Growing of Phalaroides arundinacea L. and Bromopsis inermis Leyss for Biofuel Using Sewage Sludge Compost as a Fertilizer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biomass Dry Matter (DM) Yield of Perennial Herbaceous Plants
2.2. Plant Biomass Quality Parameters Which Determine Their Quality as a Solid Biofuel
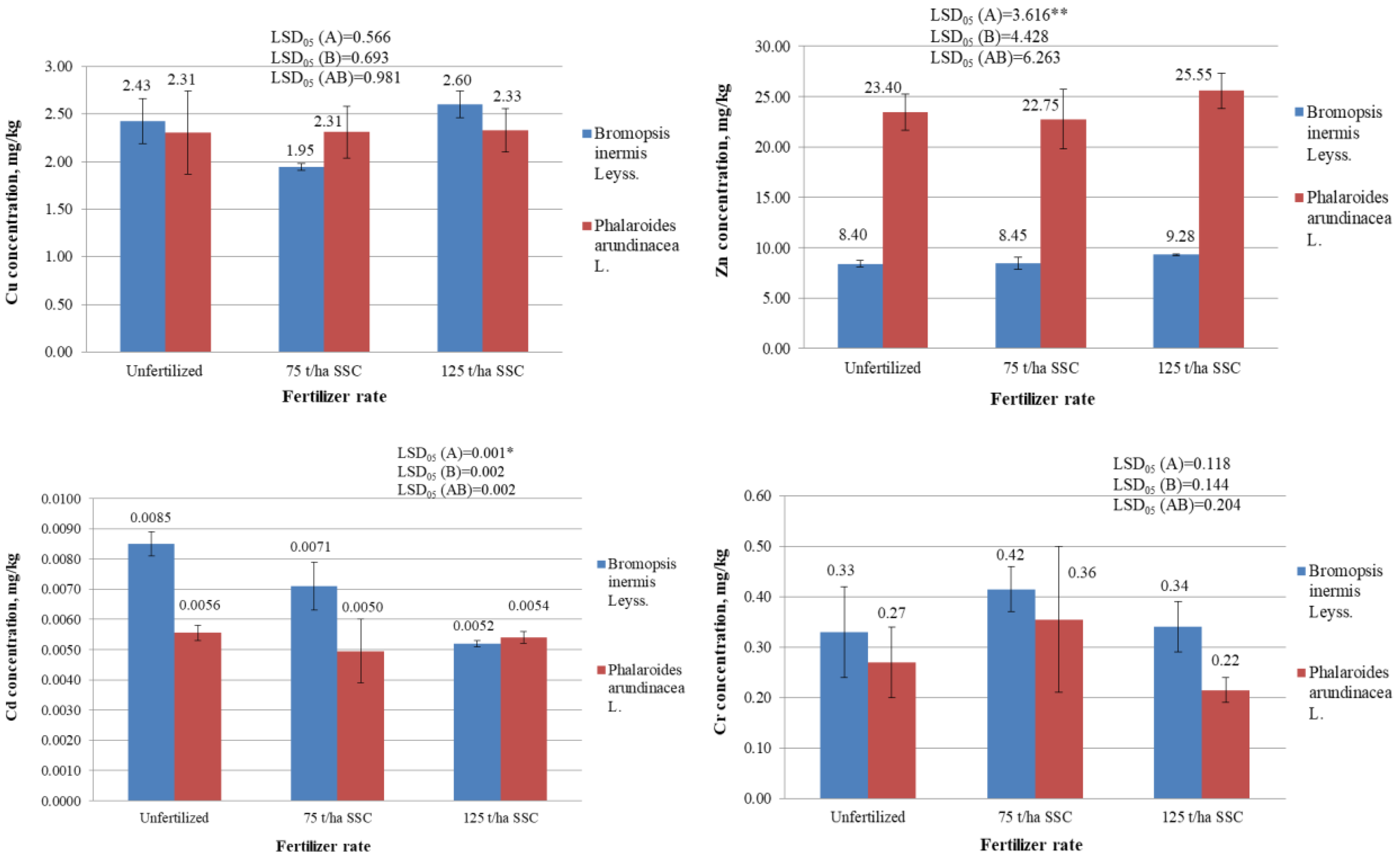
2.3. Concentration of Heavy Metals in Plant Biomass, mg/kg
2.3.1. Zinc (Zn) Concentration in Plant Biomass, mg/kg
2.3.2. Cadmium (Cd) Concentration in Plant Biomass, mg/kg
2.3.3. Lead (Pb) Concentration in Plant Biomass, mg/kg
2.3.4. Copper (Cu), Chromium (Cr), and Nickel (Ni) Concentration in Plant Biomass, mg/kg
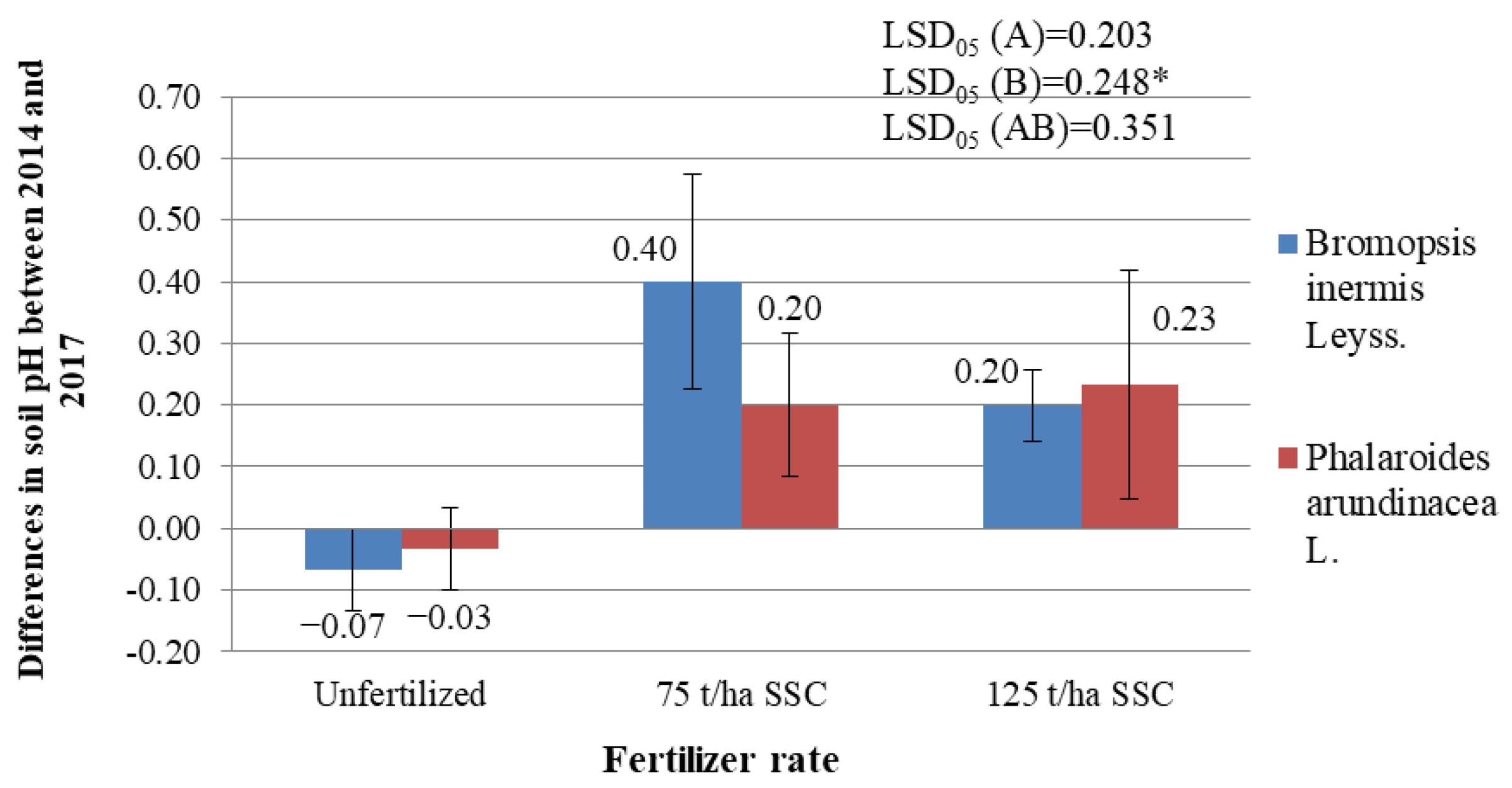
2.4. Differences in Values of the Agrochemical Parameters of the Soil between the Years 2014 and 2017
2.4.1. Differences in Soil pH
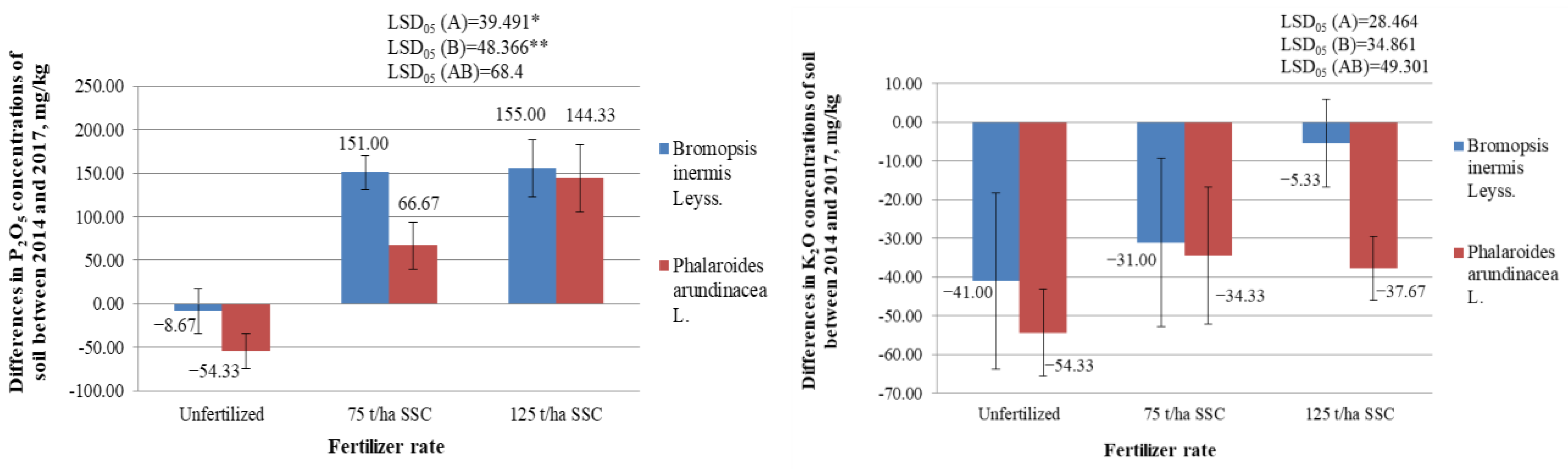
2.4.2. Differences in Mobile Phosphorus (P2O5) Concentrations in the Soil, mg/kg
2.4.3. Differences in Mobile Potassium (K2O) Concentrations in the Soil, mg/kg
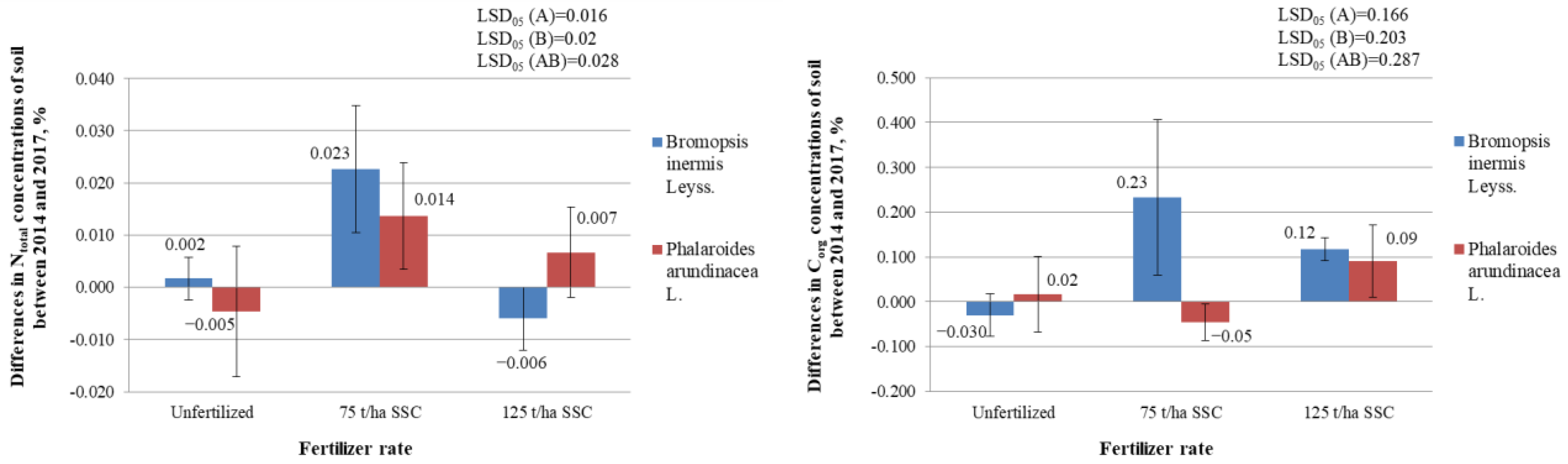
2.4.4. Differences in Total Nitrogen (Ntotal) (%) and in Organic Carbon (Corg.) (%) Concentrations in the Soil
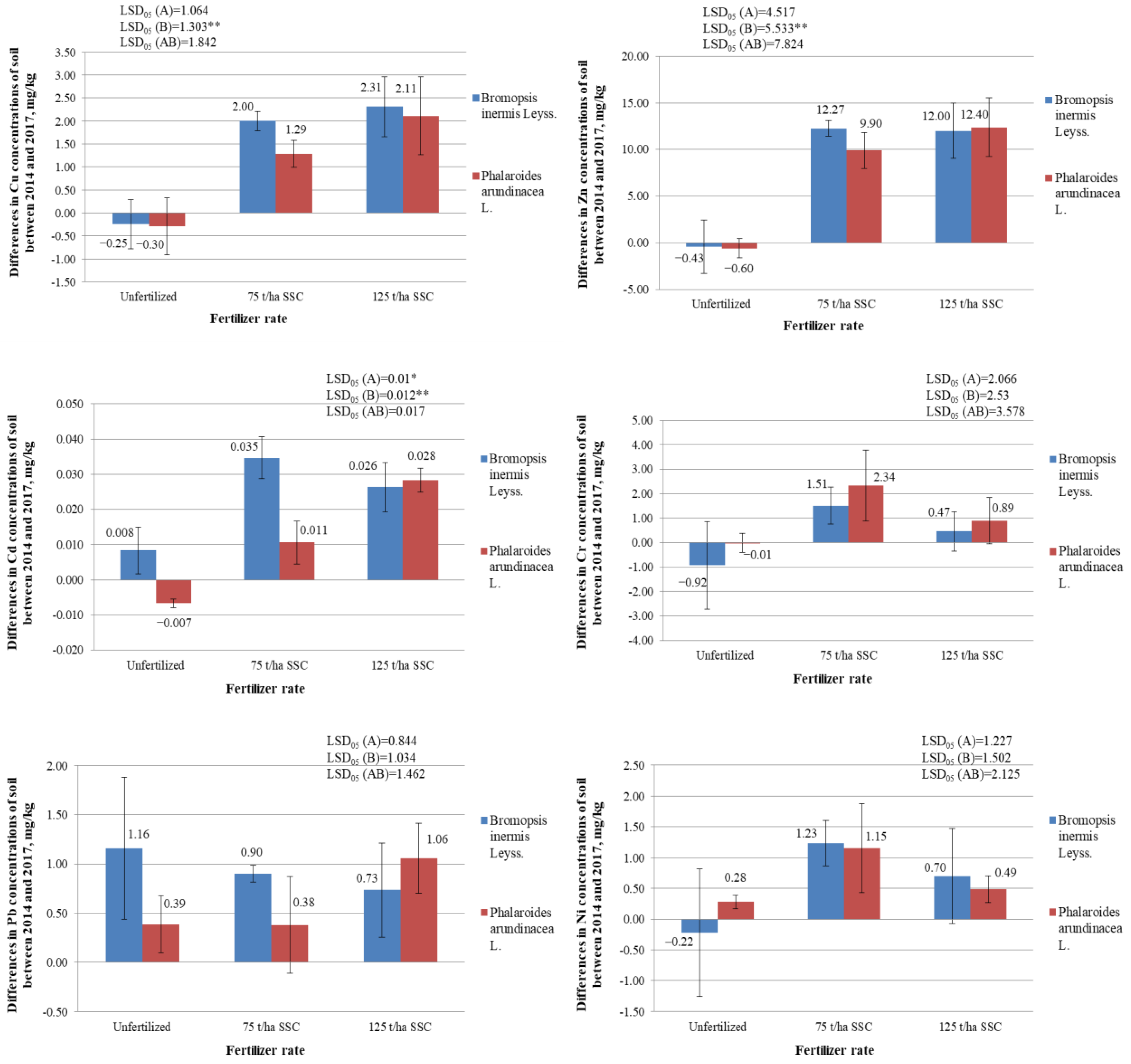
2.5. Differences in Concentrations of Heavy Metals (Cu, Zn, Cd, Cr, Pb, and Ni) in the Soil between the Years 2014 and 2017, mg/kg
2.5.1. Differences in Concentrations of Copper (Cu) in the Soil, mg/kg
2.5.2. Differences in Concentrations of Zinc (Zn) in the Soil, mg/kg
2.5.3. Differences in Concentrations of Cadmium (Cd) in the Soil, mg/kg
2.5.4. Differences in Concentrations of Chromium (Cr) in the Soil, mg/kg
2.5.5. Differences in Concentrations of Lead (Pb) in the Soil, mg/kg
2.5.6. Differences in Concentrations of Nickel (Ni) in the Soil, mg/kg
3. Materials and Methods
3.1. Field Experiment
- Bromopsis inermis Leyss. (variety ‘Galinda’);
- Phalaroides arundinacea L. (variety ‘Alaušas’).
- Unfertilized (control);
- N90P60K90 (90 kg/ha N, 60 kg/ha P2O5, 90 kg/ha K2O);
- 25 t/ha SSC DM;
- 75 t/ha SSC DM;
- 125 t/ha SSC DM.
3.2. Sewage Sludge Compost (SSC)
3.3. Plant Biomass Parameters
3.3.1. Biomass Dry Matter (DM) Yield of Perennial Herbaceous Plants
3.3.2. Plant Biomass Quality Parameters Which Determine Its Quality as a Solid Biofuel
3.3.3. Heavy Metals in Plant Biomass
3.4. Soil Parameters
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trejo, M.; Bhuyar, P.; Unpaprom, Y.; Dussadee, N.; Ramaraj, R. Advancement of fermentable sugars from fresh elephant ear plant weed for efficient bioethanol production. Environ. Dev. Sustain. 2022, 24, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Trejo, M.; Bhuyar, P.; Velu, G.; Pérez, E.Z.; Unpaprom, Y.; Trail, A.; Ramaraj, R. The effect of various pretreatments conditions on the distribution of fermentable sugar from dried elephant ear plant. Fuel 2022, 324 Pt B, 124624. [Google Scholar] [CrossRef]
- Lithuanian Energy Institute, Ministry of Education and Science. Atsinaujinantys Energijos šaltiniai. Mokslo ir Technologijų Populiarinimo Projekto “Apie Energija Mastyk Kitaip” Leidinys. 2008. Available online: http://www.lei.lt/_img/_up/File/atvir/erlic/index_files/Atsinaujinantys_energijos_saltiniai.pdf (accessed on 5 January 2016). (In Lithuanian).
- Sodsai, P.; Rachdawong, P. The current situation on CO2 emissions from the steel industry in Thailand and mitigation options. Int. J. Greenh. Gas Control 2012, 6, 48–55. [Google Scholar] [CrossRef]
- Dussadee, N.; Unpaprom, Y.; Ramaraj, R. Grass silage for biogas production. Adv. Silage Prod. Util. 2016, 16, 153. [Google Scholar]
- Sokka, L.; Sinkko, T.; Holma, A.; Manninen, K.; Pasanen, K.; Rantala, M.; Leskinen, P. Environmental impacts of the national renewable energy targets—A case study from Finland. Renew. Sustain. Energy Rev. 2016, 59, 1599–1610. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Life cycle assessment of bioenergy systems: State of the art and future challenges. Bioresour. Technol. 2011, 102, 437–451. [Google Scholar] [CrossRef] [PubMed]
- WP3 Economic Policy Instruments. Vilnius. 2007. Available online: http://www.ecuba.it/user/upload_inc_fls/D04_PTA_LEI_LT.pdf (accessed on 18 September 2023). (In Lithuanian).
- Lithuanian Biomass Energy Association LITBIOMA. Lietuvos Atsinaujinančių Energijos Išteklių Naudojimo Skatinimo Veiksmų Planas 2010–2020 m. Taikomasis Mokslinis Tyrimas. Ataskaita (galutinė). Vilnius. 2008. Available online: http://www.ena.lt/doc_atsi/Atsi_EI.pdf (accessed on 8 January 2016). (In Lithuanian).
- Ambrulevičius, R. Biomasės Deginimas Mažos Bei Vidutinės Galios Katilinėse ir Emisijų Problemos. Energetika 2010, 56, 103–109. (In Lithuanian) [Google Scholar]
- Njakou Djomo, S.; El Kasmioui, O.; De Groote, T.; Broeckx, L.S.; Verlinden, M.S.; Berhongaray, G.; Fichot, R.; Zona, D.; Dillen, S.Y.; King, J.S.; et al. Energy and climate benefits of bioelectricity from low-input short rotation woody crops on agricultural land over a two-year rotation. Appl. Energy 2013, 111, 862–870. [Google Scholar] [CrossRef]
- Keoleian, G.A.; Volk, T.A. Renewable energy from willow biomass crops: Life cycle energy, environmental and economic performance. Crit. Rev. Plant Sci. 2005, 24, 385–406. [Google Scholar] [CrossRef]
- Borkowska, H.; Molas, R. Two extremely different crops, Salix and Sida, as sources of renewable bioenergy. Biomass Bioenergy 2012, 36, 234–240. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, H.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) biomass for multiple energy purposes. Glob. Chang. Biol. Bioenergy 2016, 9, 202–214. [Google Scholar] [CrossRef]
- Djomo, S.N.; Ac, A.; Zenone, T.; De Groote, T.; Bergante, S.; Facciotto, G.; Sixto, H.; Ciria, P.C.; Weger, J.; Ceulemans, R. Energy performances of intensive and extensive short rotation cropping systems for woody biomass production in the EU. Renew. Sustain. Energy Rev. 2015, 41, 845–854. [Google Scholar] [CrossRef]
- Mažvila, J. The Productivity of Lithuania’s Land/Compiled; Lithuanian Research Centre for Agriculture and Forestry: Kėdainiai, Lithuania, 2011; 280p. (In Lithuanian) [Google Scholar]
- Lietuvos Respublikos Žemės Fondas. 2017. Available online: http://zis.lt/wp-content/uploads/2017/04/ZF_2017.pdf (accessed on 18 September 2023). (In Lithuanian).
- Bakšienė, E.; Titova, J. Effects of cultivation technologies on woody biomass yield of various willow (Salix spp.) cultivars. Zemdirb. Agric. 2018, 105, 339–348. [Google Scholar] [CrossRef]
- Titova, J.; Baltrėnaitė, E. Physical and Chemical Properties of Biochar Produced from Sewage Sludge Compost and Plants Biomass, Fertilized with that Compost, Important for Soil Improvement. Waste Biomass Valorization 2020, 12, 3781–3800. [Google Scholar] [CrossRef]
- Helby, P.; Rosenqvist, H.; Roos, A. Retreat from Salix. Swedish experience with energy crops in the 1990s. Biomass Bioenergy 2006, 30, 422–427. [Google Scholar] [CrossRef]
- Ericsson, K.; Rosenqvist, H.; Ganko, E.; Pisarek, M.; Nilsson, L. An agro-economic analysis of willow cultivation in Poland. Biomass Bioenergy 2006, 30, 16–27. [Google Scholar] [CrossRef]
- Mola-Yudego, B.; González-Olabarria, J.R. Mapping the expansion and distribution ofwillow plantations for bioenergy in Sweden: Lessons to be learned about the spread of energy crops. Biomass Bioenergy 2010, 34, 442–448. [Google Scholar] [CrossRef]
- Milovanović, J.; Babović, N.; Đorđević, A.; Spasić, S.; Marišová, E.; Končeková, L.; Kotrla, M.; Tóthová, M. External and Internal Factors Influencing the Growth and Biomass Production of Short Rotation Woods Genus Salix and Perennial Grass Miscanthus; Jureková, Z., Dražić, G., Eds.; Faculty of Applied Ecology FUTURA, Singidunum University: Belgrade, Serbia, 2011; 176p. [Google Scholar]
- Šarūnaitė, L.; Kadžiulienė, Ž.; Tilvikienė, V.; Lazauskas, S. Daugiametės Žolės Bioenergetikai; Baltijos Jūros Regiono Bioenergetikos Skatinimo Projekto Leidinys; Lithuanian Research Centre for Agriculture and Forestry: Akademija, Kėdainiai Distr., Lithuania, 2011; 9p. (In Lithuanian) [Google Scholar]
- Bivilienė, A. Lietuvoje Augančių Daugiamečių Žolių Genetinė Įvairovė; Spaudvita: Akademija, Kėdainiai District, Lithuania, 2010; 36p. (In Lithuanian) [Google Scholar]
- Gudžinskas, Z. Lietuvos Induočiai Augalai; Botanikos Instituto Leidykla: Vilnius, Lithuania, 1999; 212p. (In Lithuanian) [Google Scholar]
- Šedys, J. Lengvoms Dirvoms Tinkamų Daugiamečių Varpinių Žolių Selekcijai Rūšių Parinkimas. 1970–1976 m. Scientific Report. Vokė Branch, Institute of Agriculture: Vilnius, Lithuania, 1977. unpublished work. (In Lithuanian) [Google Scholar]
- Šedys, J. Beginklės Dirsuolės Veislė ‘Galinda‘. Veislės Išvedimo Vokės Filiale 1979–1992 Metais Ataskaita. Scientific Report. Vokė Branch, Institute of Agriculture: Vilnius, Lithuania, 1993. unpublished work. (In Lithuanian). [Google Scholar]
- Susawaengsup, C.; Jaradrattanapaiboon, A.; Sornsakdanuphap, J.; Choengpanya, K.; Jaradrattanapaiboon, Y.; Tongkoom, K.; Bhuyar, P. Effect of Fertilization Combined with Shading on Growth and Aromatic Constituents of Niamhom (Stro-bilanthes nivea Craib) Using an Internet of Things (IoT) Controlled Irrigation System. Horticulturae 2022, 8, 1130. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.; Aich, A.; Verma, A.K.; Bhuyar, P.; Nadda, A.K.; Mulla, S.I.; Kalia, S. Chicken Feather Waste Hydrolysate as a Potential Biofertilizer for Environmental Sustainability in Organic Agriculture Management. Waste Biomass Valorization 2023, 14, 2783–2799. [Google Scholar] [CrossRef]
- Ozdemir, S.; Turp, S.M.; Oz, N. Simultaneous drysorption of heavy metals by porous adsorbents during sludge composting. Environ. Eng. Res. 2020, 25, 258–265. [Google Scholar] [CrossRef]
- Quaye, A.K.; Volk, T.A. Biomass production and soil nutrients in organic and inorganic fertilized willow biomass production systems. Biomass Bioenergy 2013, 57, 113–125. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Popławska, A. The possibility of using sewage sludge for energy crop cultivation exemplified by reed canary grass and giant miscanthus. Soil Sci. Annu. 2019, 70, 21–33. [Google Scholar] [CrossRef]
- Kołodziej, B.; Antonkiewicz, J.; Bielińska, E.J.; Witkowicz, R.; Dubis, B. Recovery of microelements from municipal sewage sludge by reed canary grass and giant miscanthus. Int. J. Phytoremediat. 2022, 25, 441–454. [Google Scholar] [CrossRef]
- Wadzinska, B. Some effects of Sida hermaphrodita R. cultivation on sewage sludge. Pol. J. Environ. Stud. 2003, 12, 119–122. [Google Scholar]
- Kacprzak, M.; Ociepa, A.; Bien, J. The influence of soil fertilization on the amounts of ashes and contents of heavy metals in biomass ashes. Arch. Combust. 2010, 30, 125–131. [Google Scholar]
- Bowszys, T.; Wierzbowska, J.; Sternik, P.; Busse, M. Effect of the application of sewage sludge compost on the content and leaching of zinc and copper from soils under agricultural use. J. Ecol. Eng. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Sevel, L.; Nord-Larsen, T.; Ingerslev, M.; Jørgensen, U.; Raulund-Rasmussen, K. Fertilization of SRC Willow, I: Biomass Production Response. BioEnergy Res. 2013, 7, 319–328. [Google Scholar] [CrossRef]
- Phillips, C.J.; Marden, M.; Suzanne, L.M. Observations of root growth of young poplar and willow planting tipes. N. Z. J. For. Sci. 2014, 44, 1–15. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Lazauskas, J. Augalininkystė Lietuvoje 1895–1995 m; Lithuanian Institute of Agriculture: Vilnius, Lithuania, 1998; 388p. (In Lithuanian) [Google Scholar]
- Jakienė, E.; Liakas, V.; Klimas, E.; Bačkaitis, J. Energetinių Žolinių ir Sumedėjusių Augalų Auginimo Technologijos; Aleksandro Stulginskio Universitetas: Akademija, Lithuania, 2013; 199p. (In Lithuanian) [Google Scholar]
- Kadžiulienė, Ž.; Šarūnaitė, L.; Kryževičienė, A.; Stukonis, V.; Šlepetys, J.; Dabkevičius, Z. Žolinių augalų ir miško želdinių biomasės energinėms reikmėms formavimosi dinamika per pirmuosius penkerius metus. In Agrariniai ir Miškininkystės Mokslai: Naujausi Tyrimų Rezultatai ir Inovatyvūs Sprendimai; Mokslinės Konferencijos Pranešimų Rinkinis, Nr. 2; 2021; pp. 89–91. Available online: https://www.lammc.lt/data/public/uploads/2017/08/konferencijos-pranesimai-2012.pdf (accessed on 8 January 2016). (In Lithuanian)
- Vares, V.; Kask, U.; Muiste, P.; Pihu, T.; Soosaar, S. Biokuro Naudotojo Žinynas; Vares, V., Ed.; Žara: Vilnius, Lithuania, 2007; 169p. (In Lithuanian) [Google Scholar]
- Buinevičius, K. Tęršalų susidarymas biokuro degimo metu. Report material. In Proceedings of the Seminar “Biokuro Apskaitos Taisyklių Pakeitimai ir Naujienos 2015 m”, Kaune, Lietuva, 10 February 2015. (In Lithuanian). [Google Scholar]
- Buinevičius, K. Biokuro pelenai—Technologiniai ir gamtosauginiai aspektai. Report material. In Proceedings of the Tarptautinė Biomasės Energetikos Konferencija 2014 m, Vilnius, Lietuva, 4 November 2014. (In Lithuanian). [Google Scholar]
- Lietuvos Energetikos Institutas, Bioenergy Promotion ir Lietuvos Žemdirbystės Institutas. Kietoji Biomasė. Baltijos Jūros Regiono Bioenergetikos Skatinimo Projektas. 2009. Available online: http://www.lei.lt/_img/_up/File/atvir/bioenerlt/index_files/Bio.pdf (accessed on 9 February 2016). (In Lithuanian).
- Gold, V.M.; Gajevskij, N.A.; Golovanova, T.I.; Belonog, N.P.; Gorbaneva, T.B. Fiziologija Rastenij [Φизиoлoгия растений]; Krasnoyarsk, Russia, 2008; 148p, Available online: https://bio.sfu-kras.ru/files/1839_Konspekt_lekcii_Fiziologiya_rastenii.pdf (accessed on 17 November 2023). (In Russian)
- Adomaitis, T.R.; Antanaitis, A.; Eitminavičius, L.; Lubytė, J.; Matusevičius, K.; Mažvila, J. Sunkieji Metalai Lietuvos Dirvožemiuose ir Augaluose; Kitos spalvos: Kaunas, Lithuania, 2001; 343p. (In Lithuanian) [Google Scholar]
- Barber, S.A. Biologicheskaja Dostupnost‘ Pitatel‘nyh Veshestv v Pochve: Mechanicheskij Podhod; Chavkin, E.E., Ed.; Agropromizdat: Moscow, Russia, 1988; 376p. (In Russian) [Google Scholar]
- Bergman, W. Farbatlas. Ernahrungsstorungen bei Kulturpflanzen; VEB Gustav Fischer Verlag: Jena, Germany, 1986; 306p. [Google Scholar]
- Kabata–Pendias, A.; Pendias, X. Mikroelementy v Pochvah i Rasteniah; Saet, J.E., Ed.; 439 s; M. Mir.: Moscow, Russia, 1989. (In Russian) [Google Scholar]
- Rosikon, K.; Fijalkowski, K.; Kacprzak, M. Phytoremediation Potential of Selected Energetic Plants (Miscanthus giganteus L. and Phalaris arundinacea L.) in Dependence on Fertilization. J. Environ. Sci. Eng. A 2015, 4, 587–595. [Google Scholar] [CrossRef]
- Mažvila, J.; Staugaitis, G.; Vaišvila, Z.; Aleknavičius, P.; Juozokas, A.; Mockevičius, R.; Lukšienė, L. Lietuvos Žemės Našumas; Monografija: Akademija, Kėdainiai District, Lithuania, 2011; 280p. (In Lithuanian) [Google Scholar]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef]
- Order of the Minister of the Environment of the Republic of Lithuania. Dėl Lietuvos Respublikos Aplinkos Ministro 2001 m. Birželio 29 d. Įsakymas Nr. 349 “Dėl Normatyvinio Dokumento LAND 20-2001 “Nuotėkų Dumblo Naudojimo Tręšimui Reikalavimai” Patvirtinimo”; (The State Gazette, 2001, Nr. 61-2196); Lietuvos Respublikos Aplinkos Ministras: Vilnius, Lithuania, 2015. (In Lithuanian)
- Dudka, S.; Piotrowska, M.; Chlopecka, A. Effect of elevated concentracions of Cd and Zn in soil on spring wheat yield and the metal contens of the plants. Water Air Soil Pollut. 1994, 76, 333–341. [Google Scholar] [CrossRef]
- Kurienė, A. Chemija. Trumpas Chemijos Kursas; Gimtinė: Vilnius, Lithuania, 1999; 424p. (In Lithuanian) [Google Scholar]
- Ptašekas, R.; Ptašekas, M. Švinas: Toksikogenezė, Jos Tyrimai; Grybauskas, P., Ed.; Aldorija: Vilnius, Lithuania, 2002; 442p. (In Lithuanian) [Google Scholar]
- WRB. World reference base for soil resources. In World SoilResources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- ISO 11047:1998; Soil Quality Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc Flame and Electro-thermal Atomic Absorption Spectrometric Methods. International Organization for Standardization: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/24010.html (accessed on 5 October 2020).
- Vrubliauskas, S.; Pedišius, N. Kietojo biokuro standartizavimas ES ir Lietuvoje. Energetika 2005, 1, 16–22. (In Lithuanian) [Google Scholar]
- Miškinis, V.; Galinis, A.; Konstantinavičiūtė, I.; Norvaiša, E.; Tarvydas, D.; Lekavičius, V.; Alėbaitė, I. Kietojo Biokuro Apskaitos Energijos Gamybos Šaltiniuose Taisyklės; Scientific Report; Lithuanian Energy Institute: Kaunas, Lithuania, 2011; 70p, Available online: https://www.lsta.lt/files/studijos/2011%20metu/A-71_LEI_atask_Kietojo%20biokuro%20apskaita.pdf (accessed on 17 November 2023). (In Lithuanian)
- LST CEN/TS 14774-2:2007; Solid Biofuels—Methods for the Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Method. Lithuanian Standards Board: Vilnius, Lithuania, 2007.
- LST CEN/TS 15400:2007; Solid Recovered Fuels—Methods for the Determination of Calorific Value. Lithuanian Standards Board: Vilnius, Lithuania, 2007.
- Vares, V.; Kask, U.; Muiste, P.; Pihu, T.; Soosaar, S. Manual for Biofuel Users; Tallinn University of Technology: Tallinn, Estonia, 2005; p. 178. [Google Scholar]
- Tilvikiene, V.; Kadziuliene, Z.; Liaudanskiene, I.; Zvicevicius, E.; Cerniauskiene, Z.; Cipliene, A.; Raila, A.J.; Baltrusaitis, J. The quality and energy potential of introduced energy crops in northern part of temperate climate zone. Renew. Energy 2020, 151, 887–895. [Google Scholar] [CrossRef]
- ISO 10390:2005; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/40879.html (accessed on 30 April 2023).
- Egnér, H.; Riehm, H.; Domingo, W.R. Investigations on soil chemical analysis as a basis of the evaluation of plant nutrient status of soils II. Chemical extraction methods for phosphorus and potassium determination. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- LST ISO 11261:1995; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995. Available online: https://www.iso.org/standard/19239.html (accessed on 10 August 2021).
- ISO 10694:1995; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). ISO: Geneva, Switzerland, 1995. Available online: https://www.iso.org/standard/18782.html (accessed on 30 April 2023).
- Raudonius, S. Application of statistics in plant and crop research: Important issues. Zemdirb. Agric. 2017, 104, 377–382. [Google Scholar] [CrossRef]
- Clewer, A.G.; Scarisbrick, D.H. Practical Statistics and Experimental Desing for Plant and Crop Science; John Wiley & Sons, Ltd.: Chichester, UK, 2001; 332p. [Google Scholar]



| Fertilizer Rate | Biomass Yield (DM) | Total Biomass Yield (DM) of All Years of the Experiment | ||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | ||
| Bromopsis inermis Leyss. variety ‘Galinda’ | ||||
| Unfertilized | 0.83 | 1.27 | 1.78 | 3.88 |
| N90P60K90 | 2.55 | 5.15 | 7.19 | 14.89 |
| 25 t/ha SSC DM | 0.95 | 1.41 | 1.47 | 3.83 |
| 75 t/ha SSC DM | 0.98 | 1.88 | 2.19 | 5.05 |
| 125 t/ha SSC DM | 1.13 | 2.22 | 1.93 | 5.27 |
| Average | 1.29 | 2.38 | 2.91 | 6.59 |
| Phalaroides arundinacea L. variety ‘Alaušas’ | ||||
| Unfertilized | 0.40 | 1.32 | 2.11 | 3.83 |
| N90P60K90 | 1.41 | 4.42 | 8.75 | 14.58 |
| 25 t/ha SSC DM | 0.47 | 1.51 | 1.94 | 3.92 |
| 75 t/ha SSC DM | 0.44 | 1.96 | 2.43 | 4.83 |
| 125 t/ha SSC DM | 0.63 | 2.27 | 3.13 | 6.03 |
| Average | 0.67 | 2.30 | 3.67 | 6.64 |
| Ffact. | ||||
| Factor A | 67.2 ** | NI | 9.88 ** | NI |
| Factor B | 45.48 ** | 46.75 ** | 95.61 ** | 78.85 ** |
| Interaction A × B | 3.02 * | NI | NI | NI |
| LSD05 | ||||
| Species of plants (A) | 0.155 | 0.38 | 0.506 | 0.975 |
| Fertilization (B) | 0.244 | 0.601 | 0.8 | 1.542 |
| Interaction (A × B) | 0.346 | 0.85 | 1.132 | 2.181 |
| Bromopsis inermis | Bromopsis inermis | Phalaroides arundinacea | Phalaroides arundinacea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 m | II repetition | 2 | IV repetition | 1 | II repetition | 5 | IV repetition | 3 | |||
| 4 m | 4 | 4 | 3 | 1 | |||||||
| 4 m | 3 | 3 | 1 | 2 | |||||||
| 4 m | 1 | 2 | 4 | 5 | |||||||
| 4 m | 5 | 5 | 2 | 4 | |||||||
| 4 m | I repetition | 3 | III repetition | 4 | I repetition | 4 | III repetition | 3 | |||
| 4 m | 1 | 2 | 3 | 5 | |||||||
| 4 m | 2 | 5 | 1 | 1 | |||||||
| 4 m | 5 | 3 | 2 | 2 | |||||||
| 4 m | 4 | 1 | 5 | 4 | |||||||
| 4 m | 2 m | 4 m | 2 m | 4 m | 2 m | 4 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankuda, J.; Bakšienė, E.; Ražukas, A. Growing of Phalaroides arundinacea L. and Bromopsis inermis Leyss for Biofuel Using Sewage Sludge Compost as a Fertilizer. Plants 2023, 12, 3939. https://doi.org/10.3390/plants12233939
Ankuda J, Bakšienė E, Ražukas A. Growing of Phalaroides arundinacea L. and Bromopsis inermis Leyss for Biofuel Using Sewage Sludge Compost as a Fertilizer. Plants. 2023; 12(23):3939. https://doi.org/10.3390/plants12233939
Chicago/Turabian StyleAnkuda, Jelena, Eugenija Bakšienė, and Almantas Ražukas. 2023. "Growing of Phalaroides arundinacea L. and Bromopsis inermis Leyss for Biofuel Using Sewage Sludge Compost as a Fertilizer" Plants 12, no. 23: 3939. https://doi.org/10.3390/plants12233939
APA StyleAnkuda, J., Bakšienė, E., & Ražukas, A. (2023). Growing of Phalaroides arundinacea L. and Bromopsis inermis Leyss for Biofuel Using Sewage Sludge Compost as a Fertilizer. Plants, 12(23), 3939. https://doi.org/10.3390/plants12233939






