The Biological Properties of the Essential Oil from the Jordan Accession of Phagnalon sinaicum Bornm. & Kneuck.
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC and GC-MS Analysis
2.2. Antimicrobial Activity of PSEO
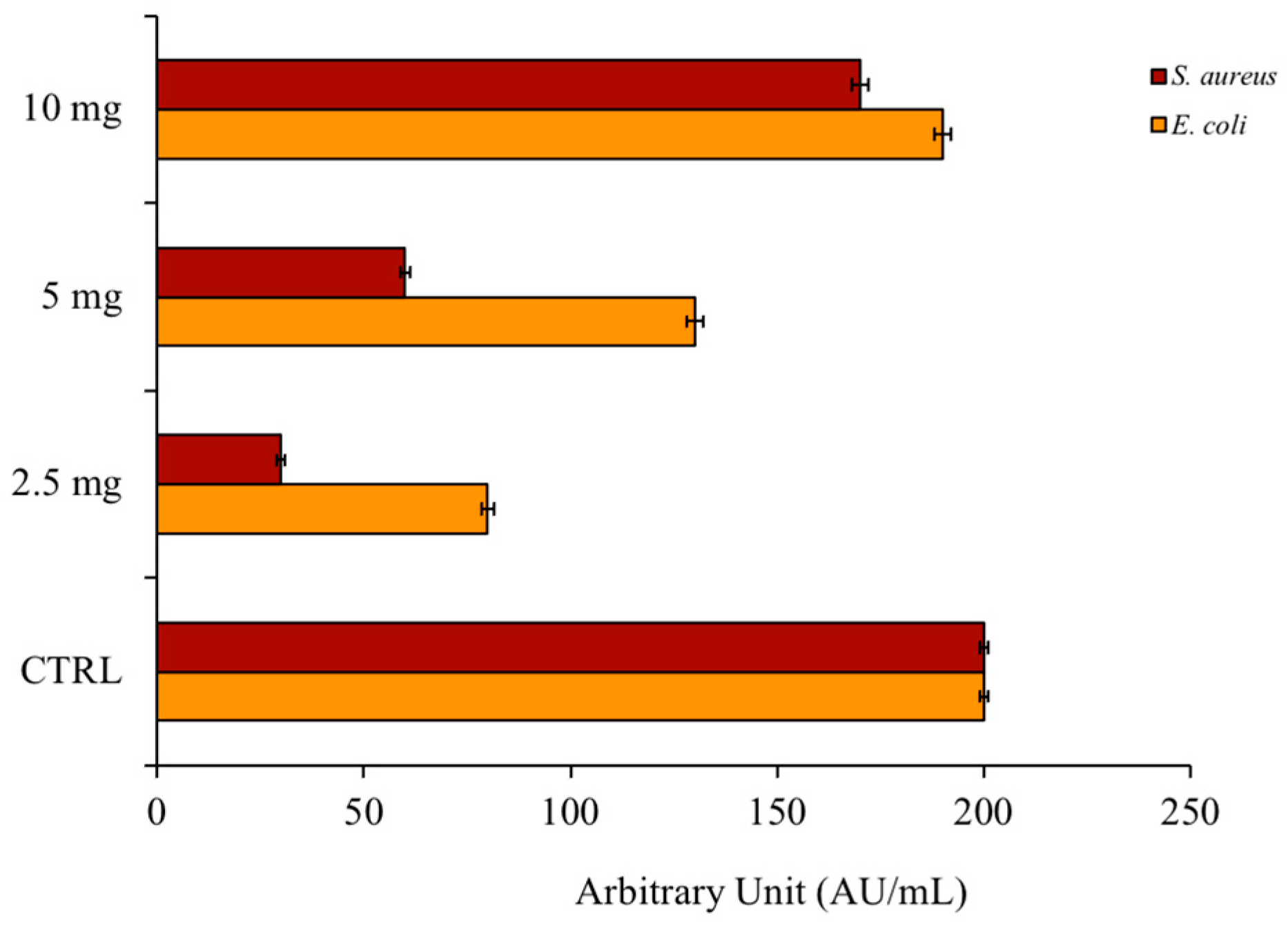
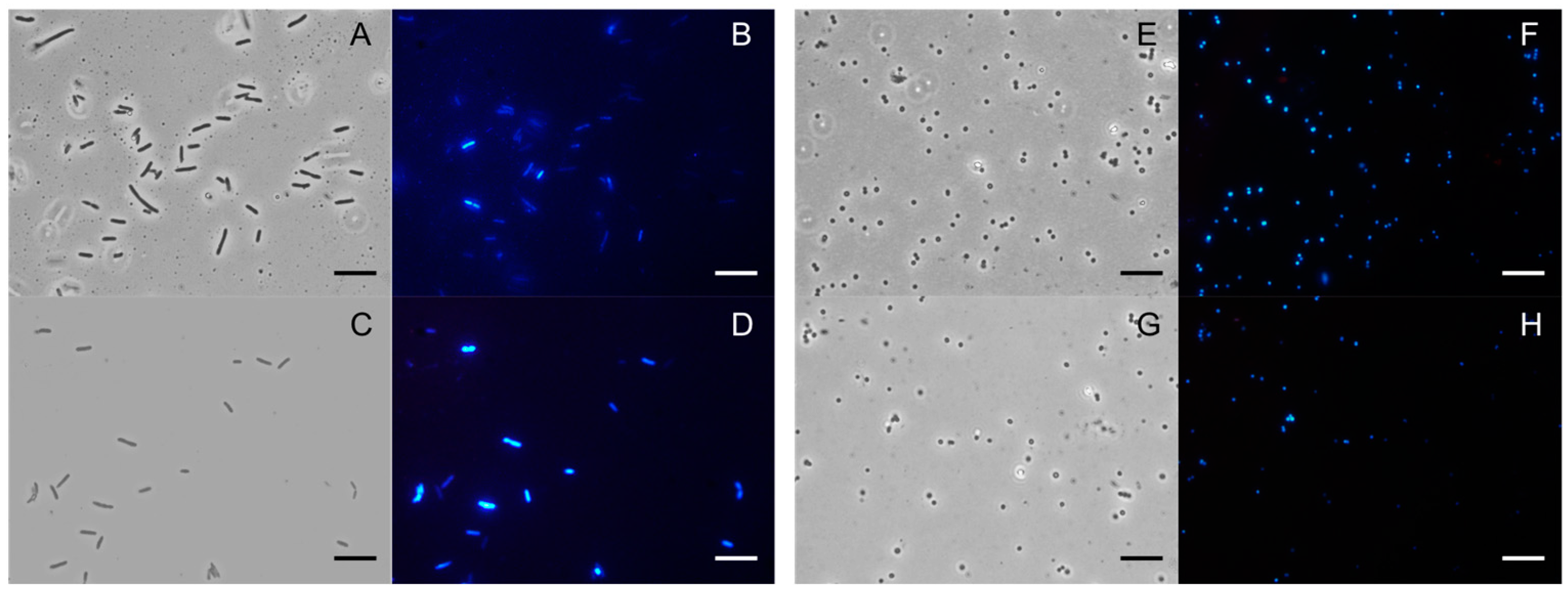
2.3. Antibiofilm Activity of PSEO
2.4. Antioxidant Activity of PSEO
3. Materials and Methods
3.1. Plant Material Collection
3.2. Isolation of Essential Oil
3.3. GC and GC-MS Analysis
3.4. Bacterial Strains
3.5. Antimicrobial Activity Assay
3.6. Determination of Minimal Inhibitory Concentration
3.7. DAPI/PI Dual Staining and Fluorescence Microscopy Image Acquisition
3.8. Antibiofilm Activity Assay
3.9. Antioxidant Activity
3.9.1. ABTS Scavenging Capacity Assay
3.9.2. Hydrogen Peroxide Scavenging Assay
3.10. Eukaryotic Cell Culture and Antioxidant Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:445949-1 (accessed on 15 May 2023).
- Ali-Shtayeh, M.S.; Yaghmour, R.M.R.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rabia, A. Palestinian plant medicines for treating renal disorders: An inventory and brief history. Altern. Complement. Ther. 2005, 11, 295–300. [Google Scholar] [CrossRef]
- Hudaib, M.; Mohammad, M.; Bustanji, Y.; Tayyem, R.; Yousef, M.; Abuirjeie, M.; Aburjai, T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 2008, 120, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Alzweiri, M.; Sarhan, A.A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, The Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef]
- Pardo De Santayana, M.; Blanco, E.; Morales, R. Plants known as te in Spain: An ethno-pharmaco-botanical review. J. Ethnopharmacol. 2005, 98, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gonzàlez-Tejero, M.R.; Casares-Porcel, M.; Sanchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Epifano, F.; Marcotullio, M.C.; Menghini, L. Constituents of Phagnalon sordidum. Chem. Nat. Comp. 2002, 38, 204–205. [Google Scholar] [CrossRef]
- Cicio, A.; Badalamenti, N.; Bruno, M. The ethnobotany, phytochemistry, and biological properties of genus Phagnalon (Asteraceae)—A review. Nat. Prod. Res. 2022, 37, 2083–2097. [Google Scholar] [CrossRef]
- Baka, Z.A.M. Antifungal activity of extracts from five Egyptian wild medicinal plants against late blight disease of tomato. Arch. Phytopathol. Plant Prot. 2014, 47, 1988–2002. [Google Scholar] [CrossRef]
- Baka, Z.A.M. Plant extract control of the fungi associated with different Egyptian wheat cultivars grains. J. Plant Prot. Res. 2014, 54, 231–237. [Google Scholar] [CrossRef]
- Baka, Z.A.M. Efficacy of wild medicinal plant extracts against predominant seed-borne fungi of broad bean cultivars. Acta Phytopathol. Entomol. Hung. 2015, 50, 45–65. [Google Scholar] [CrossRef]
- Chávez-gonzález, M.L.; Rodríguez-herrera, R.; Aguilar, C.N. Essential oils: A natural alternative to combat antibiotics resistance. In Antibiotic Resistance, Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–337. [Google Scholar]
- Coenye, T.; Nelis, H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 2010, 83, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Qaiser, M.; Lack, H.W. The Genus Phagnalon (Asteraceae, Inuleae) in Arabia. Willdenowia 1985, 15, 3–22. [Google Scholar]
- El-Dahmy, S.I.; Abdel Aal, M.; Abd El-Fatah, H.; Fid, F. Thymol derivatives from Phagnalon sinaicum Bornm and Kneuck. Acta Pharm. Hung. 1994, 64, 115–116. [Google Scholar]
- Vaglica, A.; Badalamenti, N.; Ilardi, V.; Bruno, M. The chemical composition of the aerial parts essential oil of four Phagnalon species collected in Sicily (Italy) and Greece. Nat. Prod. Res. 2023, in press. [Google Scholar] [CrossRef]
- Chikhi, I.; Allali, H.; Costa, J. Chemical composition and biological activities of essential oils of Phagnalon sordidum (L.) Rchb. (Asteraceae) from Algeria. Agric. Conspec. Sci. 2019, 84, 271–281. [Google Scholar]
- Brunel, M.; Vitrac, C.; Costa, J.; Mzali, F.; Vitrac, X.; Muselli, A. Essential oil composition of Phagnalon sordidum (L.) from Corsica, chemical variability and antimicrobial activity. Chem. Biodivers. 2016, 13, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Erdogan Orhan, I.; Senol, F.S.; Demirci, B.; Ozturk, N.; Baser, K.H.C.; Sener, B. Phytochemical characterization of Phagnalon graecum Boiss. by HPLC and GC-MS with its enzyme inhibitory and antioxidant activity profiling by spectrophotometric methods. Food Anal. Methods 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.J.; Cruz, M.T.; Salgueiro, L. Chemical characterization and bioactivity of the essential oil from Santolina insularis, a Sardinian endemism. Nat. Prod. Res. 2023, 36, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Marinas, I.C.; Oprea, E.; Gaboreanu, D.M.; Gradisteanu Pircalabioru, G.; Buleandra, M.; Nagoda, E.; Badea, I.A.; Chifiriuc, M.C. Chemical and biological studies of Achillea setacea Herba essential oil-first report on some antimicrobial and antipathogenic features. Antibiotics 2023, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Elhady, S.S.; Diri, R.M.; Fekry, M.I.; Bishr, M.; Salama, O.; El-Zalabani, S.M. Salicylic acid spraying affects secondary metabolites and radical scavenging capacity of drought-stressed Eriocephalus africanus L. Agronomy 2022, 12, 2278. [Google Scholar] [CrossRef]
- Badalamenti, N.; Porrello, A.; Merra, R.; Ilardi, V.; Bruno, M. The chemical composition of the aerial parts essential oil of Phagnalon sinaicum collected in the Negev Desert (Israel). Nat. Prod. Res. 2023, in press. [Google Scholar] [CrossRef]
- Badalamenti, N.; Russi, S.; Bruno, M.; Maresca, V.; Vaglica, A.; Ilardi, V.; Zanfardino, A.; Di Napoli, M.; Varcamonti, M.; Cianciullo, P.; et al. Dihydrophenanthrenes from a Sicilian Accession of Himantoglossum robertianum (Loisel.) P. Delforge showed antioxidant, antimicrobial, and antiproliferative activities. Plants 2021, 10, 2776. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Caillet, S.; Ursachi, L.; Shareck, F.; Lacroix, M. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Staphylococcus aureus. J. Food Sci. 2009, 74, M499–M508. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]
- Drago, L. Bacteria and biofilm in respiratory tract infections. Infez. Med. 2009, 17 (Suppl. 2), 3–9. [Google Scholar]
- European Pharmacopoeia 10.3. 2020. Determination of Essential Oils in Herbal Drugs, 2.8.12., 307. Available online: https://www.edqm.eu/ (accessed on 26 November 2023).
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Piacente, S.; Maresca, V.; Cianciullo, P.; Capasso, L.; et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Maresca, V.; Sorbo, S.; Varcamonti, M.; Basile, A.; Zanfardino, A. Proteins of the fruit pulp of Acca sellowiana have antimicrobial activity directed against the bacterial membranes. Nat. Prod. Res. 2021, 35, 2942–2946. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.D.; Luccia, B.D.; Vitiello, G.; D’Errico, G.; Carpentieri, A.; Pezzella, A.; Pizzo, E.; Notomista, E.; Varcamonti, M.; Zanfardino, A. Characterisation of EFV12 a bio-active small peptide produced by the human intestinal isolate Lactobacillus gasseri SF1109. Benef. Microbes 2020, 11, 815–824. [Google Scholar] [CrossRef] [PubMed]



| No. | Compounds a | LRI b | LRI c | Area d (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 931 | 938 | 0.4 |
| 2 | β-Phellandrene | 971 | 978 | 0.6 |
| 3 | β-Pinene | 982 | 980 | 0.1 |
| 4 | α-Terpinene | 1018 | 1022 | 1.1 |
| Monoterpene Hydrocarbons | 2.2 | |||
| 5 | Eucalyptol | 1034 | 1040 | 2.1 |
| 6 | Santolina alcohol | 1038 | 1047 | 21.4 |
| 7 | Artemisia ketone | 1061 | 1080 | 13.2 |
| 8 | Artemisia alcohol | 1092 | 1100 | 0.2 |
| 9 | α-Thujone | 1102 | 1125 | 8.8 |
| 10 | β-Thujone | 1127 | 1135 | 13.8 |
| 11 | Linalool | 1110 | 1176 | 1.0 |
| 12 | Terpinen-4-ol | 1180 | 1185 | 0.4 |
| 13 | α-Terpineol | 1199 | 1199 | 0.8 |
| Oxygenated Monoterpenes | 61.7 | |||
| 14 | α-Copaene | 1372 | 1371 | 1.0 |
| 15 | trans-β-Farnesene | 1455 | 1458 | 0.6 |
| 16 | Germacrene D | 1480 | 1480 | 5.2 |
| 17 | Bicyclogermacrene | 1494 | 1490 | 4.7 |
| 18 | β-Sesquiphellandrene | 1523 | 1531 | 16.9 |
| Sesquiterpene Hydrocarbons | 28.4 | |||
| 19 | Spathulenol | 1571 | 1571 | 2.1 |
| 20 | α-Cadinol | 1653 | 1652 | 1.9 |
| Oxygenated Sesquiterpenes | 4.0 | |||
| 21 | Hexanal | 802 | 808 | 0.3 |
| 22 | 2-Hexenal | 832 | 862 | 0.7 |
| Other Compounds | 1.0 | |||
| Total Composition (%) | 97.3 |
| Strains | MIC [mg/mL] |
|---|---|
| S. aureus | 6 |
| S. warneri | 10 |
| S. pasteuri | 10 |
| B. cereus | 10 |
| E. coli | 3 |
| P. aeruginosa | 4 |
| M. smegmatis | >10 |
| Sample | IC50 of ABTS (mg/mL) | Sample | IC50 of H2O2 (mg/mL) |
|---|---|---|---|
| PSEO | 2 | PSEO | 0.025 |
| Ascorbic acid | 0.00003 | Resveratrol | 0.00005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, N.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Bruno, M.; Zanfardino, A. The Biological Properties of the Essential Oil from the Jordan Accession of Phagnalon sinaicum Bornm. & Kneuck. Plants 2023, 12, 4007. https://doi.org/10.3390/plants12234007
Badalamenti N, Di Napoli M, Castagliuolo G, Varcamonti M, Bruno M, Zanfardino A. The Biological Properties of the Essential Oil from the Jordan Accession of Phagnalon sinaicum Bornm. & Kneuck. Plants. 2023; 12(23):4007. https://doi.org/10.3390/plants12234007
Chicago/Turabian StyleBadalamenti, Natale, Michela Di Napoli, Giusy Castagliuolo, Mario Varcamonti, Maurizio Bruno, and Anna Zanfardino. 2023. "The Biological Properties of the Essential Oil from the Jordan Accession of Phagnalon sinaicum Bornm. & Kneuck." Plants 12, no. 23: 4007. https://doi.org/10.3390/plants12234007
APA StyleBadalamenti, N., Di Napoli, M., Castagliuolo, G., Varcamonti, M., Bruno, M., & Zanfardino, A. (2023). The Biological Properties of the Essential Oil from the Jordan Accession of Phagnalon sinaicum Bornm. & Kneuck. Plants, 12(23), 4007. https://doi.org/10.3390/plants12234007








