The Response of Oxytropis aciphylla Ledeb. Leaf Interface to Water and Light in Gravel Deserts
Abstract
1. Introduction
2. Results
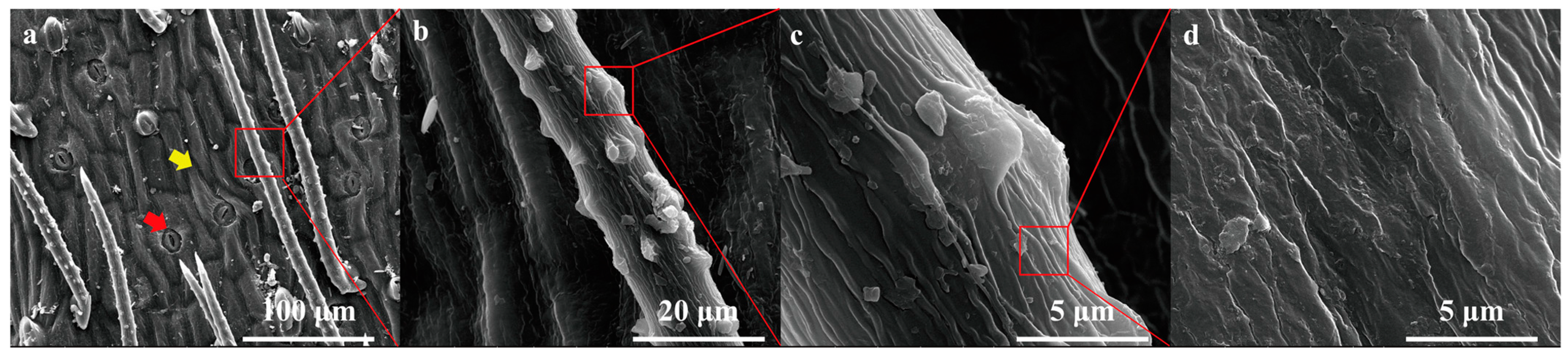
2.1. Leaf Surface Morphological Features
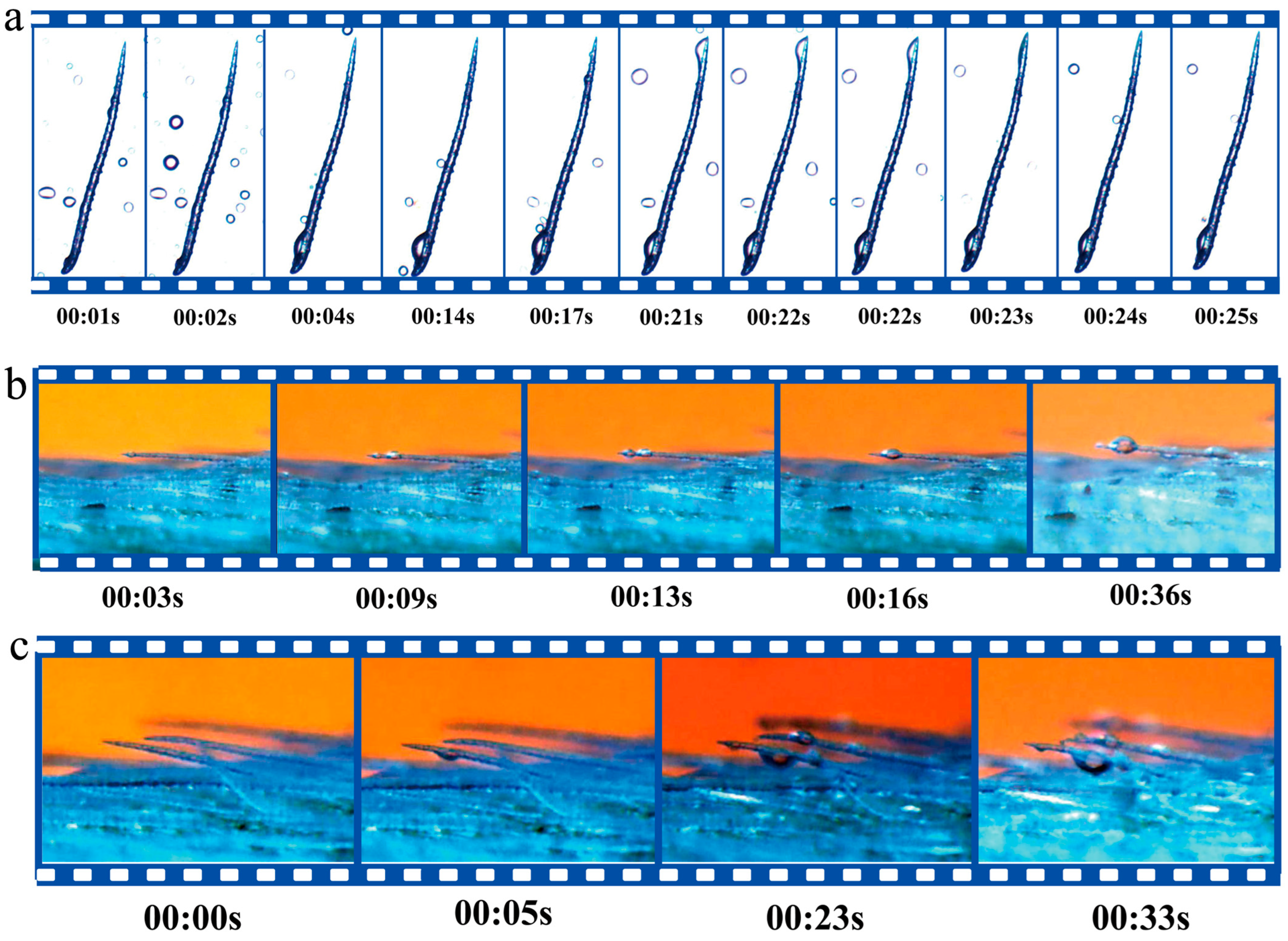
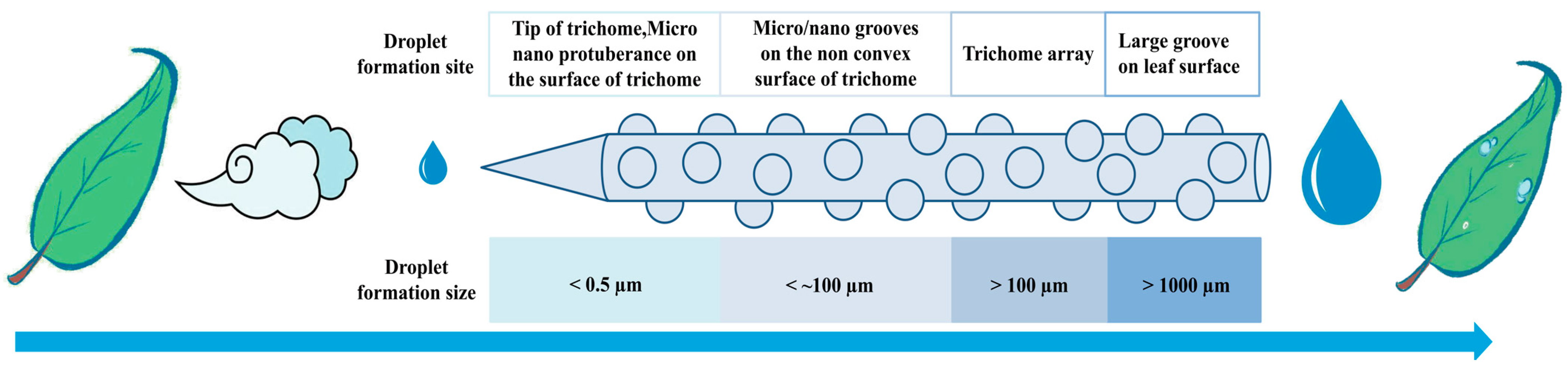
2.2. The Mechanism of Water Collection by Leaf Trichomes
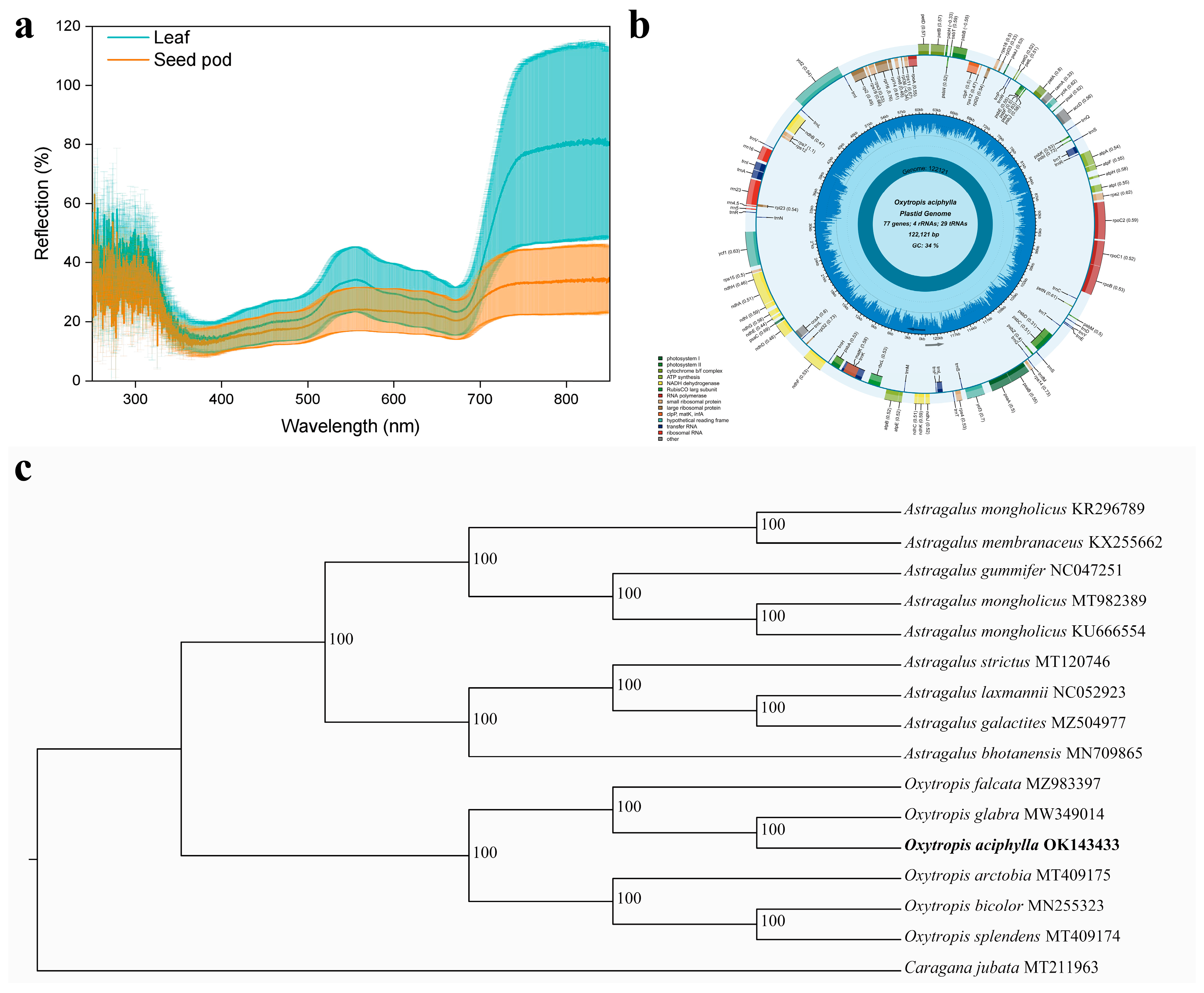
2.3. The Spectral Characteristics of the Leaves and Chloroplast Genome Features
3. Discussion
3.1. Dew as a Vital Water Source in Arid Ecosystems
3.2. Fiber Interface Structure as a Key for Moisture Transport
3.3. Fiber Interface Structure Can Generate Light Selection
4. Materials and Methods
4.1. Leaf Collection
4.2. Leaf Tissue Observation
4.3. Leaf Surface Characteristics
4.4. Leaf Structure Measurement
4.5. Leaf Water Collection Experiment
4.6. Chloroplast Genome Structural Analysis and Phylogenetic Tree Construction
4.7. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silvertown, J.; Araya, Y.; Gowing, D. Hydrological niches in terrestrial plant communities: A review. J. Ecol. 2015, 103, 93–108. [Google Scholar] [CrossRef]
- Klemm, O.; Schemenauer, R.S.; Lummerich, A.; Cereceda, P.; Marzol, V.; Corell, D.; Van Heerden, J.; Reinhard, D.; Gherezghiher, T.; Olivier, J. Fog as a fresh-water resource: Overview and perspectives. Ambio 2012, 41, 221–234. [Google Scholar] [CrossRef]
- Yates, D.; Hutley, L. Foliar uptake of water by wet leaves of Sloanea woollsii, an Australian subtropical rainforest tree. Aust. J. Bot. 1995, 43, 157–167. [Google Scholar] [CrossRef]
- Bei, Z.; Zhang, X.; Tian, X. The Mechanism by Which Umbrella-Shaped Ratchet Trichomes on the Elaeagnus angustifolia Leaf Surface Collect Water and Reflect Light. Biology 2023, 12, 1024. [Google Scholar] [CrossRef]
- Passioura, J. Roots and drought resistance. In Developments in Agricultural and Managed Forest Ecology; Elsevier: Amsterdam, The Netherlands, 1983; Volume 12, pp. 265–280. [Google Scholar]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Snyman, H. Root distribution with changes in distance and depth of two-year-old cactus pears Opuntia ficus-indica and O. robusta plants. South Afr. J. Bot. 2006, 72, 434–441. [Google Scholar] [CrossRef]
- Xu, G.-Q.; Li, Y.; Xu, H. Seasonal variation in plant hydraulic traits of two co-occurring desert shrubs, Tamarix ramosissima and Haloxylon ammodendron, with different rooting patterns. Ecol. Res. 2011, 26, 1071–1080. [Google Scholar] [CrossRef]
- Sheng, J.-h.; Qiao, Y.-x.; Liu, H.-y.; Zhai, Z.-x.; Guo, Y.-h. A study on the root system of Haloxylon Aammodendron (CA Mey.) Bunge. Acta Agrestia Sin. 2004, 12, 91. [Google Scholar]
- Li, J.; Yu, B.; Zhao, C.; Nowak, R.S.; Zhao, Z.; Sheng, Y.; Li, J. Physiological and morphological responses of Tamarix ramosissima and Populus euphratica to altered groundwater availability. Tree Physiol. 2013, 33, 57–68. [Google Scholar] [CrossRef]
- Qong, M.; Takamura, H.; Hudaberdi, M. Formation and internal structure of Tamarix cones in the Taklimakan Desert. J. Arid Environ. 2002, 50, 81–97. [Google Scholar] [CrossRef]
- Gui, D.; Zeng, F.; Liu, Z.; Zhang, B. Characteristics of the clonal propagation of Alhagi sparsifolia Shap. (Fabaceae) under different groundwater depths in Xinjiang, China. Rangel. J. 2013, 35, 355–362. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, F.-J.; Arndt, S.-K.; He, J.-X.; Luo, W.-C.; Song, C. Patterns of root architecture adaptation of a phreatophytic perennial desert plant in a hyperarid desert. S. Afr. J. Bot. 2013, 86, 56–62. [Google Scholar] [CrossRef]
- Zeng, F.; Song, C.; Guo, H.; Liu, B.; Luo, W.; Gui, D.; Arndt, S.; Guo, D. Responses of root growth of Alhagi sparsifolia Shap. (Fabaceae) to different simulated groundwater depths in the southern fringe of the Taklimakan Desert, China. J. Arid Land 2013, 5, 220–232. [Google Scholar] [CrossRef]
- Bei, Z.; Zhang, L.; Tian, X. Characterization of the complete chloroplast genome of Oxytropis aciphylla Ledeb. (Leguminosae). Mitochondrial DNA Part B 2022, 7, 1756–1757. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Huang, D.I.; Cronk, Q.C. Plann: A command-line application for annotating plastome sequences. Appl. Plant Sci. 2015, 3, 1500026. [Google Scholar] [CrossRef]
- Zheng, S.; Poczai, P.; Hyvönen, J.; Tang, J.; Amiryousefi, A. Chloroplot: An online program for the versatile plotting of organelle genomes. Front. Genet. 2020, 11, 576124. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.-R.; Si, W.; Qu, W.-R.; Yang, T.-G.; Wu, Z.-H.; Jiao, P.-P. Complete chloroplast genome sequence of Oxytropis glabra (Leguminosae). Mitochondrial DNA Part B 2021, 6, 2478–2479. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert ecosystems: Environment and producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Gries, D.; Zeng, F.; Foetzki, A.; Arndt, S.K.; Bruelheide, H.; Thomas, F.M.; Zhang, X.; Runge, M. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ. 2003, 26, 725–736. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, W.; Luo, L.; Wang, L. Dew formation characteristics in the gravel desert ecosystem and its ecological roles on Reaumuria soongorica. J. Hydrol. 2021, 603, 126932. [Google Scholar] [CrossRef]
- Agam, N.; Berliner, P.R. Dew formation and water vapor adsorption in semi-arid environments—A review. J. Arid Environ. 2006, 65, 572–590. [Google Scholar] [CrossRef]
- Li, X.-Y. Effects of gravel and sand mulches on dew deposition in the semiarid region of China. J. Hydrol. 2002, 260, 151–160. [Google Scholar] [CrossRef]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef]
- Kaiser, W.M. Effects of water deficit on photosynthetic capacity. Physiol. Plant. 1987, 71, 142–149. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.M. Effects of leaf hair points of a desert moss on water retention and dew formation: Implications for desiccation tolerance. J. Plant Res. 2012, 125, 351–360. [Google Scholar] [CrossRef]
- Pina, A.L.; Zandavalli, R.B.; Oliveira, R.S.; Martins, F.R.; Soares, A.A. Dew absorption by the leaf trichomes of Combretum leprosum in the Brazilian semiarid region. Funct. Plant Biol. 2016, 43, 851–861. [Google Scholar] [CrossRef]
- Vitarelli, N.C.; Riina, R.; Cassino, M.F.; Meira, R.M.S.A. Trichome-like emergences in Croton of Brazilian highland rock outcrops: Evidences for atmospheric water uptake. Perspect. Plant Ecol. Evol. Syst. 2016, 22, 23–35. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Cheng, Y.; Ren, J. Leaf epidermal water-absorbing scales and their absorption of unsaturated atmospheric water in Reaumuria soongorica, a desert plant from the northwest arid region of China. J. Arid Environ. 2016, 128, 17–29. [Google Scholar] [CrossRef]
- Waseem, M.; Nie, Z.F.; Yao, G.Q.; Hasan, M.; Xiang, Y.; Fang, X.W. Dew absorption by leaf trichomes in Caragana korshinskii: An alternative water acquisition strategy for withstanding drought in arid environments. Physiol. Plant. 2021, 172, 528–539. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, H.; Huang, Z.; Tian, X.; Nie, F.-Q.; Zhao, Y.; Zhai, J.; Jiang, L. Directional water collection on wetted spider silk. Nature 2010, 463, 640–643. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y. Bioinspired micro-/nanostructure fibers with a water collecting property. Nanoscale 2014, 6, 7703–7714. [Google Scholar] [CrossRef]
- Bai, H.; Sun, R.; Ju, J.; Yao, X.; Zheng, Y.; Jiang, L. Large-scale fabrication of bioinspired fibers for directional water collection. Small 2011, 7, 3429–3433. [Google Scholar] [CrossRef]
- Bai, H.; Tian, X.; Zheng, Y.; Ju, J.; Zhao, Y.; Jiang, L. Direction controlled driving of tiny water drops on bioinspired artificial spider silks. Adv. Mater. 2010, 22, 5521–5525. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, Y.; Wang, T.; Jiang, L.; Zheng, Y. Directional size-triggered microdroplet target transport on gradient-step fibers. J. Mater. Chem. A 2014, 2, 7156–7160. [Google Scholar] [CrossRef]
- Feng, S.; Hou, Y.; Xue, Y.; Gao, L.; Jiang, L.; Zheng, Y. Photo-controlled water gathering on bio-inspired fibers. Soft Matter 2013, 9, 9294–9297. [Google Scholar] [CrossRef]
- Du, M.; Zhao, Y.; Tian, Y.; Li, K.; Jiang, L. Electrospun multiscale structured membrane for efficient water collection and directional transport. Small 2016, 12, 1000–1005. [Google Scholar] [CrossRef]
- Chen, H.; Ran, T.; Gan, Y.; Zhou, J.; Zhang, Y.; Zhang, L.; Zhang, D.; Jiang, L. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater. 2018, 17, 935–942. [Google Scholar] [CrossRef]
- Feng, S.; Delannoy, J.; Malod, A.; Zheng, H.; Quéré, D.; Wang, Z. Tip-induced flipping of droplets on Janus pillars: From local reconfiguration to global transport. Sci. Adv. 2020, 6, eabb4540. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Li, Z.-B.; Kong, H.-Y.; He, J.-H. Fractal analysis of polar bear hairs. Therm. Sci. 2015, 19, 143–144. [Google Scholar] [CrossRef]
- Zhu, W.-H.; Pan, Y.-Y.; Li, Z.-B.; Wang, Q.-L. One-dimensional heat conduction equation of the polar bear hair. Therm. Sci. 2015, 19, 179–181. [Google Scholar] [CrossRef]
- Shi, N.N.; Tsai, C.-C.; Camino, F.; Bernard, G.D.; Yu, N.; Wehner, R. Keeping cool: Enhanced optical reflection and radiative heat dissipation in Saharan silver ants. Science 2015, 349, 298–301. [Google Scholar] [CrossRef]
- Ye, C.; Li, M.; Hu, J.; Cheng, Q.; Jiang, L.; Song, Y. Highly reflective superhydrophobic white coating inspired by poplar leaf hairs toward an effective “cool roof”. Energy Environ. Sci. 2011, 4, 3364–3367. [Google Scholar] [CrossRef]
- Woolley, J.T. Reflectance and transmittance of light by leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar] [CrossRef]
- Gates, D.M. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Pieruschka, R.; Huber, G.; Berry, J.A. Control of transpiration by radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 13372–13377. [Google Scholar] [CrossRef]
- Meng, H.H.; Gao, X.Y.; Huang, J.F.; Zhang, M.L. Plant phylogeography in arid Northwest China: Retrospectives and perspectives. J. Syst. Evol. 2015, 53, 33–46. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Q.; Du, M.; Zhang, H.; Jia, L.; Zhang, Z.; Ma, Z.; Sun, K. Micromorphological leaf epidermal traits as potential taxonomic markers for infrageneric classification of Oxytropis (Fabaceae). PhytoKeys 2022, 201, 51. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bei, Z.; Zhang, X.; Zhang, F.; Yan, X. The Response of Oxytropis aciphylla Ledeb. Leaf Interface to Water and Light in Gravel Deserts. Plants 2023, 12, 3922. https://doi.org/10.3390/plants12233922
Bei Z, Zhang X, Zhang F, Yan X. The Response of Oxytropis aciphylla Ledeb. Leaf Interface to Water and Light in Gravel Deserts. Plants. 2023; 12(23):3922. https://doi.org/10.3390/plants12233922
Chicago/Turabian StyleBei, Zhanlin, Xin Zhang, Fang Zhang, and Xingfu Yan. 2023. "The Response of Oxytropis aciphylla Ledeb. Leaf Interface to Water and Light in Gravel Deserts" Plants 12, no. 23: 3922. https://doi.org/10.3390/plants12233922
APA StyleBei, Z., Zhang, X., Zhang, F., & Yan, X. (2023). The Response of Oxytropis aciphylla Ledeb. Leaf Interface to Water and Light in Gravel Deserts. Plants, 12(23), 3922. https://doi.org/10.3390/plants12233922


.png)


