Abstract
Cotton (Gossypium spp. L.) is a major origin of natural fiber, and is projected at 117 million bales worldwide for 2021/22. A variety of biotic and abiotic stresses have considerable negative impacts on cotton. The significantly decreased applications of chemical insecticidal sprays in the agro-ecosystem have greatly affected the biodiversity and dynamics of primary and secondary insects. Various control measures were taken around the globe to increase production costs. Temperature, drought, and salinity, and biotic stresses such as bacteria, viruses, fungi, nematodes, insects, and mites cause substantial losses to cotton crops. Here, we summarize a number of biotic and abiotic stresses upsetting Bt cotton crop with present and future biotechnology solution strategies that include a refuge strategy, multi-gene pyramiding, the release of sterile insects, seed mixing, RNAi, CRISPR/Cas9, biotic signaling, and the use of bioagents. Surveillance of insect resistance, monitoring of grower compliance, and implementation of remedial actions can lead to the sustainable use of cotton across the globe.
1. Introduction
Plants are exposed to various biotic and abiotic stresses across their lifespan. Additionally, due to the current scenario of climatic change around the globe, the impact of these stresses has increased drastically, showing a remarkable influence on the yield of most crops [1]. Abiotic stress of, for instance, cold, salinity, high temperature, scarcity, heavy-metal noxiousness, and oxidative trauma are the major intimidations to the failure of crops in terms of growth and productivity, which could cause more than 50% of yield fatalities [2]. The seriousness of biotic stresses, not only cause losses in yield and low quality, but also increases the production costs due to the requirement for extra measures to be applied to control them [3].
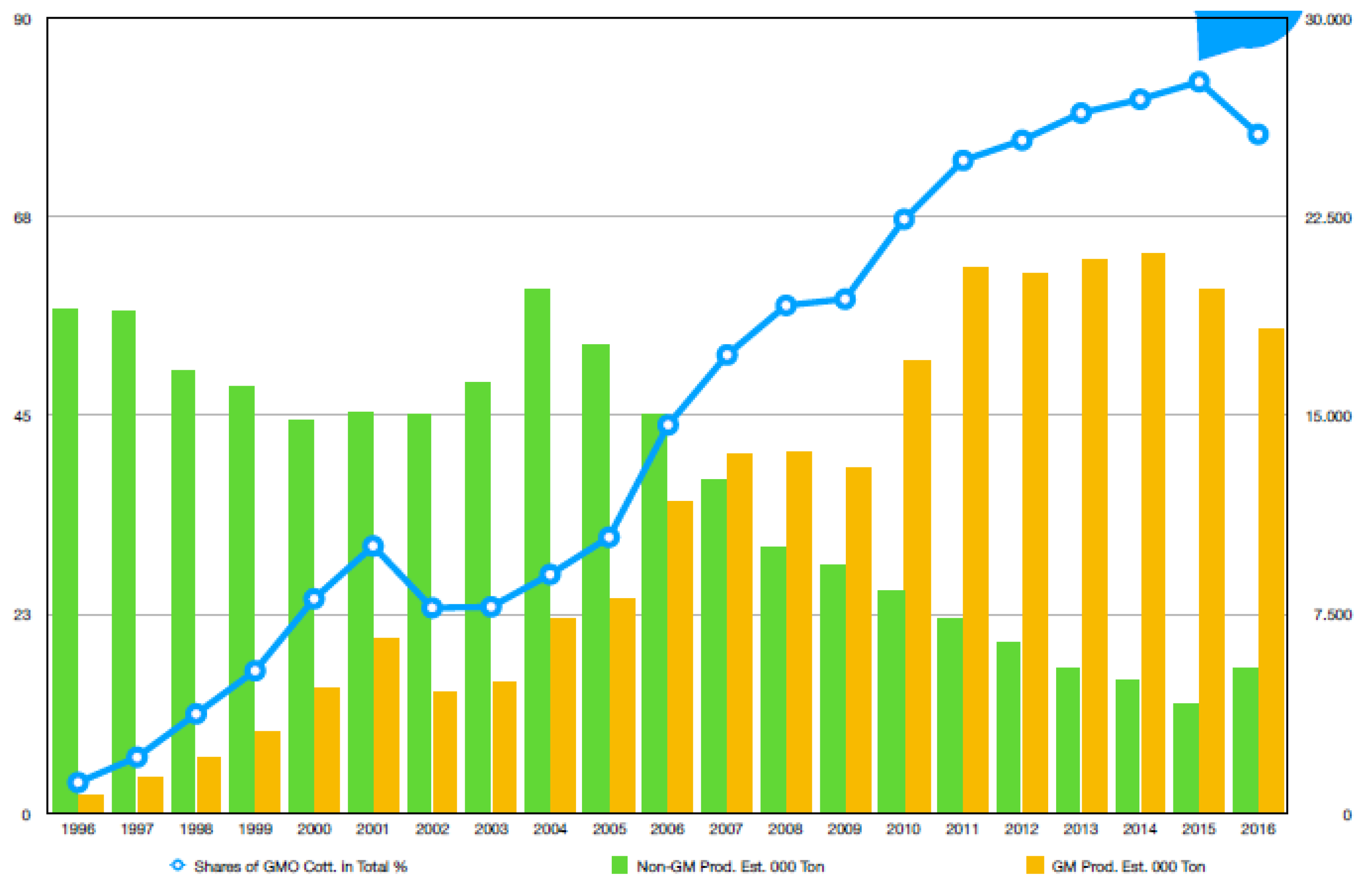
Among all the available species of cotton, only four species are used for field production. Nevertheless, the highland cotton (Gossypium hirsutum L.) and sea-island cotton (G. barbadense), belonging to plant family Malvaceae, are the major cultivated species grown in various areas of the world [4,5]. These two species contribute to 90% and 5% of the total worldwide cotton-planting acreages for fiber production in the world, respectively [6,7]. It is estimated that cotton is cultivated in an area of approximately 10,000 ha in excess of 80 countries per year [8], and 13% of which are in developed countries, while the remaining 87% are in developing countries where cotton is considered to be white gold [9]. A statistical data map of worldwide cotton-producing countries is shown in Figure 1.
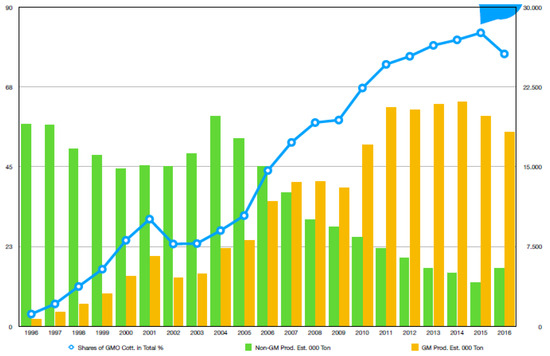
Figure 1.
Global Bt- and non-Bt-cotton production and shares of Bt cotton.
The cotton plant generally possesses higher resistance to different abiotic stresses when compared with other crops [10]. However, during its life cycle, the plant could be subjected to various abiotic stresses and biotic stresses, including but not limited to pests [11].
Pest damage has long been a global problem in cotton production. Even though only a small fraction of insect pests has economic importance, the cotton plant may encounter an estimation of up to 1326 insect pests around the world throughout its whole growth season (www.cicr.gov.in). Extensive research has been conducted to investigate the prevalence of pests in cotton fields, primarily focusing on their economic impact on cotton yields [12,13]. Among the significant insect pests responsible for losses in cotton production are the cotton jassid (Amrasca biguttula), cotton aphid (Aphis gossypii), thrips (Frankliniella schultzei), spotted bollworm (Earias vittela), pink bollworm (Pectinophora gossypiella), American bollworm (Helicoverpa armigera), cotton mealy bug (Phenacoccus solenopsis), fall armyworm (Spodoptera frugiperda), and whitefly (Trialeurodes vaporariorum) [14,15]. These insect pests, along with diseases such as root rots, leaf blight, leaf spot, target spot, and verticillium wilt, contribute to economic losses ranging from 15% to 30% in cotton production, with some instances reaching up to 50%, which are attributed to direct damage or the transmission of plant diseases [14]. For instance, in Brazil, annual losses in agricultural production due to insect pests can average 7.7%, equivalent to approximately USD 17.7 billion [16].
The recent review summarizes the development and application of Bt cotton for over two decades; analyzes and discusses the impacts of Bt cotton on agro-ecosystems, and major issues relating to Bt-cotton production; and focuses on the current problems of Bt-cotton production, including the mechanisms of evolution of pest tolerance against Bt cotton, for the purpose of improving Bt efficacy for better control strategies of the target pests to ensure cotton-production stability in the future. First, we briefly review the perception and implementation of Bt cotton in production; then, we discuss the dynamics of bio-communities in Bt-cotton fields, including the target, non-target pests, and the bio-communities; third, we examine the evolution of pest resistance to Bt toxin (PRBT); and, finally, we deliberate approaches to improve the efficacies of Bt resistance and control against its target pests and to delay the development of pest tolerance to Bt toxin. We believe this review will help and promote the future research and development of Bt cotton and pest-control management.
2. Global Perception and Adoption of Bt Cotton for Better Yield and Pest Control
Due to the potential limitations of conventional strategies for pest management in cotton production, genetically engineered cotton expressing the insect-specific Bt-toxin proteins, from the naturally occurring soil-born bacterium B. thuringiensis (Bt), was applied in cotton production. The insecticidal properties of Bt toxins from Bt have been recognized by human society for a long time even dated to early in ancient Egypt, while the isolation of Bt strains and their use in pest control started in the beginning of the 20th century [14,17]. For detailed information regarding the identification, the research activities of these bacteria, and the properties and applications of the different Bt toxins please see the review paper of Sanahuja et al. [17]. To date, almost 100 different subspecies of B. thuringiensis have been identified which produce the insecticidal toxin named Cry, Cyt, or Vip [18]. Cotton bollworms are susceptible to Cry1Ac and Cry2Ab toxins, while the corn borer is highly sensitive to Cry1Ab and the corn rootworm to Cry3Bb toxin. The cry gene was initially incorporated into tomato and tobacco genomes for the control of Lepidopteran insect pests and subsequently in other major crops including cotton, corn, soybean, and rice, etc. [19]. After the importance of the insect-pest problem was realized in cotton production, Bt cotton was adopted by major cotton-producing countries at different times.
The first commercial release of Bt cotton took place in the USA in 1996, and was conducted by Monsanto in collaboration with Delta and Pineland Company (D and PL). In the following years, Bt-cotton-planting acreage increased rapidly, occupying 15% in 1997, 37% in 2001, and 85% in 2019 of the total cotton-production areas in the USA due to its performance of increasing cotton yields, while reducing pesticide applications. This highly dramatic adoption rate of Bt cotton in the USA ensued a glaring reduction of pesticide costs, enhanced resistance of Bt-cotton crop against tobacco budworms, cotton bollworms, and pink bollworms, and, finally, resulted in significant profitability [11].
China has made a large investment in biotechnology, which was accelerated in the late years of the 1980s by the “863 project” initialed by the Ministry of Science and Technology of China. Due to an immense pressure of the problem of the insect pests in the northern region of the cotton-planting area in China (Huanghuaihai Valley Region), scientists in the Chinese Academy of Agricultural Sciences worked on plant transformation techniques and started to look at this as a promising tool of introducing Bt cry genes (cry1Ab and cry1Ac) into cotton genomes to combat this severe insect-pest problem. In the mid-1990s, Monsanto together with D and PL started to collaborate with local Chinese companies to introduce some Bt-cotton cultivars on a commercial scale into the Chinese market. In fact, in 1996 Bt cotton was approved for commercial release in the market by the Chinese Biosafety Committee [20]. Afterwards, Bt-cotton acreage also grew rapidly and steadily in China, and it was estimated that Bt-cotton-planting acreage rapidly increased from 1.5 million hectares to a total of 3.5 million hectares in 2001. In a five-year period of survey, the rate of adoption of Bt cotton in China was extremely high due to its increased yield per ha, reduced pesticide costs and incidences of pesticide poisoning, and better pest control [21,22].
Pakistan is an agricultural country and ranks 4th among the major cotton producers worldwide. The perception and adoption of Bt cotton experienced a problematic period in terms of safety, quality, yield, and effectiveness against insect pests [23]. Initially, the Government of Pakistan approved the use of Bt cotton on a trial basis in different cotton-growing areas therein. A survey conducted in 2009 in the major cotton-growing province of Punjab showed that, at the farmer level, Bt cotton was more effective and promising than non-Bt cotton [23,24] even though there were still attacks of some insect pests such as cotton bollworms (Helicoverpa armigera), pink bollworm (Pectinophora gossypiella), spotted bollworms (Earias vittella and E. insulana), tobacco cutworm (Spodoptera litura), beet armyworms (S. exigua), and some sucking insects (Bemisia tabaci, Thrips tabaci, Amrasca devastans and Aphis gossypii), as well as high incidence of cotton leaf curl virus (CLCV). The benefits of Bt-cotton planting included significant reductions in total pesticide application for pest control, and, in labor input, higher yield and cost efficiency. But at that time the farmers still used to use chemicals to ensure effective control of insect-pest attacks. This showed some farmers’ reluctance to adopt Bt cotton [25,26]. After some time, however, Bt-cotton-planting acreage increased steadily, with some traditional wheat and sugarcane growing areas being shifted to planting Bt cotton. A long-term observational study from 2003 to 2013 revealed that, of the two main cotton-producing regions, Punjab had a much higher adoption rate of Bt cotton than Sindh [27].
In India, cotton is also a major crop. The major efforts to use Bt cotton to harness the damage of the bollworm started in the late 1990s with the import of Bt cotton from Monsanto. The initial field trials of Bt cotton showed 40% more yield with 50% chemical insecticide reduction of Bt cotton compared to non-Bt cotton [28]. In February 2002, the Indian Council of Agricultural Research (ICAR), Ministry of Environment, and the Genetic Engineering and Approval Committee mutually approved three Bt-hybrid cottons MECH 162-Bt, MECH 184-Bt, and MECH 12-Bt, developed by Monsanto for commercial distribution. Soon after the approval, these hybrids were planted in six different Indian states, Andhra Pradesh, Gujarat, Karnataka, Madhya Pradesh, Maharashtra, and Tamil Nadu [29,30]. Although, that year experienced unfavorable conditions for overall cotton production due to heavy rainfall followed by dry weather, which led to a very low pest pressure, and a significant decline in chemical sprays was observed, indicating a substantial effect of Bt cotton in comparison to non-Bt cotton on the control of the bollworm complex [29]. In the following seasons, Bt-cotton-planting acreages rapidly increased from 0.56 million ha in 2004/5 to 3.7 million ha in 2006/7. It was estimated that during the period from 2001 to 2011, the production of Bt hybrid cotton increased from 1.56 million bales to 3.56 million bales [28]. Then, the Cotton Advisory Board of India (CAB) reported a significant decline of acreage of Bt cotton from 95% to below 90% during the period of 2013/14–2016/17 due to the stagnant yield of Bt cotton and the increased attacks of pink bollworm in these Bt-cotton fields. The attacks infected an acreage of 8.77 million ha of the total 10.82 million ha of cotton-production acreages therein. This decline stopped in 2017, and the Bt-cotton percentage recovered in the year 2017/18 by 8%, which indicated a high adoption rate of Bt cotton in India [31].
Cotton is grown in different states of the USA. The insect-resistant Bt cotton containing Cry1Ac was commercially introduced into the market by Monsanto. The adoption and cultivation of Bt cotton eradicated the detrimental insect–cotton boll weevi and significantly decreased the use of pesticides. With this success later on, both Cry1Ac and Cp4-EPSPS genes containing cotton were successfully introduced into the market [32]. Since 2000, Brazil has become one of the leading countries in agricultural production. Brazil produces different economic crops such as sugarcane, soyabean, coffee, corn, and cotton. During the 1970s−1980s, cotton was grown on small farms in the cerrado province of Brazil, but it was affected by the emergence of cotton boll weevi. Later on in the 1990s, transgenic cotton was adopted on a large scale. Since then, the Bt-cotton production has increased in Brazil [33]. The cultivation of cotton has increased in Turkey since the 1990s. Cotton is grown in different regions of Turkey like Antalya and Anatolia especially in the Aegean regions. Turkey is a pioneer in producing natural-colored and organic cotton around the globe because it has not applied transgenic technology to its cotton production [34]. Bt cotton was commercialized for the first time in Mexico during 1996. After the adoption of Bt cotton in 2008, the 96% cotton field was planted with Bt cotton. In Argentina, after the adoption of the transgenic soyabean, Bt cotton was adopted and cultivated in Argentina. Firstly, Bt cotton was adopted by small farms and families. The cultivation significantly reduced the cost of pesticides and increased the output. Argentina is now one of the global leaders in Bt-cotton production [35].
3. Effect of Bt-Cotton Fields on Bio-Community Interactions
3.1. Effect on Target Pest
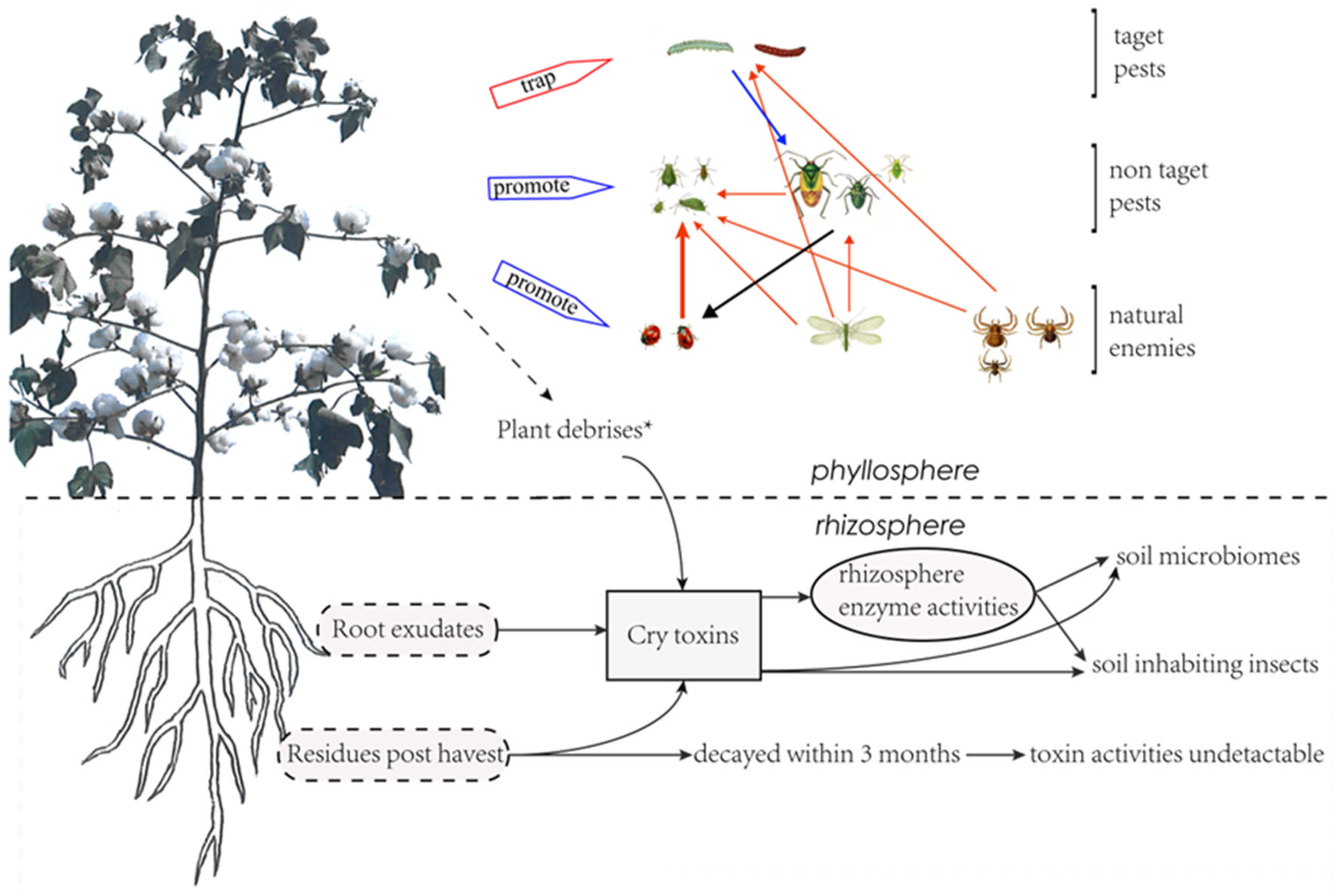
Cotton is the main host crop of cotton bollworms. From June to July each year, cotton bollworms (H. armigera) migrate to the cotton field and lay their eggs around the flower organs [36]. The hatched larvae feed on the young tissues and thus cause damage to the plant. The toxin protein in Bt cotton kills the feeding larvae, which makes it impossible for cotton bollworms to complete their life cycles. Thus, the density of eggs and larvae of the cotton bollworm steadily decreased, along with the increase in planting years and proportion of Bt cotton, as revealed in monitoring from 1992 to 2006 in China [37]. The large-scale planting of Bt cotton not only effectively controls the damages of the cotton bollworm to cotton, but also makes the Bt-cotton field a deathtrap for cotton bollworms in the agricultural ecosystem (Figure 2), in which cotton serves not only as a major host of second-generation cotton bollworms, but also as core source of third-generation cotton bollworms. This trap can break the host chains of cotton bollworms, making their threats to other host crops including corn, peanut, and soybean gradually decrease [36,37,38]. The fact that the severity of the cotton bollworm in corn, peanut, sunflower, and other crops increased significantly, along with the reduction of the Bt-cotton-planting scale in Yellow River Valley after 2010, confirmed the “death trap” effect of the Bt field.
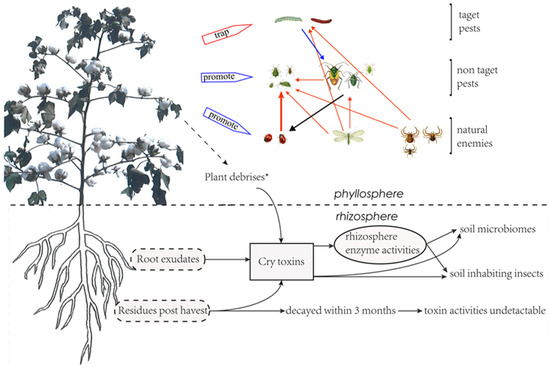
Figure 2.
Changes in pest-community interactions due to Bt cotton and Bt toxins. ★ Plant debrises include defoliation, pollen falling, and sqare and boll shedding.
Pink bollworms (P. gossypiella) are another major pest of cotton plants, which mainly attack the flowers, buds, developing bolls, and seeds, leading to a significant decrease in yield and lint quality [22,39]. They also attack other plants including kapoks and mallows, while in agricultural ecosystems, comparatively, the cotton plant is their exclusive host. This characteristic makes the population of this pest decrease rapidly along with the expansion of planting acreage of Bt cotton [40]. Thus, when Bt cotton was under an integrated pest-management (IPM) framework, the regional catastrophic risks of target pests and some other sporadic pests in cotton fields are brought under effective control at significant cost due to pesticide sprays [41,42].
3.2. Effect of Bt Cotton on Non-Targeted Pests
Bt-cotton planting has no significant effect on the individual development and population dynamics of non-target pests or natural predators, but is a strong indicator of the reduction in pesticide spray [42,43]. The reduction of pesticide sprays in Bt-cotton fields resulted in the enhancement of the biodiversity of insect species, including the population dynamics of non-targeted pests and their natural predators [36], and the interaction between them. It was observed in a study that the populations of the three main natural predators in cotton fields were ladybirds, lacewings (Chrysopa perla), and spiders, whom significantly increased along with Bt-cotton-plant scale from 1990 to 2010. Their predation behaviors were also enhanced and significantly decreased the natural populations of their prey, pest aphids, in Bt-cotton fields [36] (Figure 2). When these predators migrated to the fields of corn, peanuts, and soybeans, they also played a significant role in the natural control of aphid populations in these fields [44]. The second significant impact of reduced sprays in Bt field is that it allows the non-target secondary pests to become a major problem. The typical example is of mirid bugs, which are usually considered a secondary pest in cotton fields [44,45]. Mirid bugs have a habit of gathering in areas adjacent to the flowers and buds of host plants. At the flowering stage of the cotton plant, they coincidently migrate to the cotton field with the cotton bollworms, and this leaves them lethally trapped by the chemical sprays that control cotton bollworms. Therefore, in a conventional cotton field, controlling mirid bugs is usually not taken into consideration when primary pests are present. The predating behavior of natural enemies in the cotton field has little impact on the population development of mirid bugs [42,46]. Thus, decreased spraying of agrochemicals in Bt-cotton fields favors the development of the mirid bug population and eventually promotes them to become primary pests [17,44,47]. The outbreak of mirid bugs may bring arthropod pests under suppression [47] (Figure 2).
3.3. Effect of Bt-cotton Residues on Soil Bio-Communities
The constitutive expression of the Bt toxin in the transgenic Bt-cotton plants and the water-soluble property of the Bt toxin [43,48,49] may lead to concerns of Bt-toxin persistence in soil. The presence of cry-gene residues could be associated with the large-scale release of Bt-crops. Usually, there are plenty of leaves, stalks, roots, and falling bolls left in the Bt-cotton field after harvest, and it may take several months for these residues to decompose. The concerns might include two aspects, one is the persistence of these Bt-toxin-protein and cry-gene residues in the field and their dynamics therein, and the second is the impacts of these residues on agricultural ecosystems. Thus, studies have been conducted both in controlled laboratory conditions and in open field environments [14,50]. Tests of soil samples taken consecutively outside and inside Bt-cotton fields after three to six years of Bt planting indicated that three months after harvest the soil samples contained extremely low levels of Bt toxin, resulting in no detectable biological activities [50,51]. However, when the soils were sampled three months after the last season’s tillage, which was conducted with shredders and disk plowings, most of the plant residues were found decayed. The dynamics of the Bt toxin in those plant residues and its biological activity were unclear. Results from Bt-rice and Bt-corn suggested no significant differences in the soil’s neutral phosphatase activity [52], and there was no noteworthy variations in the decomposition of Bt-corn residues and the composition of organism communities in the soil [53]. In a controlled assay, about 50% of the introduced Bt toxin persisted in soil for at least 56 days; the activities of soil urease, acid phosphomonoesterase, and invertase were inspired, while that of soil arylsulfatase was inhibited [54]. Long-term studies indicated that consecutive lodging of Bt cotton might lead to the persistence of the Bt toxin in soil [55] and impose a negative impact on soil microbial and biochemical properties [56]. These controversial results indicate that it is still essential to evaluate the lasting impact of Bt crops in various circumstances.
4. Abiotic and Biotic Constraints to Bt-Cotton Production
4.1. Temperature
Unstable resistance of Bt cotton to insect pests has been observed during the boll-development stage, particularly under prolonged high-temperature stresses [57]. Low relative humidity together with high-temperature conditions contribute greatly to the reduction of Bt toxin content in Bt cotton leaves [57,58]. It was also observed that leaf Bt-toxin content was negatively correlated with leaf C/N ratio, which was enhanced by high temperature and nitrogen levels [59]. High-temperature stress had a similar impact on the Bt-toxin concentration in Bt-cotton plants [60]. The reduction of Bt-toxin content was thus responsible for the observed fluctuation of insect-pest-control efficacy under high-temperature conditions.
4.2. Drought
An estimation of about 30–50% annual crop loss worldwide was due to environmental stresses [61], and drought is considered as a substantial factor for crop-productivity losses [62].
Under increased drought stress, the contents of Bt toxins like Cry1Ac and Cry2Ab in Bt cotton declined, and the condition recovery was correlated to a pronounced increase in Bt-toxin content, indicating an instability of Bt-toxin production under abiotic stresses [63]. In addition, a study based on a collection of 32 cotton cultivars revealed that the Bt-toxin-content decline was correlated to the crop resistance to bollworms under drought-stress conditions [64].
4.3. Salinity
High salinity is a major threat to agricultural lands leading to land degradation, soil biology disturbance [65], growth retardations [66], yield losses, and quality attributes of cotton fibers globally. Sodium and chlorides are the most important ions contributing to soil-salinity-inducing plant disorders [67].
To remediate salt-affected soils, cotton has been planted in saline or coastal areas in Eastern China as a pilot crop for soil rehabilitation. The growth and development of H. armigera was studied in Bt-cotton fields under such conditions [68]. The results revealed that no significant difference was observed for the growth and development of H. armigera larvae under certain content ranges of saline conditions, but high-salt stress altered the larval growth and development, and adult oviposition behavior on Bt cotton [68]. This result provides some insights for the pest management of H. armigera for Bt cotton in saline-soil conditions.
4.4. Climate Change
In this century, the growing concentration of atmospheric CO2 and rising temperatures are the major challenges of global climate change. The forecast is of increased CO2 from the current 400 ppm to between 750 and 1300 ppm by the end of this century, while, in the same period, the global mean surface air temperature is anticipated to rise about 1.8–6.0 °C (IPCC, 2014). The resistance mechanisms of cotton insect pests in the context of climate change are not well studied. The elevated CO2 leads to effector-triggered immunity (ETI) and PAMP-triggered immunity (PTI) defenses [69]. Autumn is neglected due to climate change, and this causes alteration in insect reproduction rate, migration, and induction of diapause [70]. The genetic adaption to the changing climate is necessary to avoid population extinction, but genetic changes due to climate change are not well documented [71]. Studies on physiological responses of subsequent generations of insects exposed to increasing CO2 and temperature are rare [72].
The outcomes of O3 on leaf nutritional quality are not well studied but higher leaf senescence lowers the quality for insect herbivores, while alteration in the secondary chemistry and microclimate of leaves under elevated CO2 and O3 makes the plants more susceptible to insect herbivores [73,74,75]. The invasion of the fall army worm occurs only in those regions that have similar climatic conditions to the native distribution [76]. In applied ecology, the breakout of alien insects to a new land climate is an emerging issue. If evolutionary alteration of their life-history characters is fairly rapid, we cannot construct superficial forecasting for their invasion based on the properties in their original lands [77]. Climate change has increased resistance against insecticide in P. solenopsis due to the increased number of generations and shorter life cycles [78].
4.4.1. High Carbon Dioxide (CO2)
Due to elevated CO2, the plants C/N ratio is increased which effects the C-based secondary chemistry. The plants’ nutritional level, reduced because of these changes, results in low nitrogen concentration and high phenolics [79]. The raised CO2 can have diverse special effects on various trophic levels, plants, herbivores, and predators/parasitoids [80]. The elevated CO2 (E-CO2) effects the behavior of larval feeding and enhances the developmental time. It also causes reduction in adult weight, survival, and fecundity of insect herbivores, as well as altering insects’ antioxidant capabilities [81]. The chewing insects in E-CO2 grow slowly with a higher consumption and mortality rate [82]. Their fecundity is also reduced (e.g., cotton bollworm) under elevated CO2, but the number of offspring has increased in the case of aphids [83]. The larval growth of the fifth and sixth instars of H. armigera is slower with enriched CO2 as compared to ambient CO2. The female pupal weight was also lower with enriched CO2, but the duration of the pupal stage was not affected. The enriched CO2 had an adverse effect on the growth and fitness of H. armigera [84]. The consumption and metabolic rate is higher in H. armigera due to increased protease activity and carbohydrates under elevated CO2 and temperature [85]. The damage may increase because of a higher consumption rate under elevated CO2 [86]. The concentration of Bt toxin was reduced, but the concentration of gossypol, terpenoids, phenolics, and condensed tannins were increased in cotton under E-CO2 [87]. Under E-CO2, the plant response is weaker to the attack of insect herbivores [88]. The insects compensate for the nutritional deficiency due to N content dilution under E-CO2 through increasing their food intake, which causes severe damage to the host plant [89]. The E-CO2 increases the fecundity of the cotton aphid [85]. The aphid responses are species specific to E-CO2 and were the only feeding guild to respond positively to E-CO2 [90]. In future, elevated CO2 application of nitrogen fertilizer to maintain C-N balance in transgenic plants is an attractive approach [87].
4.4.2. Imminent Temperature
The prominent raised temperature caused an acceleration in insect growth, a decreased period of cohorts and productiveness, extends the dissemination of insect inhabitants, and also encourages some functional responses [91]. The effect of E-CO2 may be concurrently aggravated or alleviated in insects at a prominent high temperature [80]. The positive effect of E-CO2 on aphid performance is counteracted by a high temperature. In Bemisia tabaci, the eminent temperature and E-CO2 expressively enhanced GST and AChE expression in the first cohort group, CAT action in the third generation, and lower SOD expression in the third generation [92]. The manganese superoxide dismutase (MnSOD), peroxidase sulfate (PODS), catalase (CAT), acetylcholinesterase (AChE), and glutathione-S-transferases (GST) are important antioxidative enzymes in insects [93,94]. The protection enzymes are SOD, CAT, and POD, whereas the detoxification enzymes are GST and AChE in insect herbivores. The SOD activity is increased under E-CO2 in H. armigera [82] and A. gossypii [95].
The fluctuation in temperature influences the infestation of whitefly and jassid as compared to other environmental factors [96]. The temperature affects the activity of P450 in whitefly which ultimately influences the tolerance of whitefly to insecticides. The activity of CYP6CM1 is significantly upregulated in whitefly at 31 °C and suppressed at 35 °C [97]. The cotton jassid population is positively correlated with high temperature and negatively with low temperature [98]. The invasion of the fall army worm (FAW) occurs only to those regions that have similar climatic conditions to the native distribution [76].
Prominent CO2 and temperature enhance the consumption of food and the metabolism of larvae by increasing the activity of midgut proteases, carbohydrase’s (amylase and cellulase), and mitochondrial enzymes, and, therefore, may cause more damage to crop production. The growth and development of an insect is affected by elevated CO2 and global warming, which alters the pests and host-plant interaction [99]. Climate change also alters the insect genetics, invasion, and number of generations; therefore, there is a dire need to expand the understanding of these interaction to develop strategies to mitigate the upshots of climate variation.
4.5. Diseases
Cotton produces substantial economic return for approximately 150 countries with a planting acreage of 33 million acres providing income for approximately 100 million families [11]. It was estimated that more than 40 different diseases caused by bacteria, viruses, fungi, and nematodes have been reported on cotton plants [100] causing 10–30% annual-yield loss of cotton production worldwide. Xanthomonas citri pv. Malvacearum is a major pathogen that causes bacterial blight in G. hirsutum [101]. Fusarium wilt caused by Fusarium oxysporum f. sp. vasinfectum [102] and Verticillium wilt caused by Verticillium dahliae are two major fungal pathogenic diseases [100]. In its whole life cycle, the cotton plant may also suffer from an attack of anthracnose (Colletotrichum gossypii), ramulosis (Colletotrichum gossypii var. cephalosporioides) [103], ramularia gray mildew (Mycosphaerella areola) [104], root rots (Sclerotium rolfsii and Rhizoctonia solani) [105], leaf blight (Alternaria macrospora) [106], leaf spot (Cercospora gossypina) [107], and target spot (Corynespora cassiicola). As, currently, Bt cotton occupies the absolute majority share of the cotton-planting market, how Bt cotton responds to the diseases’ stress represents the stability of cotton production. However, the effects of these diseases on Bt cotton are still kept open to discussion, especially the stability of Bt protein expression.
4.6. Weeds
Weeds compete with crops for the available resources, such as sunlight, water, nutrients, and space, all for their growth and development. They also provide spaces and shelters for plant pathogens and pests, which may interfere with plant growth and development. Different weed-controlling strategies such as mechanical (such as manual hoeing), and chemical (such as herbicides) are adopted as integrated weed management [108,109]. Planting Bt cotton may not significantly increase the weed diversities or the risks of producing newly destructive weed species. Spraying herbicides together with tillage practices could be effective for weed managements in both conventional and Bt-cotton fields. When studied in natural wild habitats, Bt- or non-Bt-cotton seeds did not differ in their ability to germinate, establish, and survive, demonstrating that the addition of the Bt gene does not confer fitness for weeds or establish invasive cotton populations [110,111].
5. Evolution of Pest Resistance to Bt Toxins
Although the extensive cultivation of Bt cotton around the globe has brought benefits such as pest-control efficacies and economic preferences, it also imposes strong selection pressure on the target cotton pests, which has facilitated the evolution of target-pest resistance to Bt toxin (PRBT), thereby reducing its efficacy [112,113]. After over two decades’ of application of Bt cotton, the potential threats of the resistance development of bollworm to Bt toxins are increasing. This issue was recognized as a potential problem as early as the beginning of twentieth century [6]. Various studies have recorded this threat due to irregular expression of the toxin in host plants. Many studies were then conducted to survey the resistance development of pests against Bt toxins in various selected insects under laboratory or field conditions [114]. Accumulative records of the evolution of PRBT were observed in Bt-cotton fields in China [115], India [116], the USA [117], and in various geographical regions of the world, including South Africa [118], Argentina, and Brazil [119]. The documentations of the rapid evolution of PRBT in Bt crops [120,121,122,123] have encouraged researchers to attempt to understand the genetic basis of PRBT in order to develop alternative counter mechanisms.
Studies revealed that multiple mechanisms are involved in the resistance to the evolution of pests of Bt crops, including variations in toxin activation, mutations in toxin receptors, and regulation of immune systems, and for details of which please refer to the review of Xiao and Wu [113]. In the current review, we examine factors that contribute greatly to the evolution of PRBT in Bt-cotton fields. These factors include lack of regulation and/or compliance with the Environmental Protection Agency (EPA) policies, sub-lethal expression of Bt genes in plant tissues, continuous exposure to the same Bt toxins, and cross resistance to multiple Bt toxins [124].
The long-term practice of planting Bt cotton has led countries to include a variety of complex integrated governance systems to manage the PRBT evolution, and incompliances with these resistance-managing measurements will lead to the failure to manage PRBT evolution [113,125]. A recent study on the environmental and agronomic impacts of Bt cotton in Mexico over the past 20 years has shown that the management strategies used there to prevent PRBT evolution in cotton fields are successful, and no adverse effects on non-target organisms have been observed [126]. Similar strategies are recommended in other regions where Bt cotton is planted for cotton production.
The sub-lethal expression of cry-genes in plant tissues is one of the principal causes of the evolution of bollworm resistance to Bt toxins [127]. Actually, the expression of the insecticidal Bt toxins in Bt cotton is inconsistent in different genotypes [128], occurring in different parts and tissues of plants [96], showing a decreasing expression pattern as plant-age advances [129]. The factors that were reported to be responsible for low expression of cry genes were summarized in Table 1. Cross resistance could also lead to the evolution of PRBT. In a pyramiding strategy, transgenic cotton producing the two Bt toxins Cry1Ac and Cry2Ab was used to control Helicoverpa zea, a major pest in North America; it was demonstrated that selection with Cry1Ac increased the pest survival on cotton plants expressing the two toxins of Cry1Ac and Cry1Ab. Further analysis indicated typical cross-resistances between Cry1A and Cry2A toxins [130,131]. Cross resistance of H. zea to some Bt toxins has also been documented in some other pyramiding events [132,133].

Table 1.
Factors affecting the expression of cry gene in transgenic plants.
Continuous exposure to the same Bt toxins in a large-scale Bt crop will generate a large selective pressure for pest resistance, especially in the laboratory conditions, where rapid evolution of PRBT was observed when target pests are exposed continually to the same Bt toxins. The laboratory results may not represent the actual happenings in the field, but they provide early warning of potential resistance problems [138]. Long-term monitoring of Bt-cotton planting in China revealed that the sensitivity of field colonies of the cotton bollworm to Cry1Ac toxin decreased under the continuous exposure pressures; although, no failure cases of Bt cotton control were recorded [139]. A study of Tabashnik and colleagues [140] showed that the frequency of resistance alleles had increased substantially in some field populations of H. zea. Although this statement caused some debates [138], evidence of PRBT evolution gradually accumulated. Practically, resistance was observed in the insect species of B. Fusca, D.v. virgifera, H. zea, P. gossiypiella, and S. frugiperda against Cry1Ab, Cry3Bb, Cry1Ac, Cry1Ac, and Cry1Fa, respectively [141]. Fall army worm (S. frugiperda) has developed a maximum level of resistance against Cry1Ac, Cry2Ab, and Cry1Fa [142].
6. Strategies to Tackle Problems of PRBT Evolution
The emergence of resistance to Bt toxins in pest populations has prompted an immense interest of scientists to investigate the resistance mechanisms and to propose strategies to effectively manage the pest damages in Bt-cotton fields for sustainable cotton production. The strategies include phenotypic plasticity, investigation of individual cry-gene resistance in specific plant species for specific insect control [130], refuge crop strategy [143], mixing of seeds harboring different toxin genes, stacking two or more insecticidal toxin genes for one target insect [144], releasing sterile insects [145], bio-control agents, and bio-signaling [144]. Furthermore, whole-genome sequencing of the pest genome enabled researchers to identify genetic variations and QTLs using molecular markers including SSR, SNPs, and InDels to predict genetic interactions between pests and the host crop [146]. The advent of clustered regularly interspaced short palindromic repeats and caspase 9 activity (CRISPR/Cas9) [147], coupled with the above-mentioned strategies, prompted scientists to develop integrated strategies for the management of PRBT evolution to slow down the pace of advent of resistant pests of Bt cotton.
6.1. Phenotypic Plasticity
It is hypothesized that resistance development is not only a genetically controlled phenomenon, but also that of gene expression which reveals the impacts of environmental interactions [148]. Phenotypic variations of a trait depend upon the expression of other genes. A single genotype (individual gene) may be responsible for multiple phenotypes in different environments [149]. Phenotypes of a trait controlled by a gene or multiple genes were greatly influenced by macro- and micro-environments. The role of the environment in the resistance performance of Bt crops has been neglected. In the case of Bt cotton and for bollworm control, a Bt cultivar could exhibit high resistance to bollworm (implying high susceptibility of the insect to Bt toxin produced by the plant) in one environment, while low resistance (low susceptibility of the insect) is present in the other, suggesting phenotypic plasticity of Bt cotton [148]. For pink bollworms, their monophagy nature encourages them to feed on their preferred diet. This strict diet of pink bollworms may regulate their susceptibility and resistance to Cry proteins [150]. In a laboratory examination, when the larvae of corn earworm were fed with different nutrient combinations of a carbohydrate–protein diet, they showed variations in survival when challenged with Cry1Ac protein [148].
6.2. High-Dose/Refuge Strategy
High-dose/refuge strategy was probably the first worthy strategy in consideration for the management of PRBT evolution that was put into research and practical application. However, as mentioned above, the success of dose strategy requires a high concentration of the Bt toxins expressed in the plant which ensures ≥95% mortality of the heterozygous pest individuals that have one copy of the resistance allele. According to the U.S. Environmental Protection Agency, a dose 50 times higher than the concentration for killing 50% of Bt-susceptible larvae is required to assure the success of the high dose/refuge strategy. In this strategy, high expression of the cry gene reaching consistent lethal levels in all plant tissues on which the pests feed is indispensable for effective pest control [116,128]. One important fact of this high-dose strategy for management of PRBT evolution is that the dose concentration that ensures effective resistance against one target pest may not be effective against another [151]. The refuge strategies are based on three assumptions: recessive-resistant mutation to Bt toxin, low frequency of resistant mutation, and effective dilution of resistant mutations in susceptible populations which are planted near the Bt crop [113]. Most of the resistant mutations are recessive ones that must be homozygous to display phenotypes [132,152,153,154]. Then, refuge arrangement and layout in Bt-crop fields for the particular insect pest are critical for the success of a refuge strategy for the management of PRBT evolution. The effective refuges can be conventional non-Bt-cotton plants, other field crops including corn, peanut, soybean, and vegetables [143]. In the case of using different host crops of a pest species as refuges for the management of PRBT evolution, the crops that were introduced with similar Bt genes cannot be used as refuges to each other, because such crops may share a common resistance selection to the target pests [144]. This circumstance is observed in parts of the USA Cotton Belt where common hosts of H. zea, Bt corn, and cotton are planted, and in the Cerrado region of Brazil where the fall armyworm and Helicoverpa spp. are hosted by Bt corn, cotton, and soybeans [125]. The refuge strategies provide enough susceptible insects to effectively dilute the pests with resistant locus to delay the accumulation of resistant-pest population. In a four-year field study on H. armigera against Bt cotton expressing Cry1Ac toxin in six provinces of China, the results revealed an increase in the percentage of resistant insects from 0.93% in 2010 to 5.5% in 2013 [143], much lower than the model prediction of 98% in the same time spell without natural refuges. The results suggest that the natural refuge strategy effectively delays resistance development in the pest [143].
Mixing some non-Bt seeds into Bt seeds was found to be an effective method of dose strategy for the management of PRBT evolution in transgenic Bt-cotton fields. This methodology ensures that growers comply with the refuge strategy by prior mixing the Bt and non-Bt seeds. In an 11-year study in China, transgenic Bt seeds/plants were crossed/mixed with conventional non-Bt cotton seeds/plants, with the second filial generation (F2) consisting of 3/4 Bt plants producing Bt toxin, and 1/4 of non-Bt plants were planted to perform the study. The results demonstrated that the seed-mixing strategy effectively delayed the resistance of the pink bollworm (P. gossypiella) against the Cry1Ac toxin [155].
6.3. Release of Sterile Pests
Release of sterile pests in the Bt-cotton field demonstrated a significant delay in the development of resistant pink bollworm to Bt toxins [145,156]. Sterile insects were released to mate with the resistant pests under the condition of there being no refuges in Arizona. Computer simulations show that this method works effectively against pests with recessive or dominant inheritance of resistances. Over a 4-year period and large-scale adoption of this strategy, the resistance of P. gossypiella against Bt cotton did not increase [145]. When this ‘release of sterile pest’ strategy was incorporated into a multitactic eradicating program, the abundance of P. gossypiella in a Bt-cotton field was reduced by >99%, showing its effectiveness in delaying the development of resistant pink bollworm to Bt toxins [145].
6.4. Stacking of Genes and RNAi
A gene-stacking strategy, expressing two or more different insecticidal toxins in the transgenic plants against the same target pest, was proven to be highly effective in delaying resistance development [124]. In 2003 and 2006, the two Cry genes Cry2Ab and Cry1Fa were, respectively, stacked with the Cry1Ac gene and introduced into the cotton genomes. Then, Bt cotton plants that produce only the Cry1Ac toxin were replaced with the newly engineered Bt plants that produce two Bt toxins, Cry1Ac + Cry2Ab or Cry1Ac + Cry1Fa, for the control of H. zea [130,131]. The results show that such a gene-pyramiding strategy can effectively ameliorate the management of PRBT evolution. Although it does not eliminate the need for implementing strategies as ‘high-dose ⁄refuge’, it really alleviates some of the selecting pressure of the latter [124].
An attempt to stack RNAi strategy to Bt cotton has also been proved to be effective in slowing down the resistance development of the pests. In this strategy, a double-stranded RNA construct was introduced into cotton genomes, targeting the P450 monooxygenase gene, cyp6AE14, an important gene in bollworm metabolism, which enables H. armigera to digest a diet containing gossypol. When larvae were fed with such transgenic plant tissues expressing this double-stranded RNA construct, the transcription of this key cypP6AE14 gene in midgut cells of the pests was silenced, leading to retarded larvae growth due to the gossypol intoxication produced by the cotton plants [157]. When two RNAi constructs targeting H. armigera metabolism genes, the juvenile hormone acid methyltransferase (JHAMT) gene and the juvenile hormone binding protein (JHBP) gene were stacked with a Bt gene in cotton plants, respectively, in China, the pyramided cotton combining a Bt toxin and RNAi substantially delayed the resistance evolution in pests compared with using Bt cotton alone [158]. However, the challenges of improving the RNAi strategy for sustainable Bt cotton are still needed to be combatted [159].
6.5. Genome Editing
CRISPR/Cas9 technology has been demonstrated to be a promising approach to amend the genome. It significantly facilitates functional studies of both model and non-model species. This system has been used as a precise genome-editing system for various pests including Coleoptera, Diptera, Hemipitera, and Lepidoptera. CRISPR/Cas9-mediated knockout of the Lepidoptera olfactory receptor co-receptor Orco gene caused defects in plant-odor and sex-pheromone olfactory detection in homozygous individuals of the pests [160]. Genome editing of Wnt-1, a gene well known for its role in the early body planning in the Pine Caterpillar Moth, Dendrolimus punctatus, led to high embryonic mortality [161]. These results demonstrate that CRISPR/Cas9 is a simple and highly efficient technique in the development of novel pest-control strategies.
6.6. Bio-Control Agents
Employment of ecological friendly biopesticides particular to the mark organisms provides a promising alternative approach for pest control [162]. Biopesticides are consequential of plants and animals with active modules of microbial agents that include bacteria, virus, fungi, and algae [163]. Spodoptera littoralis is a polyphagous organism that assaults cotton, feeding on leaves, flower buds, fruiting points, and bolls. Damages associated with S. littoralis are severe in North Africa and Egypt. In a recent report, the spore suspensions of entomopathogenic fungi Curvularia lunata, Alternaria solani, and A. alternata exhibited promising controlling effects against S. littoralis with a mortality of 60%, 40%, and 33.3%, respectively [164]. Release of Rhynocoris longifrons, a generalist pillager of many cotton insect pests, in cotton fields was accomplished to reduce the population of H. armigera (50%), P. solenopsis (28%), D. cingulatus (18.8%), and A. gossypii (11.8%) during the rain-fed condition [165]. The release of natural enemies, Chrysoperla carnea and Trichogramma chilonis, incorporated with the use of artificial food sprays, consisting of different food attractants such as protein hydrolysate, sugar or a combination, has a great potential to encourage the population establishment of these as natural enemies. Subsequently, pest control was enhanced through the increased predation/parasitism percentage in thec cotton field [166].
6.7. Bio-Signaling and Microbial Communication
Alternative strategies other than Bt toxins would release the selective pressure of pests targeting Bt genes, and, thus, overcome the problem of the development of PRBT. In an ecosystem, plants interact dynamically with living and non-living components in their surrounding environment to elicit the adaptive and acceptable responses among different species [167]. These ecological relationships includes parasitism, symbiosis, and predation that establish complex communications among and within the species through physiological signals, pheromones, kairomones, hormones, metabolites, peptides, proteins, and RNAs [168]. Plants can sense the chemicals released by insects and prepare themselves a defense strategy through secreting anti-feedings, while the herbivorous pests may also develop mechanisms to digest such secondary metabolites or to secrete mitigants to avoid plant response to their attacks [169]. Unraveling such complex biotic relationships among different symbiotic organisms would provide tools to develop sustainable novel insect-control strategies. For example, a phytopathogenic bacteria belonging to family Xanthomonadaceae, produces a diffusible factor (DSF) acting as a cell-to-cell communication molecule eliciting an innate immune response in plants [170]. The expression of the enzyme responsible for producing DSF was successfully engineered in tobacco and the sweet orange for resistance against insect pests [169]. In another study, the mate recognition and localization of the grapevine pest Scaphoideus titanus was prevented by imitating vibrational signals with an artificial noise vibration [171]. These studies indicate that the defense system of plants against pests can be enhanced by manipulating different signals such as quorum-sensing signals through signal transduction and intracellular cascade reactions [169].
7. Conclusions
G. hirsutum is a major species for cotton production worldwide. Chemical pesticides have been used to prevent severity of insect pests and to enhance cotton yield all over the world. The adoption of genetically modified Bt cotton has presented several benefits, including reducing the load of insecticides into the cotton field, bringing better control of target pests, improving yield potential and stability, and increasing biodiversity of the non-target insects. However, it also faces some special challenges, including that the efficacy varies in different regions due to different climatic, ecological and management conditions, insect diversity, and that the target insect pests have evolved resistance against Bt cotton, which has imposed a major threat to the budget of cotton-manufacturing countries. In order to prevent or control this problem, the following approaches are recommended: (1) Novel genetic strategies together with integrated management of PRBT evolution such as a dose/refuge strategy, release of sterile insects in the field, and pyramiding of different toxin genes or genes with different pest-control mechanisms should be taken into consideration for future developments. (2) Other controlling tactics including new resources of toxin-producing genes or techniques such as RNAi and CRISPR/Cas9 are also highly recommended to overcome this insect resistance problem. (3) To succeed in improving cotton production worldwide, it is necessary to coordinate efforts from all participants around the cotton-production industry including researchers, farmers, and technicians.
In summary, cotton has become susceptible to many insect pests, and it depends on novel biotechnological solutions to prevent the losses incurred by these pests. Biotechnology solutions provide an eco-friendly system for the market of global cotton production. With the development of cotton genome sequencing and genome-editing technologies, it will bring new opportunities for cotton-pest-control strategies that shall radically change current practices to reduce losses and benefit the environment. In short, this task is still not fully completed on a global scale.
Author Contributions
A.R. and M.M.Z. wrote the initial draft of the manuscript. F.S., A.A., F.S., F.Q. and L.T.Z. made all necessary corrections and carried out final editing of manuscript. W.G., P.L. and A.H.L. proofread the manuscript. Final approval for publication was given by the group leader at the institute of cotton research Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01B114), Special Fund for Basal Research of Central Public-interest Scientific Institutions (CN) (NO.1610162023013, 1610162023002), High Quality Cotton New Variety Zhongmiansuo 703 Efficient Technology Integration Demonstration Project of Kashgar Regional Science and Technology Plan (KS2023003), Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences China Agriculture Research System of MOF and MARA.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
We would like to thank ICR-GSCAAS for the financial support.
Conflicts of Interest
Arfan Ali is employed by FB Genetics Four Brothers Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be constructed as a potential conflict of interest.
References
- Zafar, M.M.; Jia, X.; Shakeel, A.; Sarfraz, Z.; Manan, A.; Imran, A.; Mo, H.; Ali, A.; Youlu, Y.; Razzaq, A.J.; et al. Unraveling Heat Tolerance in Upland Cotton (Gossypium hirsutum L.) Using Univariate and Multivariate Analysis. Front. Plant Sci. 2021, 12, 727835. [Google Scholar] [CrossRef] [PubMed]
- Manan, A.; Zafar, M.M.; Ren, M.; Khurshid, M.; Sahar, A.; Rehman, A.; Firdous, H.; Youlu, Y.; Razzaq, A.; Shakeel, A.J. Genetic analysis of biochemical, fiber yield and quality traits of upland cotton under high-temperature. Plant Prod. Sci. 2022, 25, 105–119. [Google Scholar] [CrossRef]
- Zafar, M.M.; Mustafa, G.; Shoukat, F.; Idrees, A.; Ali, A.; Sharif, F.; Shakeel, A.; Mo, H.; Youlu, Y.; Ali, Q.; et al. Heterologous expression of cry3Bb1 and cry3 genes for enhanced resistance against insect pests in cotton. Sci. Rep. 2022, 12, 10878. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Qin, D.; Manning, M.; Averyt, K.; Marquis, M.; Tignor, M.M. Climate Change 2007-the Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Zafar, M.M.; Rehman, A.; Razzaq, A.; Parvaiz, A.; Mustafa, G.; Sharif, F.; Mo, H.; Youlu, Y.; Shakeel, A.; Ren, M.J. Genome-wide characterization and expression analysis of Erf gene family in cotton. BMC Plant Biol. 2022, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Guo, Y.Y. The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Zafar, M.M.; Ali, A.; Hafeez, A.; Sharif, F.; Guan, X.; Deng, X.; Pengtao, L.; Shi, Y.; Haroon, M.J. The pivotal role of major chromosomes of sub-genomes A and D in fiber quality traits of cotton. Front. Genet. 2021, 12, 642595. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, M.; Jia, F.; Gong, J.; Palanga, K.K.; Shi, Y.; Li, J.; Shang, H.; Liu, A.; Chen, T.; Zhang, Z.; et al. Identification of stable quantitative trait loci (QTLs) for fiber quality traits across multiple environments in Gossypium hirsutum recombinant inbred line population. BMC Genom. 2016, 17, 197. [Google Scholar] [CrossRef]
- Razzaq, A.; Zafar, M.M.; Ali, A.; Hafeez, A.; Batool, W.; Shi, Y.; Gong, W.; Yuan, Y.J. Cotton germplasm improvement and progress in Pakistan. J. Cotton Res. 2021, 4, 1. [Google Scholar] [CrossRef]
- Kamburova, V.; Salakhutdinov, I.; Abdurakhmonov, I.Y. Cotton Breeding in the View of Abiotic and Biotic Stresses: Challenges and Perspectives. In Cotton; IntechOpen: London, UK, 2022. [Google Scholar]
- USDA. United States Department of Agriculture. 2015. Available online: https://www.usda.gov/ (accessed on 18 November 2023).
- El-Gendy, R.M.; Abd-ElAzeem, E.M.; El-Shafiey, S.N. Moringa oleifera and Ruta angustifolia fixed oils and a prospective method to obstruct pupal development in cotton leafworm. J. Saudi Soc. Agri Sci 2023. [Google Scholar] [CrossRef]
- Deshmukh, K.V.; Patil, C.S.; Walunj, A.R.; Wagh, R.S.; Chimote, V.P.; Bhute, N.K. Influence of weather factors on population dynamics of lepidopteran pests of Bt cotton. Pharma Innov. J. 2023, 12, 9–12. [Google Scholar]
- Zafar, M.M.; Razzaq, A.; Farooq, M.A.; Rehman, A.; Firdous, H.; Shakeel, A.; Mo, H.; Ren, M. Insect resistance management in Bacillus thuringiensis cotton by MGPS (multiple genes pyramiding and silencing). J. Cotton Res. 2020, 3, 33. [Google Scholar] [CrossRef]
- Ardameh, M.; Olyaie-Torshiz, A.; Torabi, E.; Taherian, M. The efficacy of some pesticides on cotton yield, damage symptoms, and population of the cotton shredder bug, Creontiades pallidus (Hemiptera: Miridae) under field conditions. J. Crop Prot. 2023, 12, 253–263. [Google Scholar]
- Toscano-Miranda, R.; Toro, M.; Aguilar, J.; Caro, M.; Marulanda, A.; Trebilcok, A. Artificial-intelligence and sensing techniques for the management of insect pests and diseases in cotton: A systematic literature review. J. Agric. Sci. 2022, 160, 16–31. [Google Scholar] [CrossRef]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Dai, H.; Jin, Z.; Shen, H.; Guan, F.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Evaluating Cross-Resistance to Cry and Vip Toxins in Four Strains of Helicoverpa armigera with Different Genetic Mechanisms of Resistance to Bt Toxin Cry1Ac. Front. Microbiol. 2021, 12, 670402. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Ali, A.; Zafar, M.M.; Nawaz, A.; Xiaoying, D.; Pengtao, L.; Qun, G.; Ashraf, M.; Ren, M.; Gong, W.; et al. Pyramiding of cry toxins and methanol producing genes to increase insect resistance in cotton. GM Crop. Food 2021, 12, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Du, X.; Qiao, F.; Trujillo-Barrera, A. Technology Adoption and Learning-by-Doing: The Case of Bt Cotton Adoption in China. J. Risk Financ. Manag. 2021, 14, 524. [Google Scholar] [CrossRef]
- Pray, C.E.; Huang, J.; Hu, R.; Rozelle, S. Five years of Bt cotton in China–the benefits continue. Plant J. 2002, 31, 423–430. [Google Scholar] [CrossRef]
- Lu, Y.; Wyckhuys, K.A.; Yang, L.; Liu, B.; Zeng, J.; Jiang, Y.; Desneux, N.; Zhang, W.; Wu, K. Bt cotton area contraction drives regional pest resurgence, crop loss, and pesticide use. Plant Biotechnol. J. 2022, 20, 390–398. [Google Scholar] [CrossRef]
- Ashraf, S.; Iftikhar, M.; Khan, G.A.; Hassan, M.Z.Y. Worldwide impact of BT cotton: Implications for agricultural research in Pakistan. Greener J. Agron. For. Hortic. 2013, 1, 12–20. [Google Scholar] [CrossRef]
- Rana, M.A. When seed becomes capital: Commercialization of Bt cotton in Pakistan. J. Agrar. Change 2021, 21, 702–719. [Google Scholar] [CrossRef]
- Arshad, M.; Suhail, A.; Gogi, M.D.; Yaseen, M.; Asghar, M.; Tayyib, M.; Karar, H.; Hafeez, F.; Ullah, U.N. Farmers’ perceptions of insect pests and pest management practices in Bt cotton in the Punjab, Pakistan. Int. J. Pest Manag. 2009, 55, 1–10. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, Z.; Guo, Z. Cotton growing in Pakistan, Bangladesh and Myanmar. In Pest Management in Cotton: A Global Perspective; CABI: Wallingford, UK, 2022; pp. 53–79. [Google Scholar]
- Spielman, D.J.; Zaidi, F.; Zambrano, P.; Khan, A.A.; Ali, S.; Cheema, H.M.N.; Nazli, H.; Khan, R.S.A.; Iqbal, A.; Zia, M.A. What are farmers really planting? Measuring the presence and effectiveness of Bt cotton in Pakistan. PLoS ONE 2017, 12, e0176592. [Google Scholar] [CrossRef]
- Shah, D.K. Bt cotton in India: A review of adoption, government interventions and investment initiatives. Indian J. Agric. Econ. 2012, 67, 365–375. [Google Scholar]
- Barwale, R.; Gadwal, V.; Zehr, U.; Zehr, B. Prospects for Bt cotton technology in India. agBioForum 2004, 7, 23–26. [Google Scholar]
- Najork, K.; Friedrich, J.; Keck, M.J.A.; Values, H. Bt cotton, pink bollworm, and the political economy of sociobiological obsolescence: Insights from Telangana, India. Agric. Hum. Values 2022, 39, 1007–1026. [Google Scholar] [CrossRef]
- CAB. The Cotton Advisory Board India. 2018. Available online: https://www.ind.gov/ (accessed on 18 November 2023).
- Akbar, W.; Gowda, A.; Ahrens, J.E.; Stelzer, J.W.; Brown, R.S.; Bollman, S.L.; Greenplate, J.T.; Gore, J.; Catchot, A.L.; Lorenz, G.; et al. First transgenic trait for control of plant bugs and thrips in cotton. Pest Manag. Sci. 2019, 75, 867–877. [Google Scholar] [CrossRef]
- Raphael, J.P.A. Transgenic traits in the cotton crop in Brazil: A review. Colloq. Agrar. 2019, 15, 115–129. [Google Scholar]
- Khan, M.A.; Wahid, A.; Ahmad, M.; Tahir, M.T.; Ahmed, M.; Ahmad, S.; Hasanuzzaman, M. World cotton production and consumption: An overview. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Springer: Singapore, 2020; pp. 1–7. [Google Scholar]
- Lapegna, P.; Perelmuter, T. Genetically modified crops and seed/food sovereignty in Argentina: Scales and states in the contemporary food regime. J. Peasant. Stud. 2020, 47, 700–719. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362. [Google Scholar] [CrossRef]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef]
- Panjgotra, S.; Sudershan, A.; Sharma, V.; Sharma, P. Bt cotton. In Genetically Modified Crops and Food Security; Routledge: London, UK, 2022; pp. 32–52. [Google Scholar]
- Wu, K.-M. Environmental impact and risk management strategies of Bt cotton commercialization in China. Chin. J. Agric. Biotechnol. 2007, 4, 93–97. [Google Scholar]
- Wu, Z.; Soliman, K.; Zipf, A.; Saha, S.; Sharma, G.; Jenkins, J. Isolation and characterization of genes differentially expressed in fiber of Gossypium barbadense L. J. Cotton Sci. 2005, 9, 166–174. [Google Scholar]
- Naranjo, S.E. Impacts of Bt transgenic cotton on integrated pest management. J. Agric. Food Chem. 2010, 59, 5842–5851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, Y.; van der Werf, W.; Huang, J.; Wu, F.; Zhou, K.; Deng, X.; Jiang, Y.; Wu, K.; Rosegrant, M.W. Multidecadal, county-level analysis of the effects of land use, Bt cotton, and weather on cotton pests in China. Proc. Natl. Acad. Sci. USA 2018, 115, E7700–E7709. [Google Scholar] [CrossRef] [PubMed]
- Kranthi, K.R.; Stone, G.D. Long-term impacts of Bt cotton in India. Nat. Plants 2020, 6, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.A.; Guo, Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef]
- Naranjo, S.E. Effects of GE Crops on Non-target Organisms. In Plant Biotechnology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 127–144. [Google Scholar]
- Zhang, L.; Lu, H.; Guo, K.; Yao, S.; Cui, F. Insecticide resistance status and detoxification enzymes of wheat aphids Sitobion avenae and Rhopalosiphum padi. Sci. China Life Sci. 2017, 60, 927–930. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Jaworski, C.C.; Yang, F.; Liu, B.; Jiang, Y.; Lu, Y.; Wu, K.; Desneux, N. The outbreaks of nontarget mirid bugs promote arthropod pest suppression in Bt cotton agroecosystems. Plant Biotechnol. J. 2020, 18, 322–324. [Google Scholar] [CrossRef]
- Douville, M.; Gagne, F.; Masson, L.; McKay, J.; Blaise, C. Tracking the source of Bacillus thuringiensis Cry1Ab endotoxin in the environment. Biochem. Syst. Ecol. 2005, 33, 219–232. [Google Scholar] [CrossRef]
- Icoz, I.; Andow, D.; Zwahlen, C.; Stotzky, G. Is the Cry1Ab Protein from Bacillusthuringiensis (Bt) Taken Up by Plants from Soils Previously Planted with Bt Corn and by Carrot from Hydroponic Culture? Bull. Environ. Contam. Toxicol. 2009, 83, 48–58. [Google Scholar] [CrossRef]
- Head, G.; Surber, J.B.; Watson, J.A.; Martin, J.W.; Duan, J.J. No detection of Cry1Ac protein in soil after multiple years of transgenic Bt cotton (Bollgard) use. Environ. Entomol. 2002, 31, 30–36. [Google Scholar] [CrossRef]
- Peshin, R.; Hansra, B.S.; Singh, K.; Nanda, R.; Sharma, R.; Yangsdon, S.; Kumar, R. Long-term impact of Bt cotton: An empirical evidence from North India. J. Clean. Prod. 2021, 312, 127575. [Google Scholar] [CrossRef]
- Wu, K.M.; Guo, Y.Y. Influences of Bacillus thuringiensis Berliner Cotton Planting on Population Dynamics of the Cotton Aphid, Aphis gossypii Glover, in Northern China. Environ. Entomol. 2003, 32, 312–318. [Google Scholar] [CrossRef]
- Zwahlen, C.; Hilbeck, A.; Nentwig, W. Field decomposition of transgenic Bt maize residue and the impact on non-target soil invertebrates. Plant Soil 2007, 300, 245–257. [Google Scholar] [CrossRef]
- Sun, C.X.; Chen, L.J.; Wu, Z.J.; Zhou, L.K.; Shimizu, H. Soil persistence of Bacillus thuringiensis (Bt) toxin from transgenic Bt cotton tissues and its effect on soil enzyme activities. Biol. Fertil. Soils 2006, 43, 617–620. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Hu, W.; Wang, S.; Wang, Y.; Snider, J.L.; Zhou, Z. Combined elevated temperature and soil waterlogging stresses inhibit cell elongation by altering osmolyte composition of the developing cotton (Gossypium hirsutum L.) fiber. Plant Sci. 2017, 256, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Chen, L.J.; Zhang, Y.L.; Wu, Z.J. Microbial properties, enzyme activities and the persistence of exogenous proteins in soil under consecutive cultivation of transgenic cottons (Gossypium hirsutum L.). Plant Soil Environ. 2011, 57, 67–74. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Zhang, X.; Chen, D. The effects of the relative humidity on the insecticidal expression level of Bt cotton during bolling period under high temperature. Field Crop Res. 2012, 137, 141–147. [Google Scholar] [CrossRef]
- Khan, M.K.R.; Liu, F.; Wang, B.; Hussain, M.; Ditta, A.; Anwar, Z.; Ijaz, A. Breeding Cotton for Heat Tolerance. In Cotton Breeding and Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 113–138. [Google Scholar]
- Zhang, X.; Lü, C.-H.; Yuan, C.; Wang, G.-X.; Chen, D.-H. Relationship between leaf C/N ratio and insecticidal protein expression in Bt cotton as affected by high temperature and N rate. J. Integr. Agric. 2014, 13, 82–88. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Jin, X.; Jia, R.; Huang, Q.; Tan, Y.; Guo, A. Comparative proteomics of Bt-transgenic and non-transgenic cotton leaves. Proteome Sci. 2015, 13, 15. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wedegaertner, K.; Shekoofa, A.; Sheldon, K.; Simón, J.; Raper, T.B. Screening cotton cultivars under induced water-deficit stress in controlled environments and field settings: Expression of drought tolerance traits. J. Crop. Improv. 2022, 37, 395–416. [Google Scholar] [CrossRef]
- Parimala, P.; Muthuchelian, K. Physiological response of non-Bt and Bt cotton to short-term drought stress. Photosynthetica 2010, 48, 630–634. [Google Scholar] [CrossRef]
- Ullah, I.; Mehboob-ur-Rahman; Ashraf, M.; Zafar, Y. Genotypic variation for drought tolerance in cotton (Gossypium hirsutum L.): Leaf gas exchange and productivity. Flora 2008, 203, 105–115. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J. Effect of salinity stress on phenotypic plasticity, yield stability, and signature of stable isotopes of carbon and nitrogen in safflower. Environ. Sci. Pollut. Res. 2018, 25, 23685–23694. [Google Scholar] [CrossRef] [PubMed]
- Dong, H. Technology and field management for controlling soil salinity effects on cotton. Aust. J. Crop Sci. 2012, 6, 333. [Google Scholar]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, S.; Zhu, X.; Ji, J.; Zhang, K.; Wang, C.; Zhang, L.; Wang, L.; Cui, J. Effects of NaCl stress on the biochemical substances in Bt cotton as well as on the growth and development and adult oviposition selectivity of Helicoverpa armigera. J. Cotton Res. 2019, 2, 17–25. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Yuan, E.; Ge, F. Elevated CO2 increases R gene-dependent resistance of Medicago truncatula against the pea aphid by up-regulating a heat shock gene. New Phytol. 2018, 217, 1696–1711. [Google Scholar] [CrossRef]
- Gallinat, A.S.; Primack, R.B.; Wagner, D.L. Autumn, the neglected season in climate change research. Trends Ecol. Evol. 2015, 30, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Merilä, J. Evolution in response to climate change: In pursuit of the missing evidence. BioEssays 2012, 34, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P. Range-Expanding Pests and Pathogens in a Warming World. Annu. Rev. Phytopathol. 2015, 53, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Kiritani, K. A simple method to estimate the potential increase in the number of generations under global warming in temperate zones. Appl. Entomol. Zool. 1998, 33, 289–298. [Google Scholar] [CrossRef]
- Dermody, O.; O’Neill, B.F.; Zangerl, A.R.; Berenbaum, M.R.; DeLucia, E.H. Effects of elevated CO2 and O3 on leaf damage and insect abundance in a soybean agroecosystem. Arthropod-Plant Interact. 2008, 2, 125–135. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Yamanaka, T.; Tatsuki, S.; Shimada, M. Adaptation to the new land or effect of global warming? An age-structured model for rapid voltinism change in an alien lepidopteran pest. J. Anim. Ecol. 2008, 77, 585–596. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007, 63, 628–633. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Ge, F. Plant–aphid interactions under elevated CO2: Some cues from aphid feeding behavior. Front. Plant Sci. 2016, 7, 502. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Huang, W.; Su, L.; Wu, G.; Zhuang, J.; Zhao, W.; Hua, H.; Li, J.; Xiao, N.; Xiong, Y. Effects of elevated CO2 on the nutrient compositions and enzymes activities of Nilaparvata lugens nymphs fed on rice plants. Sci. China Life Sci. 2012, 55, 920–926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, G.; Chen, F.-J.; Ge, F. Response of multiple generations of cotton bollworm Helicoverpa armigera Hübner, feeding on spring wheat, to elevated CO2. J. Appl. Entomol. 2006, 130, 2–9. [Google Scholar] [CrossRef]
- Peltonen, P.A.; Julkunen-Tiitto, R.; Vapaavuori, E.; Holopainen, J.K. Effects of elevated carbon dioxide and ozone on aphid oviposition preference and birch bud exudate phenolics. Glob. Change Biol. 2006, 12, 1670–1679. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Chi, H.; Wang, C.; Hua, H.; Wu, G. Effects of elevated CO2 on the fitness and potential population damage of Helicoverpa armigera based on two-sex life table. Sci. Rep. 2017, 7, 1119. [Google Scholar] [CrossRef]
- Chen, F.; Ge, F.; Parajulee, M.N. Impact of Elevated CO2 on Tri-Trophic Interaction of Gossypium hirsutum, Aphis gossypii, and Leis axyridis. Environ. Entomol. 2005, 34, 37–46. [Google Scholar] [CrossRef]
- Akbar, S.M.; Pavani, T.; Nagaraja, T.; Sharma, H. Influence of CO2 and temperature on metabolism and development of Helicoverpa armigera (Noctuidae: Lepidoptera). Environ. Entomol. 2015, 45, 229–236. [Google Scholar] [CrossRef]
- Coviella, C.E.; Stipanovic, R.D.; Trumble, J.T. Plant allocation to defensive compounds: Interactions between elevated CO2 and nitrogen in transgenic cotton plants. J. Exp. Bot. 2002, 53, 323–331. [Google Scholar] [CrossRef]
- Vuorinen, T.; Nerg, A.-M.; Ibrahim, M.; Reddy, G.; Holopainen, J.K. Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 2004, 135, 1984–1992. [Google Scholar] [CrossRef]
- Salt, D.; Brooks, G.; Whittaker, J. Elevated carbon dioxide affects leaf-miner performance and plant growth in docks (Rumex spp.). Glob. Change Biol. 1995, 1, 153–156. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, F. How do aphids respond to elevated CO2? J. Asia-Pac. Entomol. 2011, 14, 217–220. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Zhang, S.; Fan, Y.; Liu, T. Effect of elevated CO2 concentration and temperature on antioxidant capabilities of multiple generations of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). J. Insect Physiol. 2017, 103, 91–97. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.H. Which acetylcholinesterase functions as the main catalytic enzyme in the Class Insecta? Insect Biochem. Mol. Biol. 2013, 43, 47–53. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, F.-J.; Sun, Y.-C.; Ge, F. Response of Cotton to Early-Season Square Abscission under Elevated CO. Agron. J. 2007, 99, 791. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, A.A.; Cheema, H.M.N.; Khan, R.S.A. Spatio-temporal and intra-plant expression variability of insecticidal gene (cry1ac) in upland cotton. Int. J. Agric. Biol. 2018, 20, 715–722. [Google Scholar]
- Guo, L.; Su, M.; Liang, P.; Li, S.; Chu, D. Effects of high temperature on insecticide tolerance in whitefly Bemisia tabaci (Gennadius) Q biotype. Pestic. Biochem. Physiol. 2018, 150, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Meena, T.; Kanwar, H. Impact of weather factors on population build up of the cotton jassid, Amrasca biguttula biguttula (Ishida) on okra under mid-hill conditions of Himachal Pradesh. Pest Manag. Econ. Zool. 2010, 18, 295–299. [Google Scholar]
- Gutierrez, A.P.; D’Oultremont, T.; Ellis, C.K.; Ponti, L. Climatic limits of pink bollworm in Arizona and California: Effects of climate warming. Acta Oecologica 2006, 30, 353–364. [Google Scholar] [CrossRef]
- Shaban, M.; Miao, Y.; Ullah, A.; Khan, A.Q.; Menghwar, H.; Khan, A.H.; Ahmed, M.M.; Tabassum, M.A.; Zhu, L. Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol. Bioch. 2018, 125, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Jalloul, A.; Sayegh, M.; Champion, A.; Nicole, M. Bacterial blight of cotton. Phytopathol. Mediterr. 2015, 54, 3–20. [Google Scholar]
- Cox, K.L., Jr.; Babilonia, K.; Wheeler, T.; He, P.; Shan, L. Return of old foes—Recurrence of bacterial blight and Fusarium wilt of cotton. Curr. Opin. Plant Biol. 2019, 50, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Salustiano, M.E.; Rondon, M.N.; Abreu, L.M.; Costa, S.D.S.; Machado, J.C.; Pfenning, L.H. The etiological agent of cotton ramulosis represents a single phylogenetic lineage within the Colletotrichum gloeosporioides species complex. Trop. Plant Pathol. 2014, 39, 357–367. [Google Scholar] [CrossRef]
- Shete, P.P.; Kasal, Y.G.; Perane, R. Screening of the cotton genotypes against Ramularia areola atk. under field condition. Plant Arch. 2018, 18, 734–736. [Google Scholar]
- Mehta, Y.R.; Marangoni, M.S.; Bocatti, C.R.; Rodrigues, H.P.; Cunha, T.S.; Galbieri, R. Systemic Acquired Resistance of Cotton, Soybean and Common Bean to Rhizoctonia solani and Sclerotium rolfsii Induced by Shale Water Seed Treatment. Am. J. Plant Sci. 2015, 6, 1493. [Google Scholar] [CrossRef]
- Cia, E.; Fuzatto, M.G.; Kondo, J.I.; Carvalho, L.H.; Ito, M.F.; Dias, F.L.F.; Gallo, P.B. Response of cotton genotypes to the incidence of Alternaria leaf spot. Summa Phytopathol. 2016, 42, 357–359. [Google Scholar] [CrossRef]
- Rothrock, C.; Kirkpatrick, T.; Frans, R.; Scott, H. The influence of winter legume cover crops on soilborne plant pathogens and cotton seedling diseases. Plant Dis. 1995, 79, 167–171. [Google Scholar] [CrossRef]
- Naqvi, R.Z.; Asif, M.; Saeed, M.; Asad, S.; Khatoon, A.; Amin, I.; Mukhtar, Z.; Bashir, A.; Mansoor, S. Development of a Triple Gene Cry1Ac-Cry2Ab-EPSPS Construct and Its Expression in Nicotiana benthamiana for Insect Resistance and Herbicide Tolerance in Plants. Front. Plant Sci. 2017, 8, 55. [Google Scholar] [CrossRef]
- Naqvi, R.Z.; Zaidi, S.S.; Mukhtar, M.S.; Amin, I.; Mishra, B.; Strickler, S.; Mueller, L.A.; Asif, M.; Mansoor, S. Transcriptomic analysis of cultivated cotton Gossypium hirsutum provides insights into host responses upon whitefly-mediated transmission of cotton leaf curl disease. PLoS ONE 2019, 14, e0210011. [Google Scholar] [CrossRef]
- Eastick, R.J.; Hearnden, M.N. Potential for weediness of Bt cotton in northern Australia. Weed Sci. 2006, 54, 1142–1151. [Google Scholar] [CrossRef]
- Rogers, D.J.; Reid, R.E.; Rogers, J.J.; Addison, S.J. Prediction of the naturalisation potential and weediness risk of transgenic cotton in Australia. Agric. Ecosyst. Environ. 2007, 119, 177–189. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Ma, Y.; Wan, P.; Liu, K.; Cong, S.; Xiao, Y.; Xu, D.; Wu, K.; Fabrick, J.A. Transposon insertion causes cadherin mis-splicing and confers resistance to Bt cotton in pink bollworm from China. Sci. Rep. 2019, 9, 7479. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, K. Recent progress on the interaction between insects and Bacillus thuringiensis crops. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20180316. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China, and India. J. Econ. Entomol. 2019, 112, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, S.; Yang, Y.; Wu, Y. Baseline Susceptibility of Field Populations of Helicoverpa armigera to Bacillus thuringiensis Vip3Aa Toxin and Lack of Cross-Resistance between Vip3Aa and Cry Toxins. Toxins 2017, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Naik, V.C.; Kumbhare, S.; Kranthi, S.; Satija, U.; Kranthi, K.R. Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders)(Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manag. Sci. 2018, 74, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef]
- van Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2013, 24, 147–151. [Google Scholar] [CrossRef]
- Grimi, D.; Ocampo, F.; Martinelli, S.; Head, G. Detection and characterization of Diatraea saccharalis resistant to Cry1A. 105 protein in a population of northeast San Luis province in Argentina. In Proceedings of the Congreso Argentino de Entomología, Misiones, Argentina, 19–22 May 2015. [Google Scholar]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P.; Doughty, H.B.; Patton, T.; Cissel, W.; Hutchison, W.D. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef]
- Nicolia, A.; Manzo, A.; Veronesi, F.; Rosellini, D. An overview of the last 10 years of genetically engineered crop safety research. Crit. Rev. Biotechnol. 2014, 34, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E. Tips for battling billion-dollar beetles. Science 2016, 354, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Andow, D.A.; Buschman, L.L. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol. Exp. Appl. 2011, 140, 1–16. [Google Scholar] [CrossRef]
- Carrière, Y.; Brown, Z.S.; Downes, S.J.; Gujar, G.; Epstein, G.; Omoto, C.; Storer, N.P.; Mota-Sanchez, D.; Jørgensen, P.S.; Carroll, S.P. Governing evolution: A socioecological comparison of resistance management for insecticidal transgenic Bt crops among four countries. Ambio 2020, 49, 1–16. [Google Scholar] [CrossRef]
- Rocha-Munive, M.G.; Soberon, M.; Castaneda, S.; Niaves, E.; Scheinvar, E.; Eguiarte, L.E.; Mota-Sanchez, D.; Rosales-Robles, E.; Nava-Camberos, U.; Martinez-Carrillo, J.L.; et al. Evaluation of the Impact of Genetically Modified Cotton After 20 Years of Cultivation in Mexico. Front. Bioeng. Biotechnol. 2018, 6, 82. [Google Scholar] [CrossRef]
- Wan, P.; Huang, Y.; Wu, H.; Huang, M.; Cong, S.; Tabashnik, B.E.; Wu, K. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS ONE 2012, 7, e29975. [Google Scholar] [CrossRef]
- Cheema, H.M.N.; Khan, A.A.; Khan, M.I.; Aslam, U.; Rana, I.A.; Khan, I.A. Assessment of Bt cotton genotypes for the Cry1Ac transgene and its expression. J. Agric. Sci. 2016, 154, 109–117. [Google Scholar] [CrossRef]
- Zaman, M.; Mirza, M.S.; Irem, S.; Zafar, Y. A temporal expression of Cry1Ac protein in cotton plant and its impact on soil health. Int. J. Agric. Biol. 2015, 17, 280–288. [Google Scholar]
- Brévault, T.; Heuberger, S.; Zhang, M.; Ellers-Kirk, C.; Ni, X.; Masson, L.; Li, X.; Tabashnik, B.E.; Carrière, Y. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. USA 2013, 110, 5806–5811. [Google Scholar] [CrossRef]
- Crespo, A.L.; Alves, A.P.; Wang, Y.; Hong, B.; Flexner, J.L.; Catchot, A.; Buntin, D.; Cook, D. Survival of Corn Earworm (Lepidoptera: Noctuidae) on Bt Maize and Cross-Pollinated Refuge Ears From Seed Blends. J. Econ. Entomol. 2016, 109, 288–298. [Google Scholar] [CrossRef]
- Jackson, R.E.; Gould, F.; Bradley, J.R., Jr.; Van Duyn, J.W. Genetic variation for resistance to Bacillus thuringiensis toxins in Helicoverpa zea (Lepidoptera: Noctuidae) in eastern North Carolina. J. Econ. Entomol. 2006, 99, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.L.; Unnithan, G.C.; Degain, B.A.; Wei, J.; Zhang, J.; Li, X.; Tabashnik, B.E.; Carrière, Y. Cross-resistance to toxins used in pyramided Bt crops and resistance to Bt sprays in Helicoverpa zea. J. Invertebr. Pathol. 2015, 132, 149–156. [Google Scholar] [CrossRef]
- Bakhsh, A.; Shahzad, K.; Husnain, T. Variation in the spatio-temporal expression of insecticidal genes in cotton. Czech. J. Genet. Plant 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Kranthi, K.R.; Naidu, S.; Dhawad, C.S.; Tatwawadi, A.; Mate, K.; Patil, E.; Bharose, A.A.; Behere, G.T.; Wadaskar, R.M.; Kranthi, S. Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Hubner)(Noctuidae: Lepidoptera). Curr. Sci. 2005, 89, 291–298. [Google Scholar]
- Ullah, I.; Asif, M.; Arslan, M.; Ashfaq, M. Temporal Expression of Cry1Ab/c Protein in Bt-Cotton Varieties their Efficacy against Helicoverpa armigera (Lepidoptera: Noctuidae) and Population Dynamics of Sucking Arthropods on Them. Int. J. Agric. Biol. 2014, 16, 879–885. [Google Scholar]
- Mamatha, K.; Jaybhaye, P. Impact of Drought Weather Condition on Bt Cotton Growth, Development and Yield. Int. J. Curr. Microbiol. Appl. Sci. 2018, 2332–2338. [Google Scholar]
- Moar, W.; Roush, R.; Shelton, A.; Ferré, J.; Macintosh, S.; Leonard, B.R.; Abel, C. Field-evolved resistance to bt toxins. Nat. Biotechnol. 2008, 26, 1072–1074. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, Y.; Chen, W.; Lu, Y.; Wu, K. Field monitoring of Helicoverpa armigera (Lepidoptera: Noctuidae) Cry1Ac insecticidal protein resistance in China (2005–2017). Pest Manag. Sci. 2019, 75, 753–759. [Google Scholar]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carriére, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Mota-Sanchez, D.; Whalon, M.E.; Hollingworth, R.M.; Carrière, Y. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 2014, 107, 496–507. [Google Scholar] [CrossRef]
- Monnerat, R.; Martins, E.; Macedo, C.; Queiroz, P.; Praça, L.; Soares, C.M.; Moreira, H.; Grisi, I.; Silva, J.; Soberon, M. Evidence of field-evolved resistance of Spodoptera frugiperda to Bt corn expressing Cry1F in Brazil that is still sensitive to modified Bt toxins. PLoS ONE 2015, 10, e0119544. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, H.; Lu, Y.; Yang, Y.; Wu, K.; Tabashnik, B.E.; Wu, Y. Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat. Biotechnol. 2015, 33, 169. [Google Scholar] [CrossRef]
- Carrière, Y.; Fabrick, J.A.; Tabashnik, B.E. Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 2016, 34, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Sisterson, M.S.; Ellsworth, P.C.; Dennehy, T.J.; Antilla, L.; Liesner, L.; Whitlow, M.; Staten, R.T.; Fabrick, J.A.; Unnithan, G.C. Suppressing resistance to Bt cotton with sterile insect releases. Nat. Biotechnol. 2010, 28, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, X.; Crossa, J.; Crouch, J.; Weng, J.; Zhai, H.; Wan, J. QTL mapping of grain length in rice (Oryza sativa L.) using chromosome segment substitution lines. Genet Res. 2006, 88, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.M.; Kunert, K.J.; Maling’a, J.; Foyer, C.H. Defining biotechnological solutions for insect control in sub-Saharan Africa. Food Energy Secur. 2020, 9, e191. [Google Scholar] [CrossRef]
- Deans, C.A.; Behmer, S.T.; Tessnow, A.E.; Tamez-Guerra, P.; Pusztai-Carey, M.; Sword, G.A. Nutrition affects insect susceptibility to Bt toxins. Sci. Rep. 2017, 7, 39705. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G. The environmental contribution to gene expression profiles. Nat. Rev. Genet. 2008, 9, 575. [Google Scholar] [CrossRef]
- Deans, C.; Sword, G.; Behmer, S. Nutrition as a neglected factor in insect herbivore susceptibility to Bt toxins. Curr. Opin. Insect Sci. 2016, 15, 97–103. [Google Scholar] [CrossRef]
- Downes, S.; Mahon, R.J.; Rossiter, L.; Kauter, G.; Leven, T.; Fitt, G.; Baker, G. Adaptive management of pest resistance by Helicoverpa species (Noctuidae) in Australia to the Cry2Ab Bt toxin in Bollgard II® cotton. Evol. Appl. 2010, 3, 574–584. [Google Scholar] [CrossRef]
- Morin, S.; Biggs, R.W.; Sisterson, M.S.; Shriver, L.; Ellers-Kirk, C.; Higginson, D.; Holley, D.; Gahan, L.J.; Heckel, D.G.; Carriere, Y.; et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 2003, 100, 5004–5009. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zeng, B.; Wang, Y.; James, A.A.; Gurr, G.M.; Yang, G.; Lin, X.; Huang, Y.; You, M. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in the global pest, diamondback moth (Plutella xylostella). Insect Biochem. Mol. Biol. 2016, 75, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Miyamoto, K.; Yamamoto, K.; Narukawa, J.; Kawai, S.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Mita, K. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2012, 109, E1591–E1598. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Xu, D.; Cong, S.; Jiang, Y.; Huang, Y.; Wang, J.; Wu, H.; Wang, L.; Wu, K.; Carrière, Y. Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm. Proc. Natl. Acad. Sci. USA 2017, 114, 5413–5418. [Google Scholar] [CrossRef]
- Wu, K. No refuge for insect pests. Nat. Biotechnol. 2010, 28, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Ni, M.; Ma, W.; Wang, X.; Gao, M.; Dai, Y.; Wei, X.; Zhang, L.; Peng, Y.; Chen, S.; Ding, L.; et al. Next-generation transgenic cotton: Pyramiding RNAi and Bt counters insect resistance. Plant Biotechnol. J. 2017, 15, 1204–1213. [Google Scholar] [CrossRef]
- Suhag, A.; Yadav, H.; Chaudhary, D.; Subramanian, S.; Jaiwal, R.; Jaiwal, P.K. Biotechnological interventions for the sustainable management of a global pest, whitefly (Bemisia tabaci). Insect Sci. 2020, 28, 1228–1252. [Google Scholar] [CrossRef]
- Koutroumpa, F.A.; Monsempes, C.; Francois, M.C.; de Cian, A.; Royer, C.; Concordet, J.P.; Jacquin-Joly, E. Heritable genome editing with CRISPR/Cas9 induces anosmia in a crop pest moth. Sci. Rep. 2016, 6, 29620. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Zhou, X.; Huang, Y.; Zhang, Z. Genome Editing of Wnt-1, a Gene Associated with Segmentation, via CRISPR/Cas9 in the Pine Caterpillar Moth, Dendrolimus punctatus. Front. Physiol. 2016, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dikshit, A. Biopesticides: An ecofriendly approach for pest control. J. Biopestic. 2010, 3, 186–188. [Google Scholar]
- Lord, J.C. Desiccant dusts synergize the effect of Beauveria bassiana (Hyphomycetes: Moniliales) on stored-grain beetles. J. Econ. Entomol. 2001, 94, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.; Ghareeb, R.Y.; Saeed, A.A. The potential of endophytic fungi as bio-control agents against the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt J. Biol. Pest Control 2019, 29, 29. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Kalidas, S.; Estelle, L.Y.L. Bioefficacy of Rhynocoris longifrons (Stal) (Heteroptera: Reduviidae) against multiple cotton pests under screen house and field conditions. Sci. Rep. 2020, 10, 6637. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Sarwar, M.; Wagan, M.S.; Muhammad, R.; Tofique, M. Conservation of bio-control agents in cotton, Gossypium hirsutum L. field by food supplements for insect pests management. Nucleus 2011, 48, 255–260. [Google Scholar]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Kan, J.; Fang, R.; Jia, Y. Interkingdom signaling in plant-microbe interactions. Sci. China Life Sci. 2017, 60, 785–796. [Google Scholar] [CrossRef]
- Kang, L. Overview: Biotic signalling for smart pest management. Philos. Trans. Royao Sciety B Biol. Sci. 2019, 374, 1767. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.-H.; Cámara, M.; He, Y.-W. The DSF family of quorum sensing signals: Diversity, biosynthesis, and turnover. Trends Microbiol. 2017, 25, 293–303. [Google Scholar] [CrossRef]
- Polajnar, J.; Eriksson, A.; Virant-Doberlet, M.; Mazzoni, V. Mating disruption of a grapevine pest using mechanical vibrations: From laboratory to the field. J. Pest Sci. 2016, 89, 909–921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).