Effects of Low-Light Environments on the Growth and Physiological and Biochemical Parameters of Indocalamus and Seasonal Variations in Leaf Active Substance Contents
Abstract
:1. Introduction
2. Materials and Methods
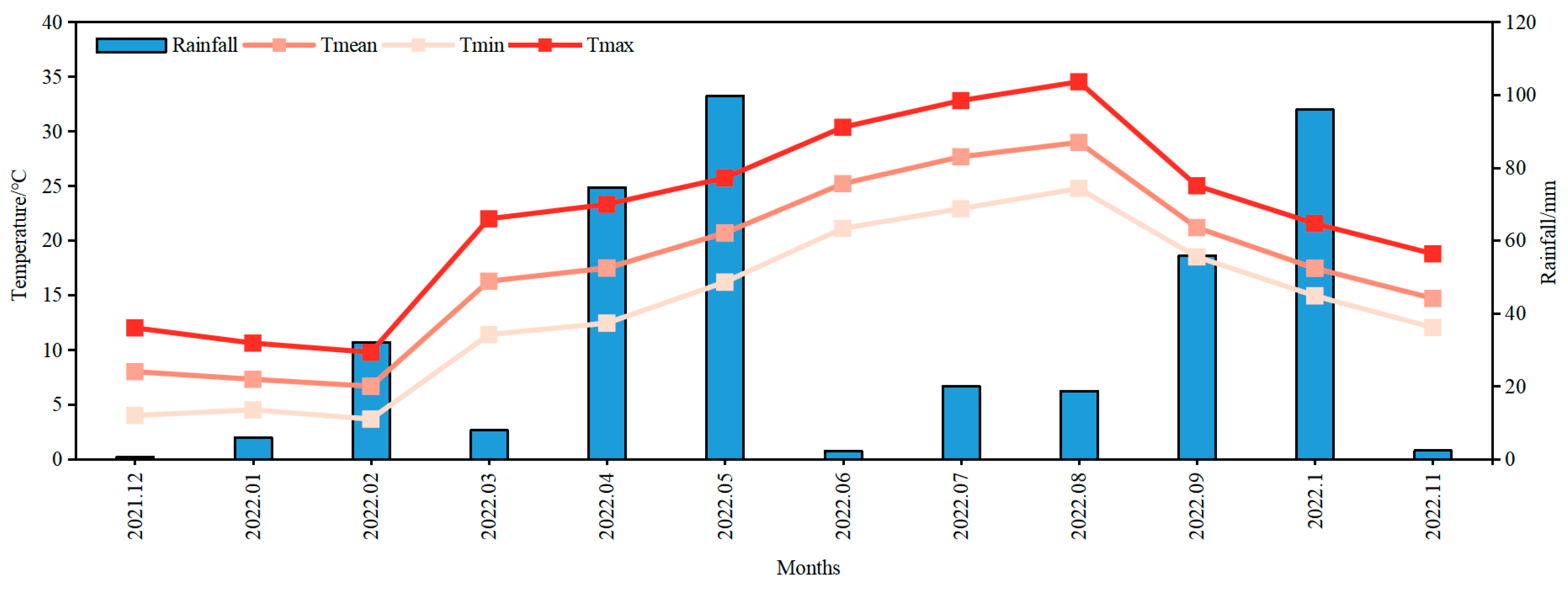
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design
2.3. Measurement Methods
2.3.1. Observation of the Number of Bamboo Shoots
2.3.2. Leaf Morphology and Quantification
2.3.3. Photosynthetic Parameters
2.3.4. Leaf Active Substances
2.4. Statistical Analysis
3. Results
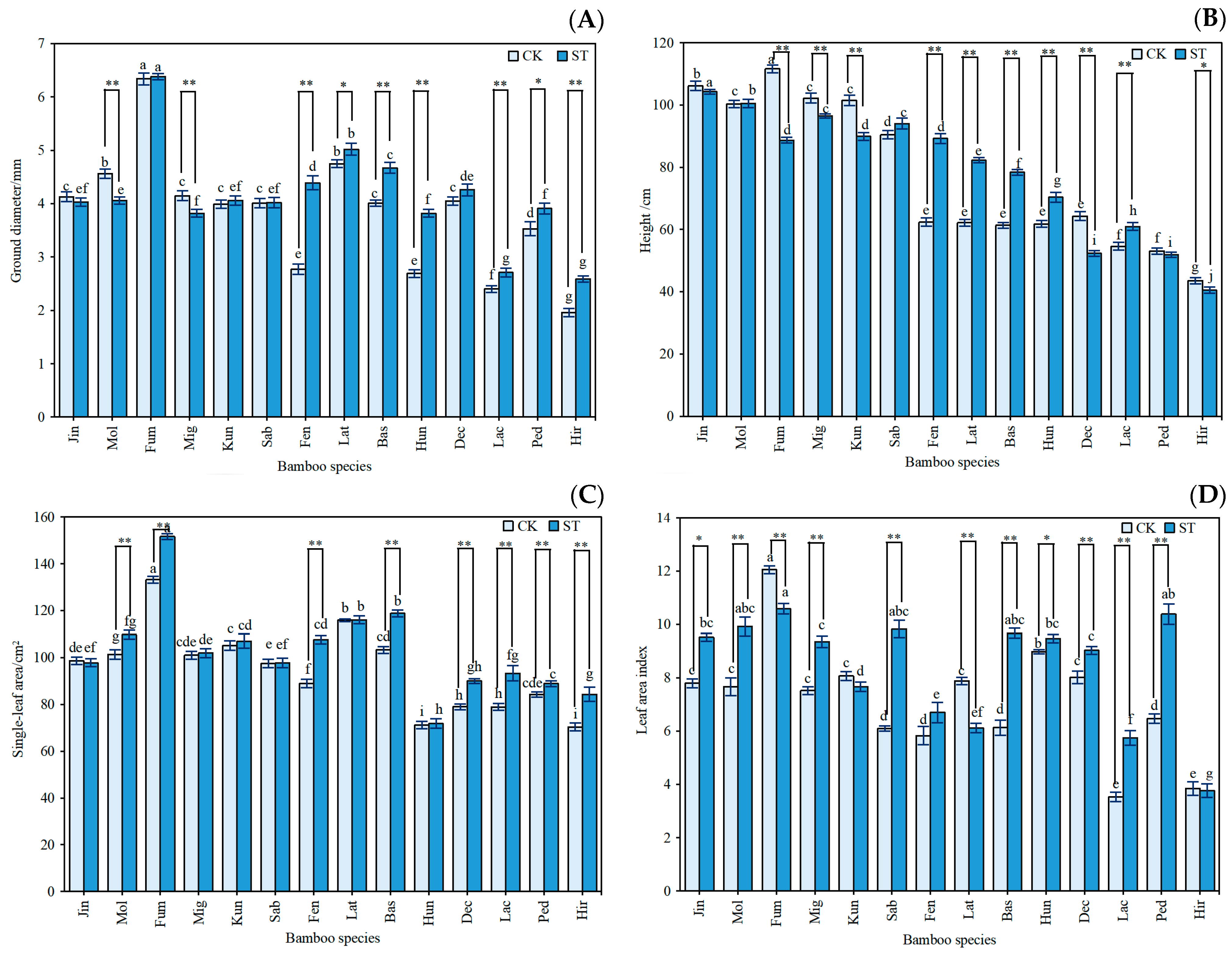
3.1. Growth Characteristics and Photosynthetic Capacity of Indocalamus
3.2. Morphological Characteristics of Indocalamus
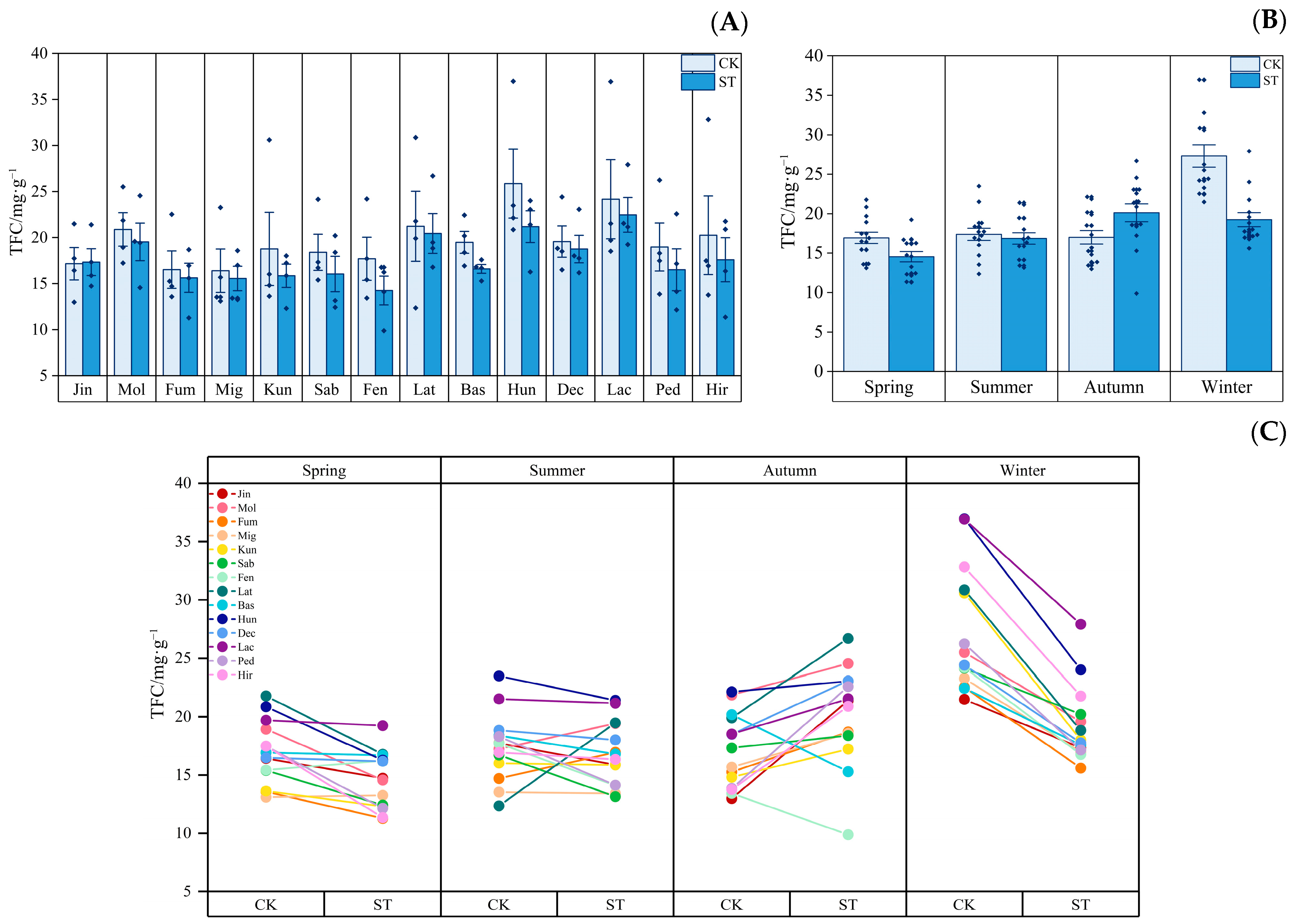
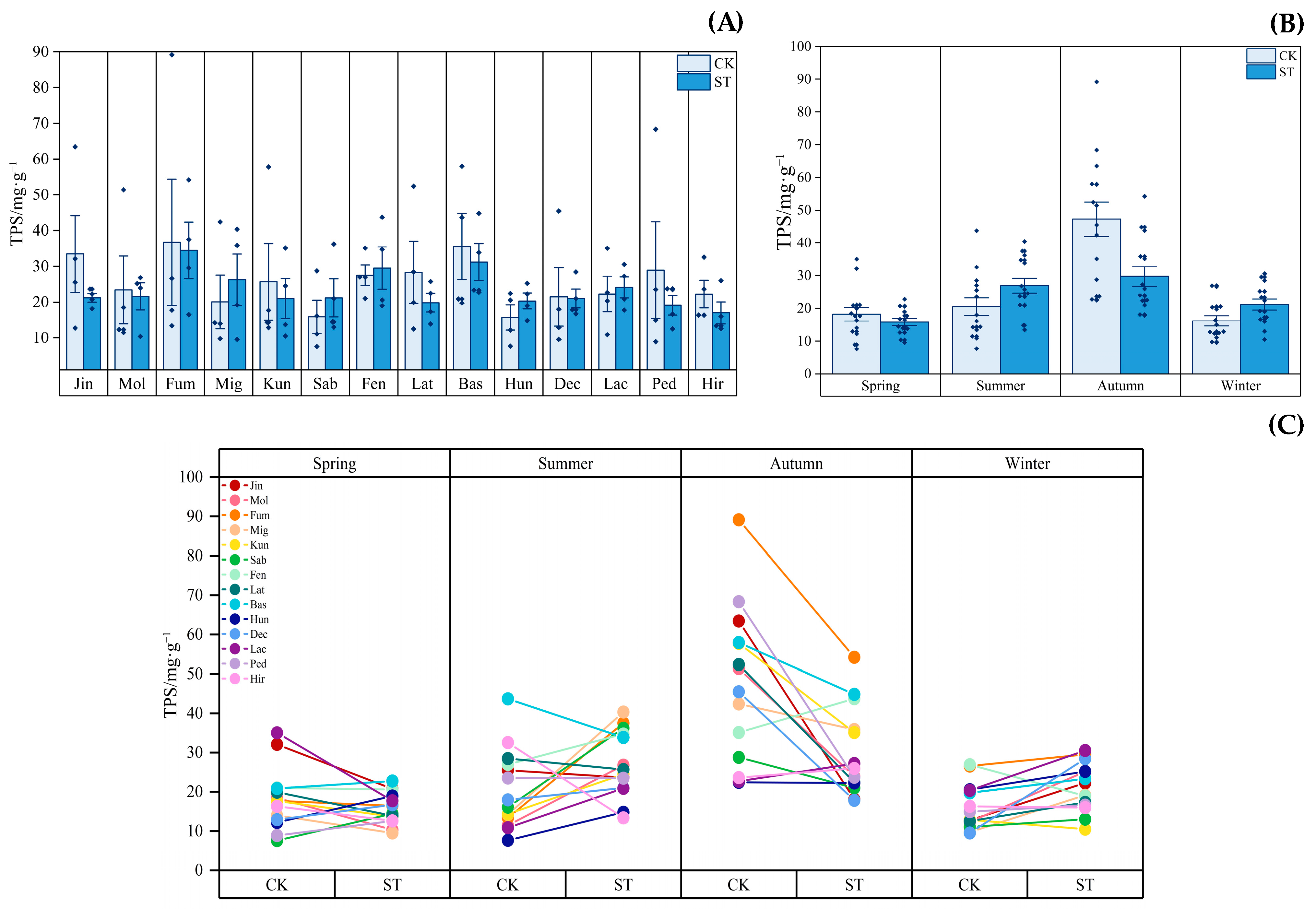
3.3. Dynamics of Leaf Active Substances
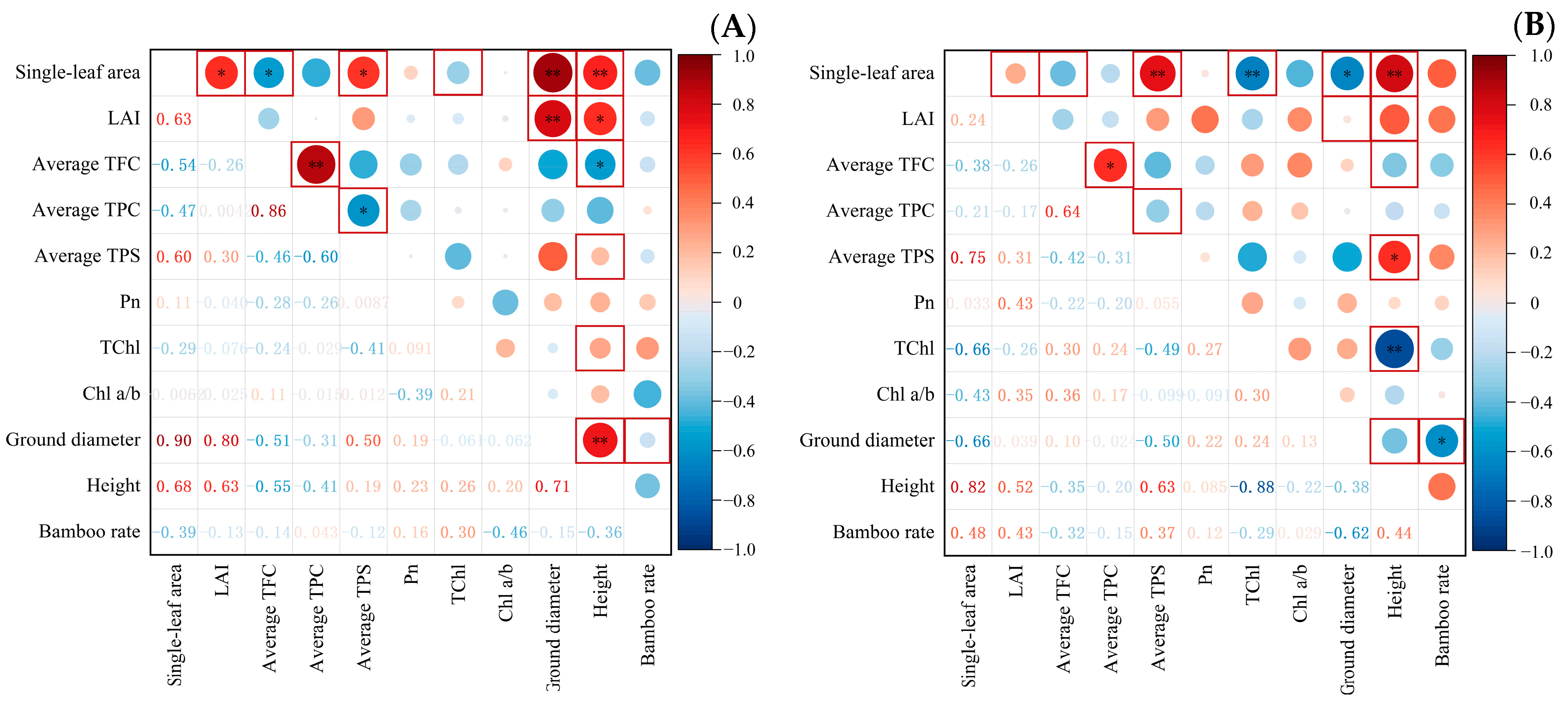
3.4. Correlation Analysis among Indicators
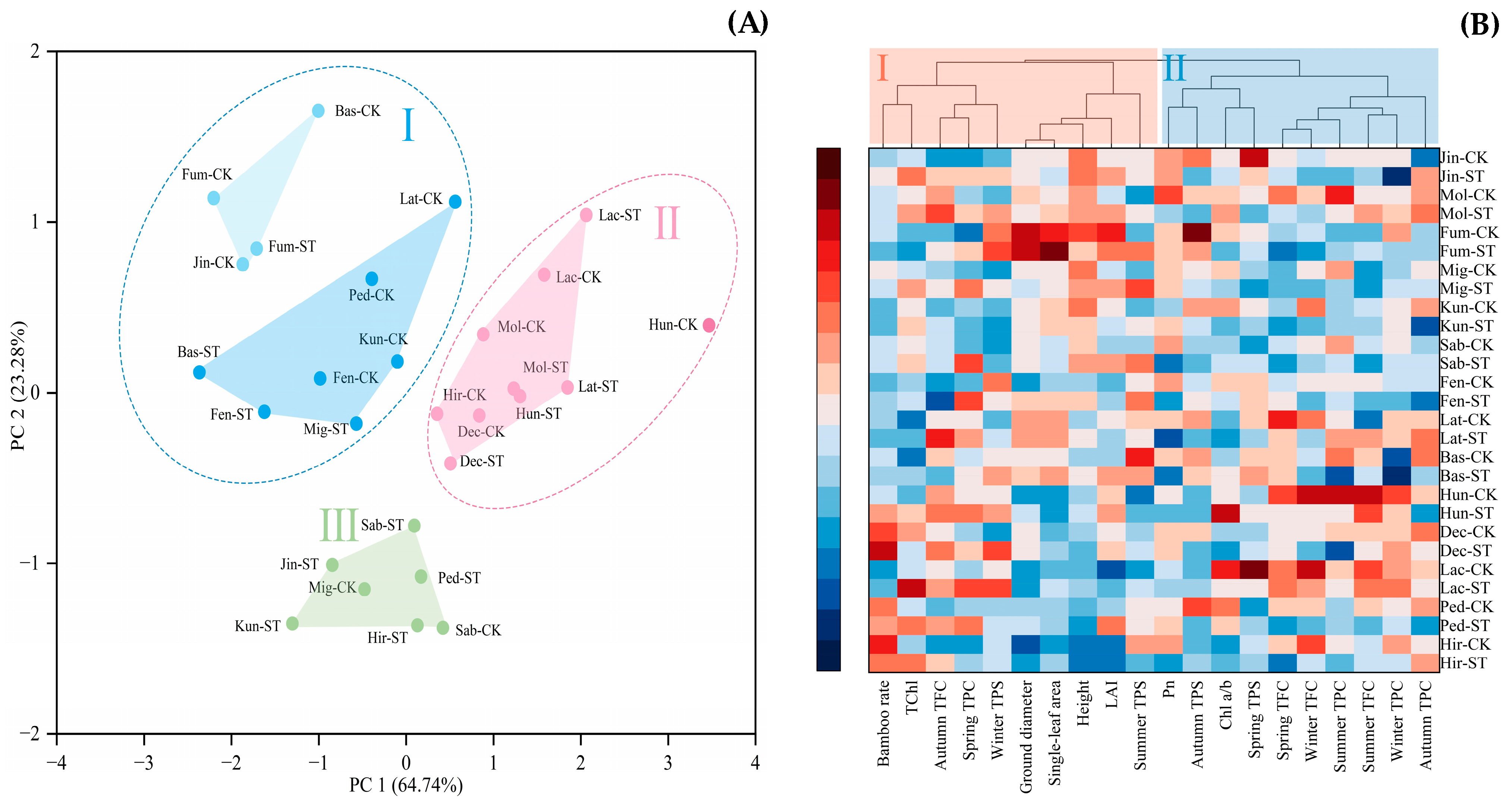
3.5. Principal Component Analysis of the Active Substances and Cluster Analysis of the Indicators
4. Discussion
4.1. Growth Morphology and Photosynthetic Capacity of Indocalamus in Response to Different Light Environments
4.2. Response of Active Substances in the Leaves of Indocalamus to Different Light Environments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| LAI | leaf area index |
| Pn | net photosynthetic rate (μmol CO2·m−2·s−1) |
| TChl | total chlorophyll (mg·g−1) |
| Chl a/b ratio | chlorophyll a/b ratio |
| TFC | total flavonoid (mg·g−1) |
| TPC | total polyphenol (mg·g−1) |
| TPS | total polysaccharide (mg·g−1) |
Appendix A
| Origin | Bamboo Specie | Abbreviations | Photos | Characterisation |
|---|---|---|---|---|
| Songming County, Yunnan, China | I. jinpingensis | Jin |  | Culms 1.5–2.0 m tall, 0.4–0.8 cm in diameter; leaf blades oblong-lanceolate, 17–27 cm long, 3.5–5.5 cm wide |
| I. guangdongensis var. mollis | Mol |  | Culms 1.5–3.0 m tall, 0.9–1.5 cm in diameter; leaf blades broadly lanceolate, 15–35 cm long, 4.0–6.5 cm wide | |
| I. kunmingensis cv. fuminer | Fum |  | Culms 2.0–3.0 m tall, 0.8–1.8 cm in diameter; leaf blades oblong-lanceolate, 14–40 cm long, 2.2–7.0 cm wide | |
| I. migoi | Mig |  | Culms 1.5–2.5 m tall, 0.5–1.2 cm in diameter; leaf blades oblong-lanceolate, 20–38 cm long, 3.5–8.0 cm wide | |
| I. kunmingensis | Kun |  | Culms 1.5–2.5 m tall, 0.5–1.0 cm in diameter; leaf blades oblong-lanceolate, 15–35 cm long, 3.0–6.0 cm wide | |
| I. sabaensis | Sab |  | Culms 1.0–1.8 m tall, 0.5–1.0 cm in diameter; leaf blades broadly lanceolate, 20–38 cm long, 3.0–7.5 cm wide | |
| I. fengsaliensis | Fen |  | Culms 1.2–2.0 m tall, 0.7–1.0 cm in diameter; leaf blades broadly lanceolate, 25–38 cm long, 3.5–8.0 cm wide | |
| I. pedalis | Ped |  | Culms 30–60 cm tall, 0.2–0.4 cm in diameter; leaf blades oblong-lanceolate, 6.5–28 cm long, 1.0–4.0 cm wide | |
| Anji County, Zhejiang Province, China | I. latifolius | Lat |  | Culms 1.2–2.0 m tall, 0.5–1.5 cm in diameter; leaf blades oblong-lanceolate, 10–45 cm long, 2.0–9.0 cm wide |
| I. bashanensis | Bas |  | Culms 1.5–3.0 m tall, 1.0–1.5 cm in diameter; leaf blades oblong-lanceolate, 25–36 cm long, 3.0–8.0 cm wide | |
| I. hunanensis | Hun |  | Culms 1.0–1.8 m tall, 0.4–0.8 cm in diameter; leaf blades linear-lanceolate, 10–28 cm long, 4.5–7.5 cm wide | |
| I. decorus | Dec |  | Culms 35–80 cm tall, 0.3–0.5 cm in diameter; leaf blades oblong-lanceolate, 15–35 cm long, 3.0–5.5 cm wide | |
| I. lacunosus | Lac |  | Culms 30–70 cm tall, 0.4–0.6 cm in diameter; leaf blades linear-lanceolate, 10–20 cm long, 3.0–5.0 cm wide | |
| I. hirtinodus | Hir |  | Culms 30–50 cm tall, 0.2–0.4 cm in diameter; leaf blades oblong-lanceolate, 8.0–15 cm long, 3.0–5.0 cm wide |
References
- Cui, J.; Yue, Y.D.; Tang, F.; Wang, J. HPTLC Analysis of the Flavonoids in Eight Species of Indocalamus Leaves. JPC-J. Planar Chromatogr.-Mod. TLC 2011, 24, 394–399. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, J.F.; Liu, Z.G.; Liu, T.X.; Yue, X.Q.; Yang, J.C. Research of the Processing Technology and Quality Standard of Indocalamus latifolius Leaves. Adv. Mater. Res. 2013, 773, 755–762. [Google Scholar] [CrossRef]
- Sun, J.; Yue, Y.; Tang, F.; Guo, X. Simultaneous HPTLC analysis of flavonoids in the leaves of three different species of bamboo. JPC-J. Planar Chromatogr.-Mod. TLC 2010, 23, 40–45. [Google Scholar] [CrossRef]
- Jin, S.L.; Yin, Y.G. In vivo antioxidant activity of total flavonoids from Indocalamus leaves in aging mice caused by D-galactose. Food Chem. Toxicol. 2012, 50, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Santosh, O.; Bajwa, H.K.; Bisht, M.S.; Chongtham, N. Application of Bamboo in the Food and Pharmaceutical Industry. In Biotechnological Advances in Bamboo; Springer: Berlin/Heidelberg, Germany, 2021; pp. 401–429. [Google Scholar]
- Zhang, Y.; He, L.; Li, Q.; Cheng, J.; Wang, Y.; Zhao, J.; Yuan, S.; Chen, Y.; Shi, R. Optimization of ultrasonic-assisted deep eutectic solvent for the extraction of polysaccharides from Indocalamus tessellatus leaves and their biological studies. Sustain. Chem. Pharm. 2022, 30, 100855. [Google Scholar] [CrossRef]
- Aribam, I.; Oinam, S.; Koul, A.; Chongtham, N. Comparative assessment of the antioxidant potential of bamboo leaves, along with some locally and commercially consumed beverages in India. Adv. Bamboo Sci. 2022, 1, 100007. [Google Scholar]
- Gong, J.; Xia, D.; Huang, J.; Ge, Q.; Mao, J.; Liu, S.; Zhang, Y. Functional components of bamboo shavings and bamboo leaf extracts and their antioxidant activities in vitro. J. Med. Food 2015, 18, 453–459. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z. Study on creation of an Indocalamus leaf flavor. Food Sci. Technol. 2015, 35, 647–651. [Google Scholar] [CrossRef]
- Sun, J.; Xun, H.; Yu, J.; Tang, F.; Yue, Y.; Guo, X. Chemical Constituents and Antibacterial Properties of Indocalamus latifolius McClure Leaves, the Packaging Material for ‘Zongzi’. Molecules 2015, 20, 15686–15700. [Google Scholar] [CrossRef]
- Ramovs, B.; Roberts, M. Understory vegetation and environment responses to tillage, forest harvesting, and conifer plantation development. Ecol. Appl. 2003, 13, 1682–1700. [Google Scholar] [CrossRef]
- Naidu, S.L.; DeLucia, E.H. Growth, Allocation and Water Relations of Shade-grown Quercus rubra L. Saplings Exposed to a Late-season Canopy Gap. Ann. Bot. 1997, 80, 335–344. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E.; Valladares, F. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 2010, 1206, 35–55. [Google Scholar] [CrossRef]
- Collins, B.; Wein, G. Stem Elongation Response to Neighbour Shade in Sprawling and Upright Polygonum Species. Ann. Bot. 2000, 86, 739–744. [Google Scholar] [CrossRef]
- Mielke, M.S.; Schaffer, B. Leaf gas exchange, chlorophyll fluorescence, and pigment indexes of Eugenia uniflora L. in response to changes in light intensity and soil flooding. Tree Physiol. 2010, 30, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic plasticity for plant development, function, and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Fang, S.; Shang, X.; Li, Y. Influence of provenance and shade on biomass production and triterpenoid accumulation in Cyclocarya paliurus. Agrofor. Syst. 2019, 93, 483–492. [Google Scholar] [CrossRef]
- Ahmad, E.; Arshad, M.; Khan, M.; Amjad, M.S.; Sadaf, H.; Riaz, I.; Sabir, S.; Ahmad, N.; Sabaoon. Secondary metabolites and their multidimensional prospective in plant life. J. Pharmacogn. Phytochem. 2017, 205, 205–214. [Google Scholar]
- Ni, Y.W.; Lin, K.H.; Chen, K.H.; Wu, C.W.; Chang, Y.S. Flavonoid Compounds and Photosynthesis in Passiflora Plant Leaves under Varying Light Intensities. Plants 2020, 9, 633. [Google Scholar] [CrossRef]
- Prinsloo, G.; Nogemane, N. The effects of season and water availability on chemical composition, secondary metabolites and biological activity in plants. Sci. Total Environ. 2018, 17, 889–902. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, Z.-S.; Cheng, F.; Ruan, X.; Jiang, D.-A.; Pan, C.-D.; Wang, Q. Seasonal Dynamics of Metabolites in Needles of Taxus wallichiana var. mairei. Molecules 2016, 21, 1403. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of chemical composition, antioxidant activity, and the effects of alfalfa flavonoids on growth performance. Oxidative Med. Cell. Longev. 2020, 2020, 11. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, C.; Zhao, L. Extraction, purification, and antioxidant activity of polysaccharides from bamboo leaves. J. For. Res. 2012, 23, 139–143. [Google Scholar] [CrossRef]
- Ferreira, J.; Schmildt, E.; Schmildt, O.; Cattaneo, L.; Alexandre, R.; Cruz, C. Comparison of methods for classification of the coefficient of variation in papaya. Rev. Ceres 2016, 63, 138–144. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar]
- Hallik, L.; Niinemets, U.; Kull, O. Photosynthetic acclimation to light in woody and herbaceous species: A comparison of leaf structure, pigment content, and chlorophyll fluorescence characteristics measured in the field. Plant Biol. 2012, 14, 88–99. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological limits to plant phenotypic plasticity. N. Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, T.; Tarin, M.W.K.; Han, Y.; Hu, W.; Rong, J.; He, T.; Zheng, Y. Effect of Various Mulch Materials on Chemical Properties of Soil, Leaves and Shoot Characteristics in Dendrocalamus Latiflorus Munro Forests. Plants 2021, 10, 2302. [Google Scholar] [CrossRef]
- Fan, L.; Li, B.; Han, Y.; Chen, L.; He, T.; Zheng, Y.; Rong, J. Lower light intensities increase shoot germination with improved leaf biosynthesis in Ma bamboo (Dendrocalamus latiflorus Munro). Forests 2022, 13, 1723. [Google Scholar] [CrossRef]
- March, R.H.; Clark, L.G. Sun-shade variation in bamboo (Poaceae: Bambusoideae) leaves. Telopea 2011, 13, 93–104. [Google Scholar] [CrossRef]
- Mulkey, S.S. Photosynthetic Acclimation and Water-Use Efficiency of Three Species of Understory Herbaceous Bamboo (Gramineae) in Panama. Oecologia 1986, 70, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Sun, M.; Zhang, Y.-J.; Cochard, H.; Cao, K.-F. Strong leaf morphological, anatomical, and physiological responses of a subtropical woody bamboo (Sinarundinaria nitida) to contrasting light environments. Plant Ecol. 2014, 215, 97–109. [Google Scholar] [CrossRef]
- Terashima, I.; Miyazawa, S.I.; Hanba, Y. Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J. Plant Res. 2001, 114, 93–105. [Google Scholar] [CrossRef]
- Fan, L.; Tarin, M.; Hu, W.; Han, Y.; Rong, J.; Chen, L.; He, T.; Zheng, Y. Changes in light intensity affect leaf gas exchange, chlorophyll fluorescence, and nonstructural carbohydrates of Ma bamboo (Dendrocalamus latiflorus Munro). Appl. Ecol. Environ. Res. 2022, 20, 1269–1284. [Google Scholar] [CrossRef]
- Lei, T.T.; Koike, T. Functional leaf phenotypes for shaded and open environments of a dominant dwarf bamboo (Sasa senanensis) in northern Japan. Int. J. Plant Sci. 1998, 159, 977–987. [Google Scholar] [CrossRef]
- Smith, M.C.; Mack, R.N. Shade tolerance of temperate Asian bamboos: A harbinger of their naturalization in Pacific Northwest coniferous forests? Biol. Invasions 2013, 15, 2081–2093. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chapter Six-The biochemistry, physiology, and evolution of the chlorophyll cycle. Adv. Bot. Res. 2019, 90, 183–212. [Google Scholar]
- Su, C.H.; Liu, G.H.; Wang, F.S.; Ding, Y.L.; Xue, J.H. Several changes of Indocalamus leaf active ingredients contents. J. Appl. Ecol. 2011, 22, 2471–2476. [Google Scholar]
- Kitamura, S. Transport of Flavonoids: From Cytosolic Synthesis to Vacuolar Accumulation. In The Science of Flavonoid; Springer: Berlin/Heidelberg, Germany, 2006; pp. 123–146. [Google Scholar]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Linatoc, A.C.; Abu Bakar, M.F.; Ibrahim, Z.T.; Audu, Y. Effect of Light Quality and Quantity on the Accumulation of Flavonoid in Plant Species. J. Sci. Technol. 2018, 10, 3. [Google Scholar] [CrossRef]
- Angmo, P.; Phuntsog, N.; Namgail, D.; Chaurasia, O.P.; Stobdan, T. Effect of shading and high temperature amplitude in greenhouse on growth, photosynthesis, yield and phenolic contents of tomato (Lycopersicum esculentum Mill.). Physiol. Mol. Biol. Plants 2021, 27, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Partial Solar Radiation Exclusion with Color Shade Nets Reduces the Degradation of Organic Acids and Flavonoids of Grape Berry (Vitis vinifera L.). J. Agric. Food Chem. 2017, 65, 10693–10702. [Google Scholar] [CrossRef] [PubMed]
- Szymborska-Sandhu, I.; Przybył, J.L.; Pióro-Jabrucka, E.; Jędrzejuk, A.; Węglarz, Z.; Bączek, K. Effect of Shading on Development, Yield and Quality of Bastard Balm Herb (Melittis melissophyllum L.). Molecules 2020, 25, 2142. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, S.; Wu, X.; Zhang, J.; He, A.; Zhao, G.; Yang, X. Effect of Light Intensity on Leaf Photosynthetic Characteristics and Accumulation of Flavonoids in Lithocarpus litseifolius (Hance) Chun. (Fagaceae). Open J. For. 2016, 6, 445–459. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, Y.; Xu, G.; Gao, Q.; Gong, L.; Zhang, Y. Influence of Harvest Season and Drying Method on the Antioxidant Activity and Active Compounds of Two Bamboo Grass Leaves. J. Food Process. Preserv. 2014, 38, 1565–1576. [Google Scholar] [CrossRef]
- Ko, H.C.; Lee, J.Y.; Jang, M.G.; Song, H.; Kim, S.J. Seasonal variations in the phenolic compounds and antioxidant activity of Sasa quelpaertensis. Ind. Crops Prod. 2018, 122, 506–512. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Yang, Z.; Shi, R.; Li, Q.; Wang, Y.; He, L.; Chen, Y. Analysis of Biochemical Components and Antioxidant Capacity of Indocalamus Leaves of Different Varieties. Sci. Technol. Food Ind. 2022, 43, 93–100. [Google Scholar]
- Ntobela, B.; Oguntibeju, O.O.; Rautenbach, F.; Etsassala, N.G.; Akinpelu, E.A.; Nchu, F. Evaluating the effect of 40% and 0% shading levels on the secondary metabolites, antifungal and anti-insect activities of extracts of Allium porrum cultivated hydroponically under greenhouse conditions. J. Med. Plants Econ. Dev. 2022, 6, a144. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Poledica, M.; Starace, G.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Pearl Grey Shading Net Boosts the Accumulation of Total Carotenoids and Phenolic Compounds That Accentuate the Antioxidant Activity of Processing Tomato. Antioxidants 2021, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Neményi, A.; Stefanovitsné-Bányai, É.; Burján, S.S.; Pék, Z.; Hegedus, A.; Gyuricza, C.; Helyes, L. Seasonal Variations in Total Antioxidant Capacity and Total Phenolics Content of Leaves of Phyllostachys Taxa Using Different Extraction Methods. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 43–50. [Google Scholar] [CrossRef]
- Ni, Q.; Xu, G.; Wang, Z.; Gao, Q.; Wang, S.; Zhang, Y. Seasonal Variations of the Antioxidant Composition in Ground Bamboo Sasa argenteastriatus Leaves. Int. J. Mol. Sci. 2012, 13, 2249–2262. [Google Scholar] [CrossRef]
- Zaman, S.; Shen, J.; Wang, S.; Wang, Y.; Ding, Z.; Song, D.; Wang, H.; Ding, S.; Pang, X.; Wang, M. Effects of Shading Nets on Reactive Oxygen Species Accumulation, Photosynthetic Changes, and Associated Physiochemical Attributes in Promoting Cold-Induced Damage in Camellia sinensis (L.) Kuntze. Horticulturae 2022, 8, 637. [Google Scholar] [CrossRef]
- Quiñones-Muñoz, T.A.; Villanueva-Rodríguez, S.J.; Torruco-Uco, J.G. Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms. Metabolites 2022, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Li, S.J.; Li, J.J. Comparison of Chemical Components in Leaves of Indocalamus. Food Res. Dev. 2008, 29, 7–10. [Google Scholar]
- Yu, X.; Ming, X.; Xiong, M.; Zhang, C.; Yue, L.; Yang, L.; Fan, C. Partial shade improved the photosynthetic capacity and polysaccharide accumulation of the medicinal plant Bletilla ochracea Schltr. Photosynthetica 2022, 60, 168–178. [Google Scholar] [CrossRef]
- Ma, Q.-H.; Wang, X.-H.; Fan, Y.-G.; Bian, L.-S.; Chen, L.; Liu, H.-H.; Ren, F.; Zhang, Y.-A. Effect of shading and forest type on morphological characteristics and bioactive compounds of fruiting bodies of Songshan Lingzhi Ganoderma tsugae (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiang, W.; Silva, E.N.; Mao, L.; Hannaway, D.; Lu, H. Optimization of shade condition and harvest time for Dendrobium candidum plants based on leaf gas exchange, alkaloids and polysaccharides contents. Plant Omics 2012, 5, 253–260. [Google Scholar]
- Acheampong, K.; Hadley, P.; Daymond, A. Photosynthetic activity and early growth of four cacao genotypes as influenced by different shade regimes under West African dry and wet season conditions. Exp. Agric. 2013, 49, 31–42. [Google Scholar] [CrossRef]
- Shiflett, S.A.; Zinnert, J.C.; Young, D.R. Seasonal patterns of light availability and light use of broadleaf evergreens in a deciduous forest understory: Potential mechanisms for expansion. Open J. Ecol. 2013, 3, 151–160. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Jiang, M.; Yue, Q.; Yang, Y.; Luo, Z.; Lv, B.; He, R.; Feng, S.; Yang, M. Effects of Low-Light Environments on the Growth and Physiological and Biochemical Parameters of Indocalamus and Seasonal Variations in Leaf Active Substance Contents. Plants 2023, 12, 3993. https://doi.org/10.3390/plants12233993
Yu W, Jiang M, Yue Q, Yang Y, Luo Z, Lv B, He R, Feng S, Yang M. Effects of Low-Light Environments on the Growth and Physiological and Biochemical Parameters of Indocalamus and Seasonal Variations in Leaf Active Substance Contents. Plants. 2023; 12(23):3993. https://doi.org/10.3390/plants12233993
Chicago/Turabian StyleYu, Weiqian, Mingyan Jiang, Qiling Yue, Yixiong Yang, Zhenghua Luo, Bingyang Lv, Rui He, Shihan Feng, and Meng Yang. 2023. "Effects of Low-Light Environments on the Growth and Physiological and Biochemical Parameters of Indocalamus and Seasonal Variations in Leaf Active Substance Contents" Plants 12, no. 23: 3993. https://doi.org/10.3390/plants12233993





