 110 Differing in Their Ability to Impart Drought Tolerance to Soybean. Curr. Microbiol. 1997, 35, 363–366. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Plant-Microbe Interactions: Laboratory Techniques; Springer Protocols Handbooks; Springer: New York, NY, USA, 2021; ISBN 978-1-07-161079-4. [Google Scholar]


| Stage | Sulfur Concentration (mM) | Number of Nodules (No.·Plant−1) | Dry Weight of Nodules (g·Plant−1) | ||||
|---|---|---|---|---|---|---|---|
| <3 mm | >3 mm | Total Number | <3 mm | >3 mm | Total Weight | ||
| V9 | 0 | 0 ± 0.0 b 1 | 0 ± 0.0 a | 0 ± 0.0 b | 0 ± 0.0000 a | 0 ± 0.0000 a | 0 ± 0.0000 a |
| 0.15 | 6 ± 1.2 ab | 3 ± 0.3 a | 9 ± 1.0 ab | 0.0062 ± 0.0012 a | 0.0049 ± 0.0004 a | 0.0112 ± 0.0010 a | |
| 0.30 | 11 ± 5.9 ab | 3 ± 2.0 a | 15 ± 6.1 ab | 0.0052 ± 0.0018 a | 0.0064 ± 0.0037 a | 0.0116 ± 0.0047 a | |
| 0.60 | 21 ± 10.6 ab | 5 ± 1.6 a | 26 ± 12.1 ab | 0.0195 ± 0.0109 a | 0.0134 ± 0.0047 a | 0.0329 ± 0.0155 a | |
| 1.20 | 26 ± 11.6 a | 3 ± 2.3 a | 30 ± 13.5 a | 0.0161 ± 0.0087 a | 0.0140 ± 0.0124 a | 0.0301 ± 0.0204 a | |
| R5 | 0 | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 c | 0 ± 0.0000 b | 0 ± 0.0000 c | 0 ± 0.0000 c |
| 0.15 | 192 ± 52.3 ab | 19 ± 8.2 a | 211 ± 57.5 ab | 0.5248 ± 0.1581 a | 0.1925 ± 0.0685 bc | 0.7344 ± 0.1966 b | |
| 0.30 | 226 ± 59.2 a | 18 ± 3.2 a | 244 ± 57.6 a | 0.5721 ± 0.1272 a | 0.2577 ± 0.0356 b | 0.8531 ± 0.1264 ab | |
| 0.60 | 156 ± 18.0 ab | 26 ± 1.4 a | 182 ± 17.5 ab | 0.5647 ± 0.0456 a | 0.5857 ± 0.0662 a | 1.1919 ± 0.0663 a | |
| 1.20 | 75 ± 24.2 bc | 28 ± 7.1 a | 103 ± 17.5 bc | 0.2255 ± 0.0408 b | 0.5796 ± 0.1147 a | 0.8695 ± 0.0903 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chen, Y.; Yang, X.; Deng, L.; Lu, X. Enhancing Soybean Yield: The Synergy of Sulfur and Rhizobia Inoculation. Plants 2023, 12, 3911. https://doi.org/10.3390/plants12223911
Hu Y, Chen Y, Yang X, Deng L, Lu X. Enhancing Soybean Yield: The Synergy of Sulfur and Rhizobia Inoculation. Plants. 2023; 12(22):3911. https://doi.org/10.3390/plants12223911
Chicago/Turabian StyleHu, Yiao, Yulin Chen, Xu Yang, Lansheng Deng, and Xing Lu. 2023. "Enhancing Soybean Yield: The Synergy of Sulfur and Rhizobia Inoculation" Plants 12, no. 22: 3911. https://doi.org/10.3390/plants12223911
APA StyleHu, Y., Chen, Y., Yang, X., Deng, L., & Lu, X. (2023). Enhancing Soybean Yield: The Synergy of Sulfur and Rhizobia Inoculation. Plants, 12(22), 3911. https://doi.org/10.3390/plants12223911






