Characterization of the F-Box Gene Family and Its Expression under Osmotic Stress in Birch
Abstract
:1. Introduction
2. Results
2.1. Identification of the BpF-Box Family and Phylogenetic Tree
2.2. Gene Sequence Analysis and the Prediction of Cis-Elements
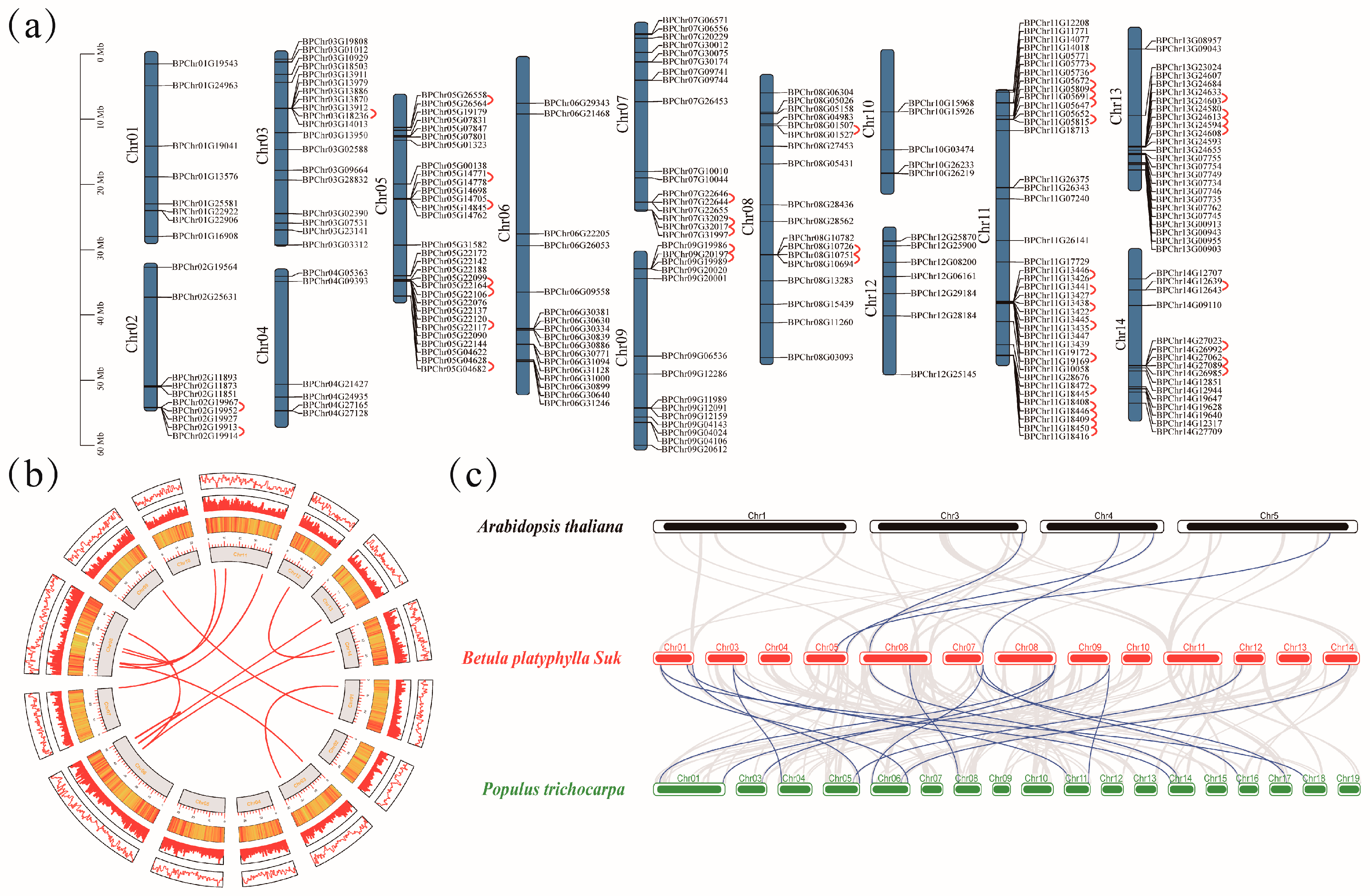
2.3. Chromosome Distribution and Gene Duplication
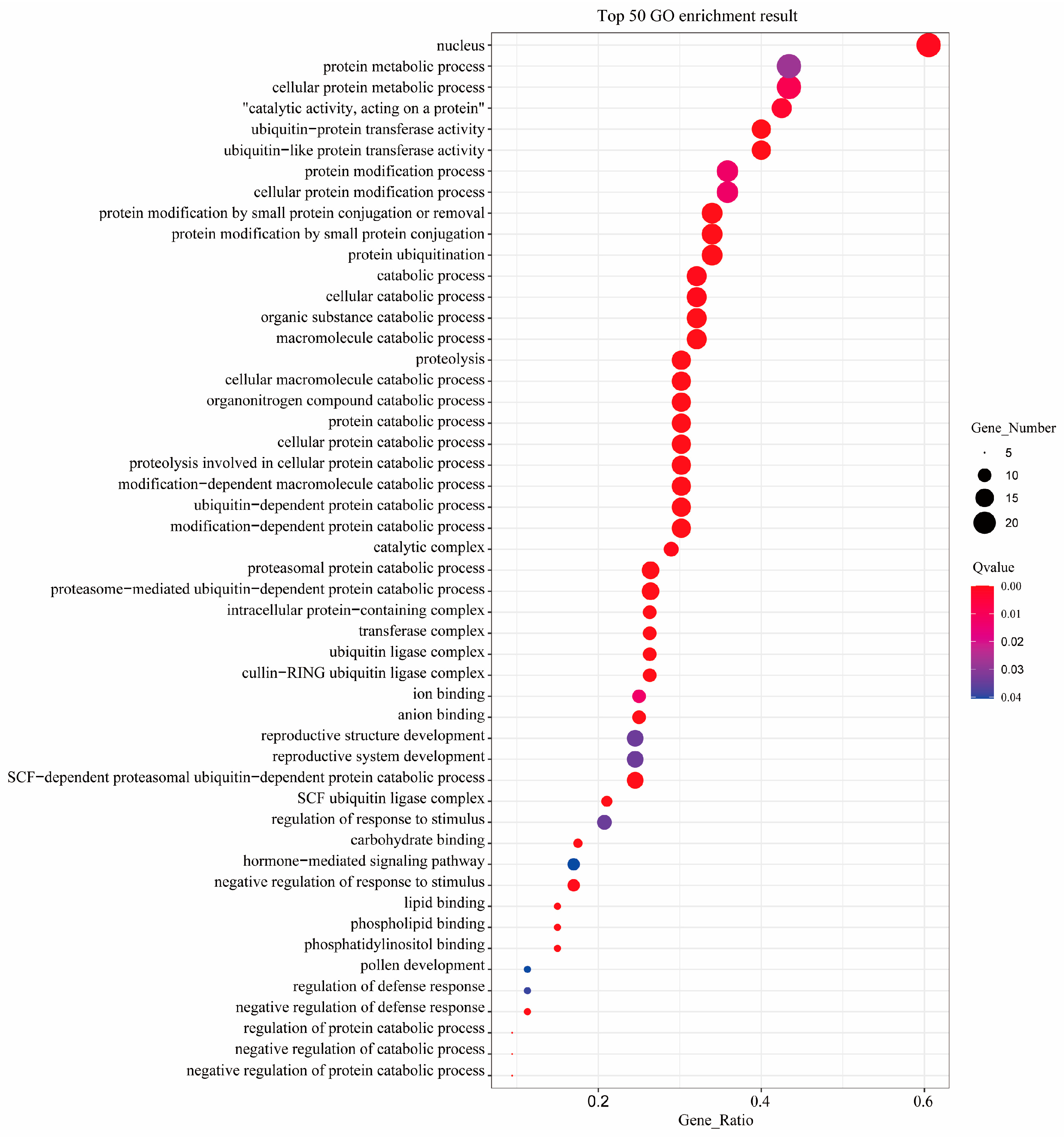
2.4. GO Annotation of BpF-Box Family Proteins
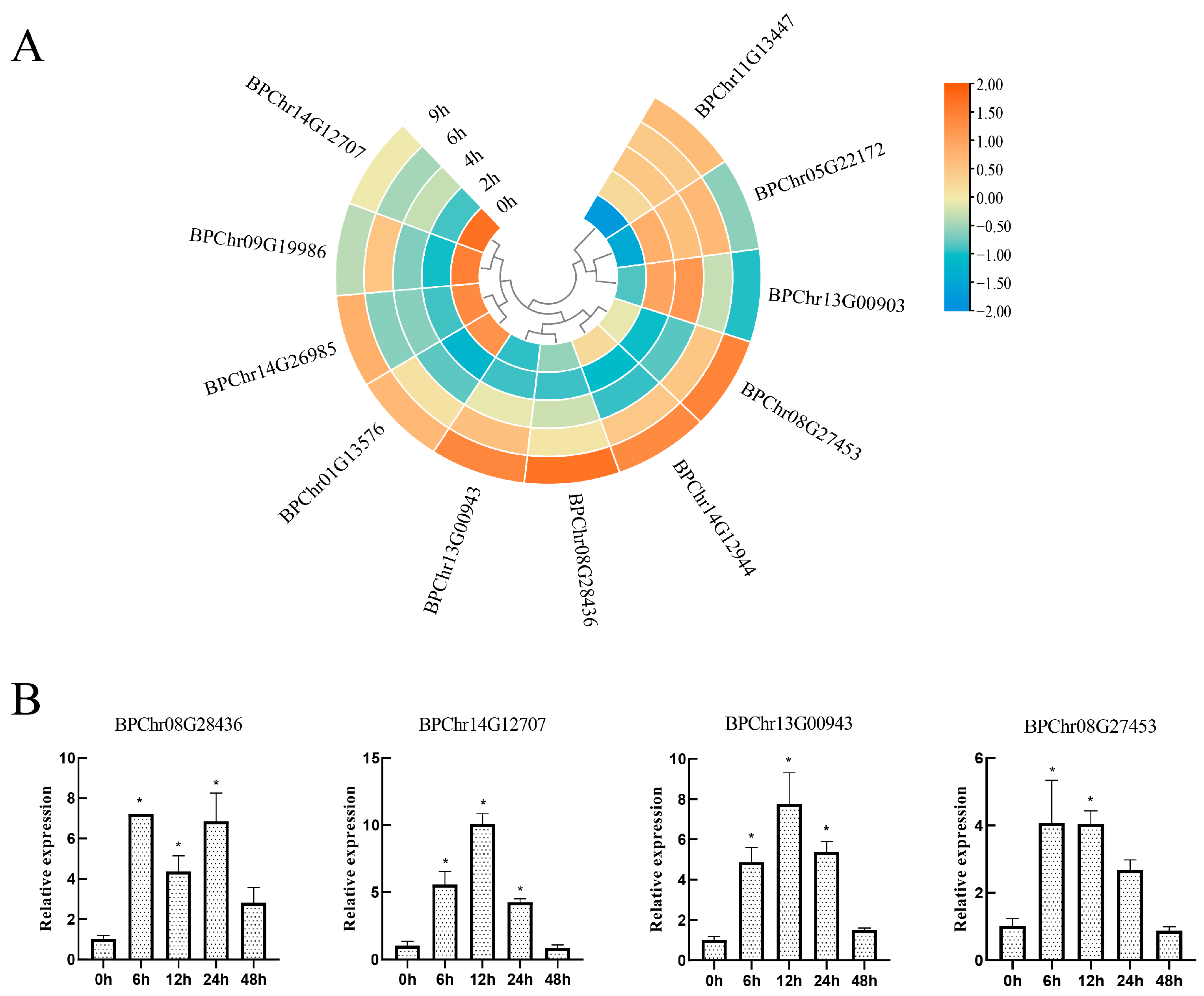
2.5. The Expression of BpF-Box Family after PEG6000 Treatments
2.6. Co-Expression Networks and Gene Ontology Analyses
2.7. BpF-Box Genes Expression under Different Hormone Treatments
3. Discussion
4. Materials and Methods
4.1. The Member of BpF-Box Genes and Construction of Phylogenetic Tree
4.2. Classification and Sequence Analysis on the BpF-Box Members
4.3. Chromosome Distribution and Gene Duplication
4.4. BpF-Box Proteins and GO Functional Annotation in Birch
4.5. Gene Expression Analysis
4.6. Using qRT-PCR to Validate Differentially Expressed Genes
4.7. Gene Co-Expression Networks and Gene Ontology Analyses
4.8. BpF-Box Genes Expression under Different Hormone Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gagne, J.M.; Downes, B.P.; Shiu, S.H.; Durski, A.M.; Vierstra, R.D. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 11519–11524. [Google Scholar] [CrossRef]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef]
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Patton, E.E.; Willems, A.R.; Tyers, M. Combinatorial control in ubiquitin-dependent proteolysis: Don’t Skp the F-box hypothesis. Trends Genet. TIG 1998, 14, 236–243. [Google Scholar] [CrossRef]
- Jin, J.P.; Ang, X.L.L.; Shirogane, T.; Harper, J.W. Identification of substrates for F-Box proteins. In Ubiquitin and Protein Degradation, Pt B; Deshaies, R.J., Ed.; Methods in Enzymology; Elsevier Academic Press Inc.: San Diego, CA, USA, 2005; Volume 399, pp. 287–309. [Google Scholar]
- Xu, G.X.; Ma, H.; Nei, M.; Kong, H.Z. Evolution of F-box genes in plants: Different modes of sequence divergence and their relationships with functional diversification. Proc. Natl. Acad. Sci. USA 2009, 106, 835–840. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, C.R.; Meng, Y.Y.; Fan, R.Q.; Zhao, W.Q.; Wang, X.D.; Yu, X.M.; Laroche, A.; Kang, Z.S.; Liu, D.Q. Identification and expression analysis of some wheat F-box subfamilies during plant development and infection by Puccinia triticina. Plant Physiol. Biochem. 2020, 155, 535–548. [Google Scholar] [CrossRef]
- Jia, Q.; Xiao, Z.X.; Wong, F.L.; Sun, S.; Liang, K.J.; Lam, H.M. Genome-Wide Analyses of the Soybean F-Box Gene Family in Response to Salt Stress. Int. J. Mol. Sci. 2017, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Yin, H.; Qiao, X.; Tan, X.; Gu, C.; Wang, B.H.; Cheng, R.; Wang, Y.Z.; Zhang, S.L. F-box genes: Genome-wide expansion, evolution and their contribution to pollen growth in pear (Pyrus bretschneideri). Plant Sci. Int. J. Exp. Plant Biol. 2016, 253, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, W.M.; Liu, W.; Han, X.J.; Wu, L.H.; Yu, M.; Qiu, X.L.; He, Z.Q.; Li, H.Y.; Zhuo, R.Y. Genome-wide characterization of the hyperaccumulator Sedum alfredii F-box family under cadmium stress. Sci. Rep. 2021, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Kipreos, E.T.; Pagano, M. The F-box protein family. Genome Biol. 2000, 1, Reviews3002. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Wang, W.Q.; Wang, W.L.; Zhang, G.Q.; Liu, Y.; Wang, Y.; Wang, W. Wheat F-Box Protein Gene TaFBA1 Is Involved in Plant Tolerance to Heat Stress. Front. Plant Sci. 2018, 9, 15. [Google Scholar] [CrossRef]
- Bao, Y.; Song, W.M.; Jin, Y.L.; Jiang, C.M.; Yang, Y.; Li, B.; Huang, W.J.; Liu, H.; Zhang, H.X. Characterization of Arabidopsis Tubby-like proteins and redundant function of AtTLP3 and AtTLP9 in plant response to ABA and osmotic stress. Plant Mol. Biol. 2014, 86, 471–483. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Li, T.T.; Liu, Y.; Ren, C.X.; Wang, M.L. Molecular cloning and expression analysis of an F-box protein gene responsive to plant hormones in Brassica napus. Mol. Biol. Rep. 2010, 37, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Kepinski, S.; Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, I.; Ismail, A.; Darwish, A.; El Kayal, W.; Subramanian, J.; Sherif, S.M. Functional characterization of a gibberellin F-box protein, PslSLY1, during plum fruit development. J. Exp. Bot. 2021, 72, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Yang, W.Q.; Zhao, B.; Wang, L.; Zhang, P.; Ouyang, Y.; Chang, Y.K.; Chen, G.Z.; Zhang, J.L.; Wang, S.J.; et al. The Kelch-F-box protein SMALL AND GLOSSY LEAVES 1 (SAGL1) negatively influences salicylic acid biosynthesis in Arabidopsis thaliana by promoting the turn-over of transcription factor SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1). New Phytol. 2022, 235, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Kalluri, U.C.; Jawdy, S.; Gunter, L.E.; Yin, T.M.; Tschaplinski, T.J.; Weston, D.J.; Ranjan, P.; Tuskan, G.A. The F-Box Gene Family Is Expanded in Herbaceous Annual Plants Relative to Woody Perennial Plants. Plant Physiol. 2008, 148, 1189–1200. [Google Scholar] [CrossRef]
- Stone, S.L. The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front. Plant Sci. 2014, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Paquis, S.; Mazeyrat-Gourbeyre, F.; Fernandez, O.; Crouzet, J.; Clément, C.; Baillieul, F.; Dorey, S. Characterization of a F-box gene up-regulated by phytohormones and upon biotic and abiotic stresses in grapevine. Mol. Biol. Rep. 2011, 38, 3327–3337. [Google Scholar] [CrossRef]
- Gao, L.T.; Jia, S.Z.; Cao, L.; Ma, Y.J.; Wang, J.L.; Lan, D.; Guo, G.Y.; Chai, J.F.; Bi, C.L. An F-box protein from wheat, TaFBA-2A, negatively regulates JA biosynthesis and confers improved salt tolerance and increased JA responsiveness to transgenic rice plants. Plant Physiol. Biochem. 2022, 182, 227–239. [Google Scholar] [CrossRef]
- Qu, L.; Sun, M.S.; Li, X.M.; He, R.Q.; Zhong, M.; Luo, D.; Liu, X.M.; Zhao, X.Y. The Arabidopsis F-box protein FOF2 regulates ABA-mediated seed germination and drought tolerance. Plant Sci. 2020, 301, 16. [Google Scholar] [CrossRef] [PubMed]
- Piisilä, M.; Keceli, M.A.; Brader, G.; Jakobson, L.; Joesaar, I.; Sipari, N.; Kollist, H.; Palva, E.T.; Kariola, T. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Wang, X.Y.; Cao, X.C.; Chen, B.Z.; Ma, C.K.; Lv, J.Y.; Sun, Z.M.; Qiao, K.K.; Zhu, L.F.; Zhang, C.J.; et al. GhTULP34, a member of tubby-like proteins, interacts with GhSKP1A to negatively regulate plant osmotic stress. Genomics 2021, 113, 462–474. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Cui, M.H.; Noh, M.S.; Jung, K.W.; Shin, J.S. The FBA motif-containing protein AFBA1 acts as a novel positive regulator of ABA response in Arabidopsis. Plant Cell Physiol. 2017, 58, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.Y.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Tian, Z.L.; Li, H.P.; Guo, Y.T.; Zhang, Y.Q.; Roberts, J.A.; Zhang, X.B.; Miao, Y.C. Genome-wide analysis and characterization of F-box gene family in Gossypium hirsutum L. BMC Genom. 2019, 20, 16. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, S.; Yao, W.J.; Cheng, Z.H.; Zhou, B.R.; Jiang, T.B. Genome-wide analysis and expression profile of the bZIP gene family in poplar. BMC Plant Biol. 2021, 21, 122. [Google Scholar] [CrossRef]
- Jia, F.J.; Wu, B.J.; Li, H.; Huang, J.G.; Zheng, C.C. Genome-wide identification and characterisation of F-box family in maize. Mol. Genet. Genom. 2013, 288, 559–577. [Google Scholar] [CrossRef]
- Lim, J.; Lim, C.W.; Lee, S.C. Functional Analysis of Pepper F-box Protein CaDIF1 and Its Interacting Partner CaDIS1: Modulation of ABA Signaling and Drought Stress Response. Front. Plant Sci. 2019, 10, 15. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Busino, L. The Biology of F-box Proteins: The SCF Family of E3 Ubiquitin Ligases. In Cullin-Ring Ligases and Protein Neddylation: Biology and Therapeutics; Sun, Y., Wei, W., Jin, J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing Ag: Cham, Switzerland, 2020; Volume 1217, pp. 111–122. [Google Scholar]
- Jiao, J.; Gao, F.; Liu, J.; Lv, Z.Y.; Liu, C.M. A structural basis for the functional differences between the cytosolic and plastid phosphoglucose isomerase isozymes. PLoS ONE 2022, 17, 14. [Google Scholar] [CrossRef]
- Abd-Hamid, N.A.; Ahmad-Fauzi, M.I.; Zainal, Z.; Ismail, I. Diverse and dynamic roles of F-box proteins in plant biology. Planta 2020, 251, 31. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.; Turner, J. COI1, a jasmonate receptor, is involved in ethylene-induced inhibition of Arabidopsis root growth in the light. J. Exp. Bot. 2010, 61, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, Z.L.; Gao, J.H.; Xing, L.; Cao, M.J.; Yu, C.M.; Hu, Y.L.; You, J.; Shi, H.T.; Zhu, Y.F.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Wang, Y.; Zhang, Y.; Ma, M.M.; Gao, Y.F.; Zhou, Z.Y.; Wang, B.Z.; Wang, T.; Lv, X.L.; Wang, X.; et al. Alleviation of drought stress and the physiological mechanisms in Citrus cultivar (Huangguogan) treated with methyl jasmonate. Biosci. Biotechnol. Biochem. 2020, 84, 1958–1965. [Google Scholar] [CrossRef]
- Dong, Q.L.; Duan, D.Y.; Zheng, W.Q.; Huang, D.; Wang, Q.; Yang, J.; Liu, C.H.; Li, C.; Gong, X.Q.; Li, C.Y.; et al. Overexpression of MdVQ37 reduces drought tolerance by altering leaf anatomy and SA homeostasis in transgenic apple. Tree Physiol. 2022, 42, 160–174. [Google Scholar] [CrossRef]
- Aerts, N.; Mendes, M.P.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Keller, K.; Chan, K.X.; Gessel, M.M.; Thines, B.C. Transcriptome analysis uncovers Arabidopsis FBOX STRESS INDUCED 1 as a regulator of jasmonic acid and abscisic acid stress gene expression. BMC Genom. 2017, 18, 15. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.C.; Yu, L.L.; Zheng, T.; Wang, S.; Yue, Z.; Jiang, J.; Kumari, S.; Zheng, C.F.; Tang, B.; et al. Genome sequence and evolution of Betula platyphylla. Hortic. Res.-Engl. 2021, 8, 12. [Google Scholar] [CrossRef]
- Gupta, S.; Garg, V.; Kant, C.; Bhatia, S. Genome-wide survey and expression analysis of F-box genes in chickpea. BMC Genom. 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, 14. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Jia, Y.Q.; Niu, Y.I.; Zhao, H.M.; Wang, Z.B.; Gao, C.Q.; Wang, C.; Chen, S.; Wang, Y.C. Hierarchical transcription factor and regulatory network for drought response in Betula platyphylla. Hortic. Res.-Engl. 2022, 9, 14. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, 12. [Google Scholar] [CrossRef]
- Yao, W.J.; Wang, S.J.; Zhou, B.R.; Jiang, T.B. Transgenic poplar overexpressing the endogenous transcription factor ERF76 gene improves salinity tolerance. Tree Physiol. 2016, 36, 896–908. [Google Scholar] [CrossRef]
- Pripp, A.H. Pearson’s or Spearman’s correlation coefficients. Tidsskr. Nor. Laegeforen. 2018, 138. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Shi, J.; Li, J.; Liu, G.; Yang, C.; Jiang, J. Characterization of the F-Box Gene Family and Its Expression under Osmotic Stress in Birch. Plants 2023, 12, 4018. https://doi.org/10.3390/plants12234018
Lv G, Shi J, Li J, Liu G, Yang C, Jiang J. Characterization of the F-Box Gene Family and Its Expression under Osmotic Stress in Birch. Plants. 2023; 12(23):4018. https://doi.org/10.3390/plants12234018
Chicago/Turabian StyleLv, Guanbin, Jingjing Shi, Jingnan Li, Guifeng Liu, Chuanping Yang, and Jing Jiang. 2023. "Characterization of the F-Box Gene Family and Its Expression under Osmotic Stress in Birch" Plants 12, no. 23: 4018. https://doi.org/10.3390/plants12234018
APA StyleLv, G., Shi, J., Li, J., Liu, G., Yang, C., & Jiang, J. (2023). Characterization of the F-Box Gene Family and Its Expression under Osmotic Stress in Birch. Plants, 12(23), 4018. https://doi.org/10.3390/plants12234018




