Transcriptome Analysis Reveals Fruit Quality Formation in Actinidia eriantha Benth
Abstract
:1. Introduction
2. Results
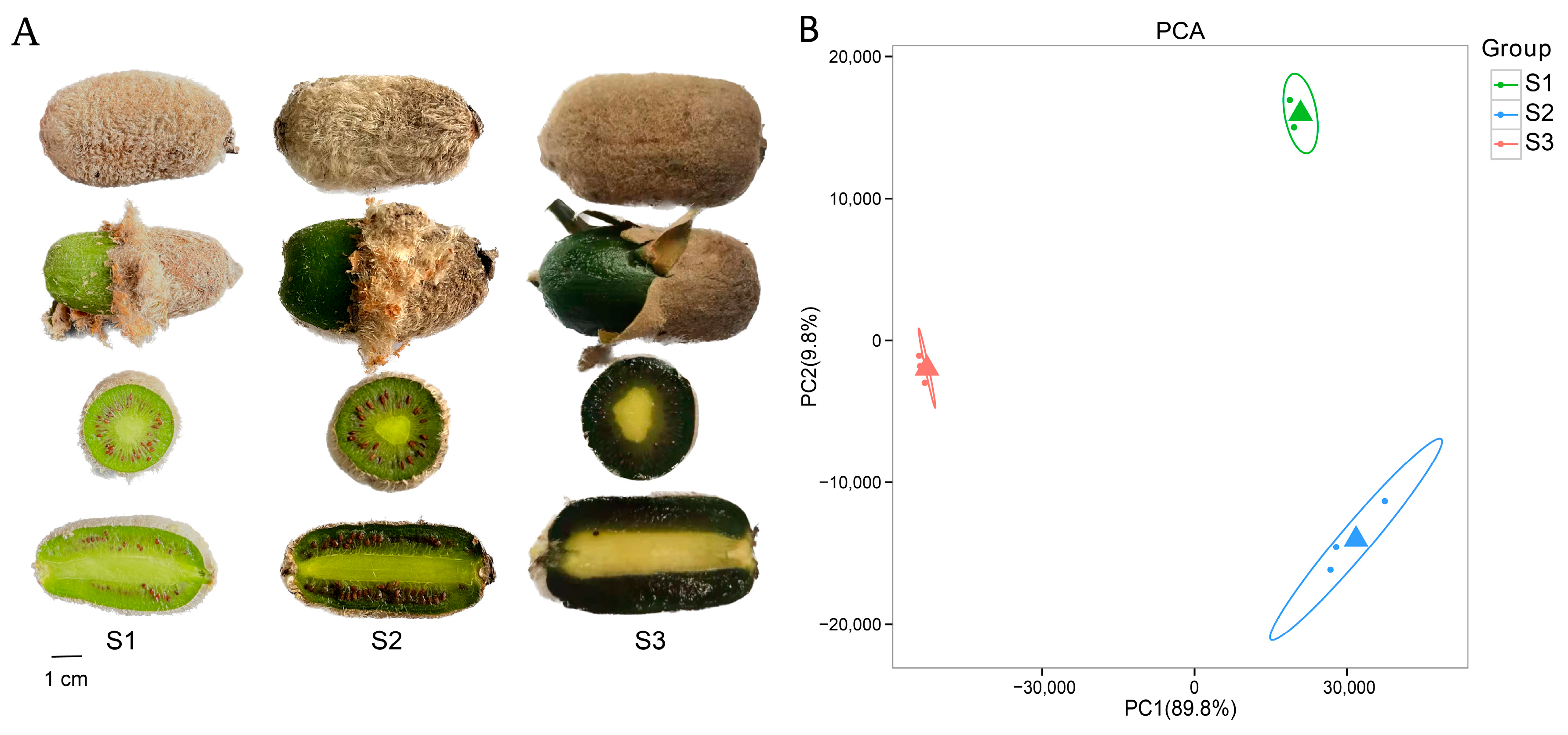
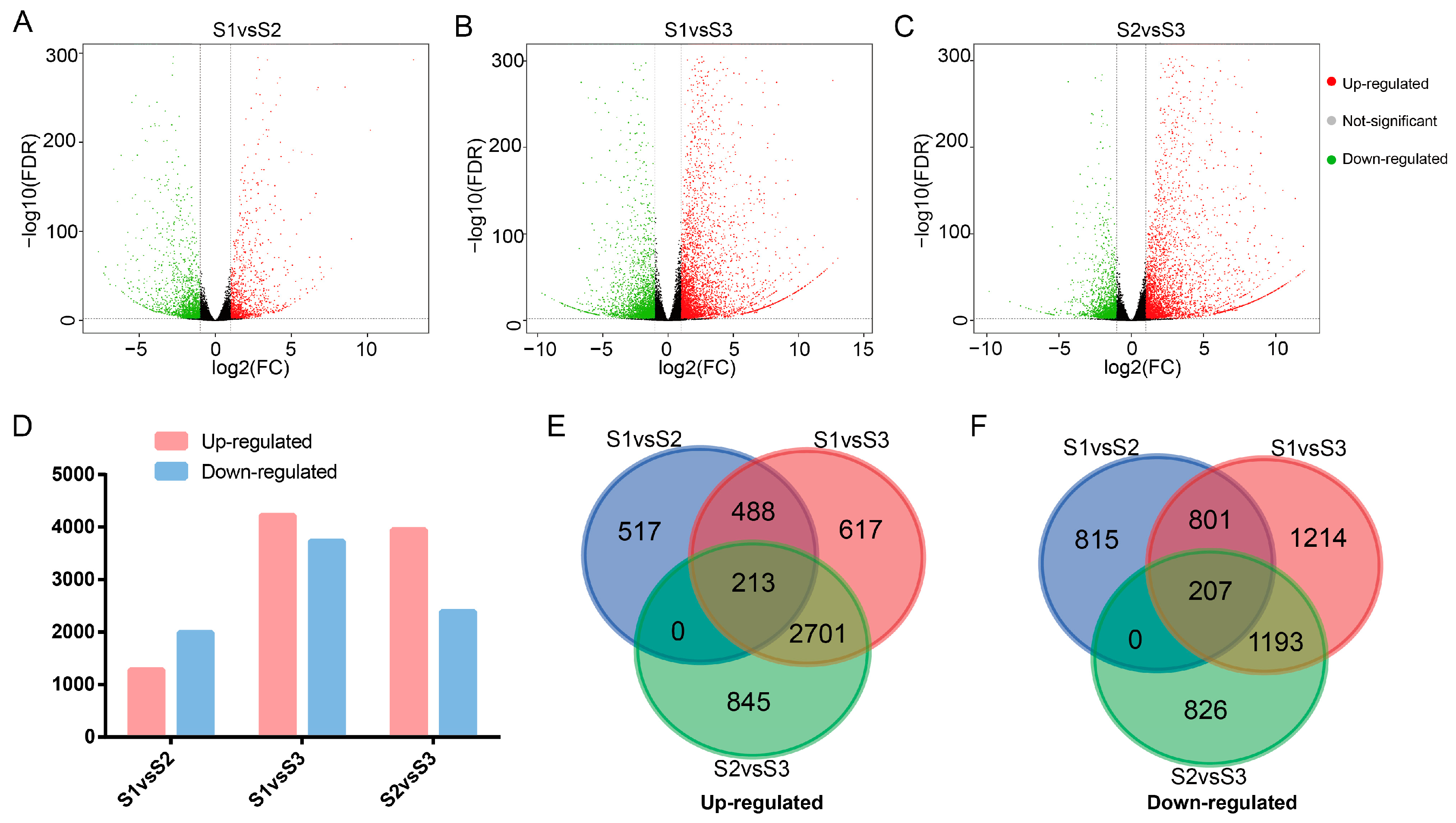
2.1. Transcriptomic Analysis of A. eriantha
2.2. Analysis of the Vc Synthesis Pathway in A. eriantha
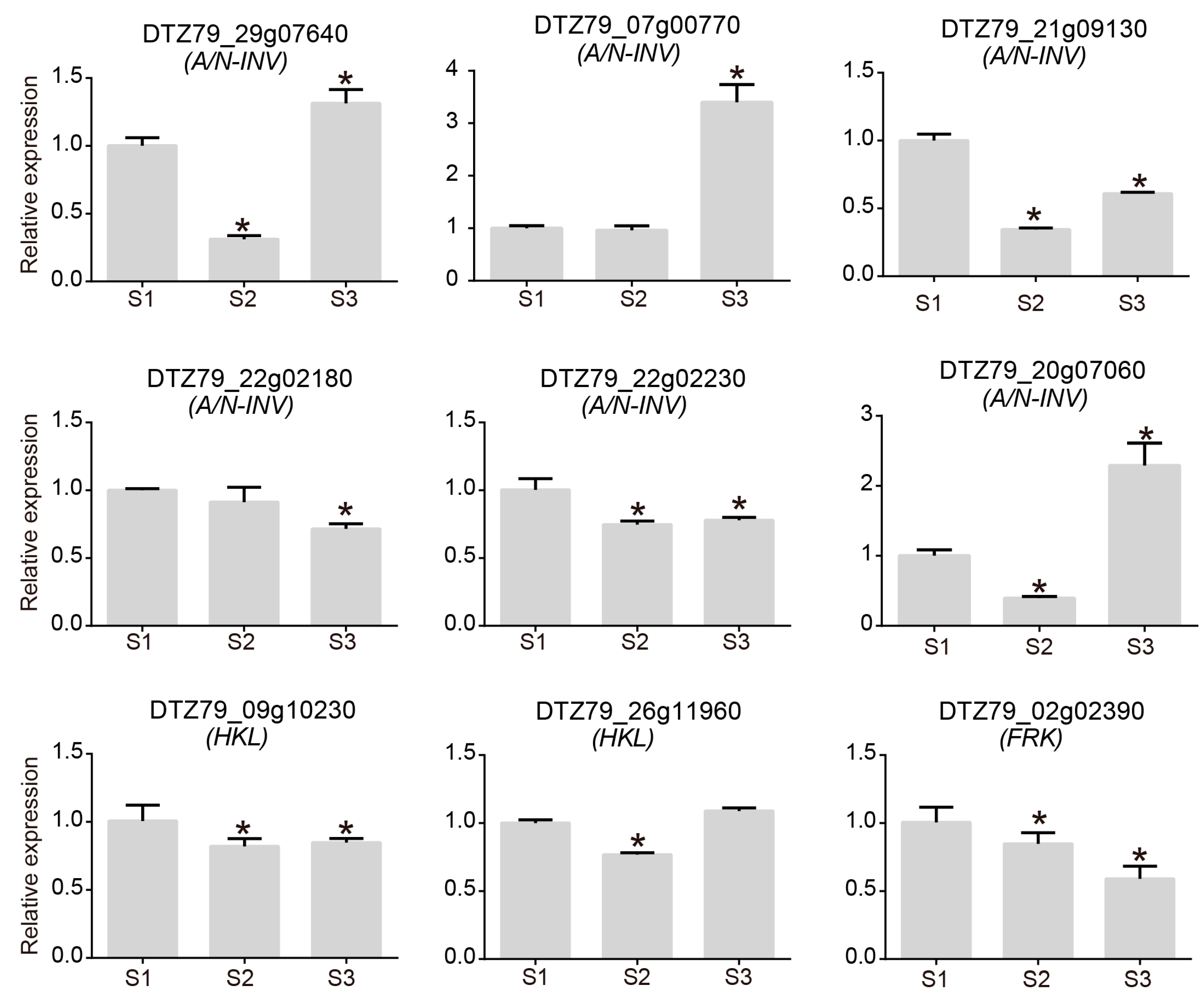
2.3. Analysis of the Sugar Metabolic Pathway in A. eriantha
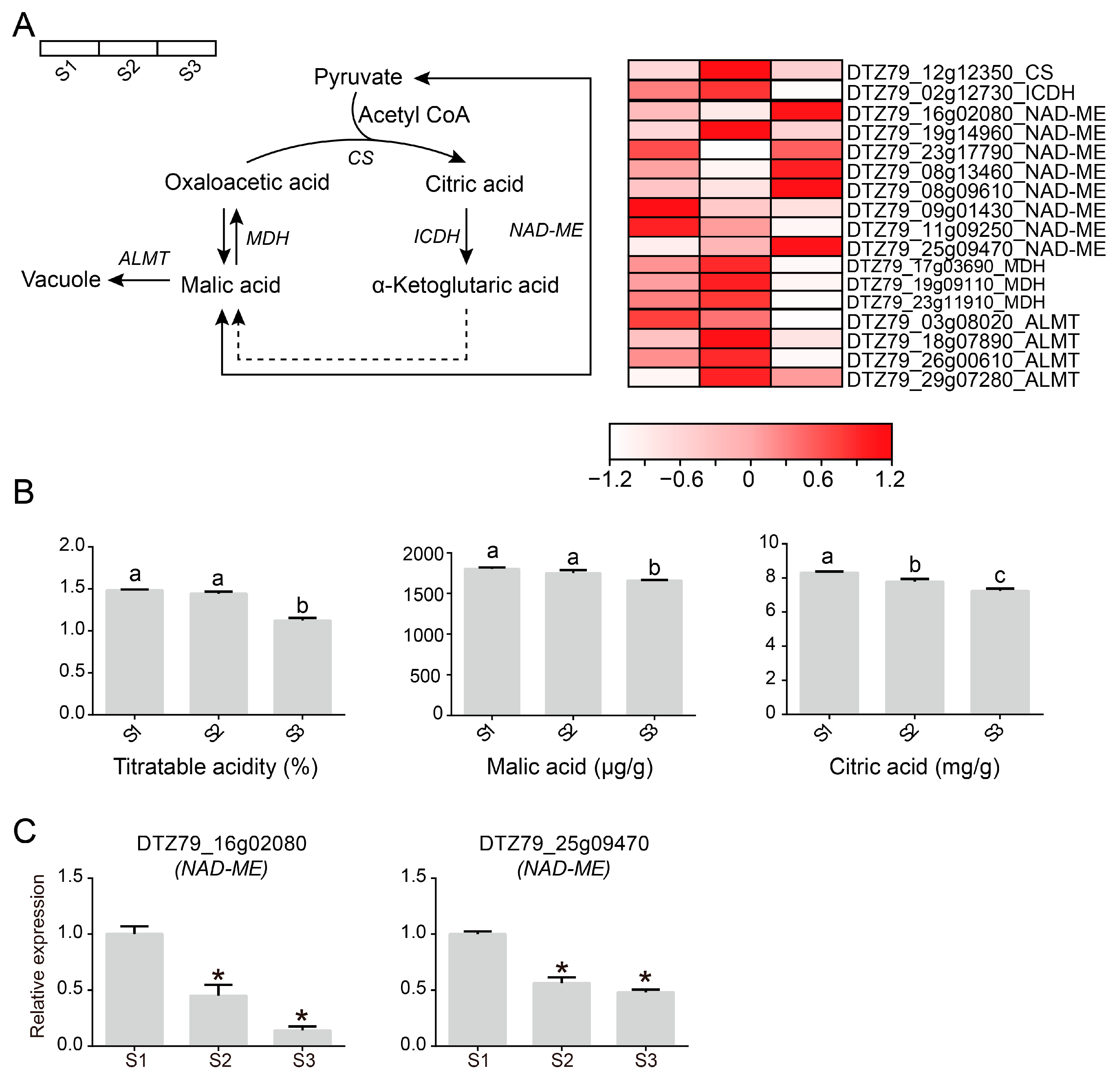
2.4. Analysis of the Organic Acid Metabolism Pathway in A. eriantha
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. cDNA Library Construction and Transcriptome Sequencing
4.3. Transcriptome Data Analysis
4.4. Differential Gene Analysis and Enrichment Analysis
4.5. Vc, Sugar and Organic Acid Determination
4.6. Quantitative Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, G.; He, Y.; Li, X.; Zhong, M.; Xu, X. Effects of bagging on fruit flavor quality and related gene expression of AsA synthesis in Actinidia eriantha. Sci. Hortic. 2019, 256, 108511. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits–ScienceDirect. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Huang, C.H.; Tao, J.J.; Liao, G.L.; Xie, M.; Xu, X.B. Dynamic changes of phenol and antioxidant capacity during fruit development of three Actinidia species (kiwifruit). Sci. Hortic.-Amsterdam 2020, 273, 109571. [Google Scholar] [CrossRef]
- Hale, T.; Hassell, R.; Phillips, T. Taste Panel Perception of Sweetness as it Correlates to Refractometer Measurements and HPLC Analysis of Sugar Content. Hortscience 2004, 39, 879. [Google Scholar] [CrossRef]
- Gang, W.; Tao, W.; Delin, P.; Zhanhui, J.; Jiyu, Z.; Zhongren, G. The Components and Contents of Organic Acid in Kiwifruit Fruits from Different Cultivars. J. Agric. 2017, 7, 81–84. [Google Scholar]
- Ackermann, J.; Fischer, M.; Amado, R. Changes in sugars, acids, and amino acids during ripening and storage of apples (cv. Glockenapfel). J. Agric. Food Chem. 1992, 40, 1131–1134. [Google Scholar] [CrossRef]
- Kroger, M.; Meister, K.; Kava, R. Low-calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues. Compr. Rev. Food Sci. Food Saf. 2010, 5, 35–47. [Google Scholar] [CrossRef]
- Pangborn, R. Relative Taste Intensities of Selected Sugars and Organic Acids. J. Food Sci. 2006, 28, 726–733. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wu, J.Y.; Qin, G.H.; Yao, G.F.; Qi, K.J.; Wang, L.F.; Zhang, S.L. The role of sucrose-metabolizing enzymes in pear fruit that differ in sucrose accumulation. Acta Physiol. Plant. 2014, 36, 71–77. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, S.; Zhang, J.; He, H.; Sun, H.; Tian, S.; Gong, G.; Zhang, H.; Levi, A.; Tadmor, Y.; et al. A Tonoplast Sugar Transporter Underlies a Sugar Accumulation QTL in Watermelon. Plant Physiol. 2018, 176, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Lingle, S.E.; Dunlap, J.R. Sucrose Metabolism in Netted Muskmelon Fruit during Development. Plant Physiol. 1987, 84, 386–389. [Google Scholar] [CrossRef]
- Richardson, A.C.; Boldingh, H.L.; Mcatee, P.A.; Gunaseelan, K.; Luo, Z.; Atkinson, R.G.; David, K.M.; Burdon, J.N.; Schaffer, R.J. Fruit development of the diploid kiwifruit, Actinidia chinensis “Hort16A”. BMC Plant Biol. 2011, 11, 182. [Google Scholar] [CrossRef]
- Gook, J.; Kenji, K.; Kataoka, B. Physical and compositional characteristics of “Mitsuko” and local hardy kiwifruits in Japan. Hortic. Environ. Biotechnol. 2012, 53, 1–8. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Montero, T.M.; Mollá, E.M.; Esteban, R.M.; López-Andréu, F.J. Quality attributes of strawberry during ripening. Sci. Hortic. 1996, 65, 239–250. [Google Scholar] [CrossRef]
- Giné Bordonaba, J.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria × ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Z.; Hu, G.; Yao, X. Genome-wide identification and characterization of AP2/ERF gene superfamily during flower development in Actinidia eriantha. BMC Genomics 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Q.; Jia, D.; Zhong, M.; Tao, J.; Liao, G.; Huang, C.; Xu, X. Characterization of Organic Acid Metabolism and Expression of Related Genes During Fruit Development of Actinidia eriantha “Ganmi 6”. Plants 2020, 9, 332. [Google Scholar] [CrossRef]
- Macrae, E.A.; Bowen, J.H.; Stec, M.G.H. Maturation of kiwifruit (Actinidia deliciosa cv Hayward) from two orchards: Differences in composition of the tissue zones. J. Sci. Food Agric. 2010, 47, 401–416. [Google Scholar] [CrossRef]
- Marsh, K.; Attanayake, S.; Walker, S.; Gunson, A.; Macrae, E.A. Acidity and taste in kiwifruit. Postharvest Biol. Technol. 2004, 32, 159–168. [Google Scholar] [CrossRef]
- Etienne, A.M.G.; Lobit, P.D.M.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 34, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Agius, F.; Amaya, I.; Botella, M.A.; Valpuesta, V. Functional analysis of homologous and heterologous promoters in strawberry fruits using transient expression. J. Exp. Bot. 2005, 56, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wolucka, B.A.; Van Montagu, M. GDP-Mannose 3′, 5′-Epimerase Forms GDP-L-gulose, a Putative Intermediate for the de Novo Biosynthesis of Vitamin C in Plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.; Strain, J.J.; Favell, D.; Fletcher, J. Review plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sc. Food Agr. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal Difference in Vitamin C Content in the Fruit of Kiwifruit and Other Actinidia Species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef]
- Cai-Hong, Z.; Peng, Z.; Zheng-Wang, J.; Sheng-Mei, W.; Fei, H.; Li-Yun, X.U.; Hong-Wen, H. Dynamic Changes of Carbohydrate and Vitamin C in Fruits of Actinidia chinensis and A. eriantha during Growth Season. Plant Sci. J. 2011, 4, 319–330. [Google Scholar] [CrossRef]
- Gao, X.; Wang, L.; Zhang, H.; Zhu, B.; Lv, G.; Xiao, J. Transcriptome analysis and identification of genes associated with floral transition and fruit development in rabbiteye blueberry (Vaccinium ashei). PLoS ONE 2021, 16, e259119. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, H.; Gu, W.; Shi, W.; Jiang, L.; Deng, L.; Liu, X. Transcriptome profiling provides insights into the fruit color development of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Protoplasma 2021, 258, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Huang, Y.; Zhang, Y.; Wang, X.; Yang, H.; Yu, O.; Dai, W.; Fang, C. Transcriptome profiling of fruit development and maturation in Chinese white pear (Pyrus bretschneideri Rehd). BMC Genom. 2013, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Ester Garcia-Gomez, B.S.J.L. Identification of QTLs linked to fruit quality traits in apricot (Prunus armeniaca L.) and biological validation through gene expression analysis using qPCR. Mol. Breed. 2019, 39, S96–S101. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-Gómez, P. Analysis of Metabolites and Gene Expression Changes Relative to Apricot (Prunus armeniaca L.) Fruit Quality During Development and Ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, T.; Wu, M.; Zhong, Y.; Cheng, Z.M. Transcriptome and Metabolome Reveal Sugar and Organic Acid Accumulation in Rosa roxburghii Fruit. Plants 2023, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Tao, J.; Zhong, W.; Jiao, X.; Chen, S.; Wu, M.; Gao, Z.; Huang, C. Nutritional Component Analyses in Different Varieties of Actinidia eriantha Kiwifruit by Transcriptomic and Metabolomic Approaches. Int. J. Mol. Sci. 2022, 6, 217. [Google Scholar] [CrossRef]
- Liao, G.; Liu, Q.; Xu, X.; He, Y.; Li, Y.; Wang, H.; Ye, B.; Huang, C.; Zhong, M.; Jia, D. Metabolome and Transcriptome Reveal Novel Formation Mechanism of Early Mature Trait in Kiwifruit. Front. Plant Sci. 2021, 12, 760496. [Google Scholar] [CrossRef]
- Xiong, Y.; Yan, P.; Du, K.; Li, M.; Xie, Y.; Gao, P. Nutritional component analyses of kiwifruit in different development stages by metabolomic and transcriptomic approaches. J. Sci. Food Agr. 2020, 100, 2399–2409. [Google Scholar] [CrossRef]
- Jeong, H.R.; Cho, H.S.; Cho, Y.S.; Kim, D.O. Changes in phenolics, soluble solids, vitamin C, and antioxidant capacity of various cultivars of hardy kiwifruits during cold storage. Food Sci. Biotechnol. 2020, 29, 1763–1770. [Google Scholar] [CrossRef]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.; Yuan, P.; Zhu, H.; Kaseb, M.O.; Anees, M.; Lu, X.; He, N.; et al. Identification of key gene networks controlling organic acid and sugar metabolism during watermelon fruit development by integrating metabolic phenotypes and gene expression profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, L.; Ogutu, C.; Liu, J.; Liu, L.; Mollah, M.; Han, Y. Functional Analysis Reveals the Regulatory Role of PpTST1 Encoding Tonoplast Sugar Transporter in Sugar Accumulation of Peach Fruit. Int. J. Mol. Sci. 2020, 21, 1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Huiqin, Z.; Ming, X.; Jinping, X.; Liqiu, Z.; Genhua, S. Characterization of fruit development of the diploid kiwifruit, Actinidia eriantha “White”. J. Fruit. Sci. 2015, 32, 238–246. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruzcastillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Yao, Y.X.; Ming, L.; Liu, Z.; You, C.X.; Wang, D.M.; Zhai, H.; Hao, Y.J. Molecular cloning of three malic acid related genes MdPEPC, MdVHA-A, MdcyME and their expression analysis in apple fruits. Sci. Hortic. 2009, 122, 404–408. [Google Scholar] [CrossRef]
- Li, S.; Yin, X.; Xie, X.; Allan, A.C.; Ge, H.; Shen, S.; Chen, K. The Citrus transcription factor, CitERF13, regulates citric acid accumulation via a protein-protein interaction with the vacuolar proton pump, CitVHA-c4. Sci. Rep. 2016, 6, 20151. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, H.; Peng, Q.; Jiang, Q.; Wang, H.; Fang, T.; Liao, L.; Wang, L.; He, H.; Han, Y. Assessment of Sugar Components and Genes Involved in the Regulation of Sucrose Accumulation in Peach Fruit. J. Agr. Food Chem. 2016, 64, 6723–6729. [Google Scholar] [CrossRef]
- Guo, C.; Wang, P.; Zhang, J.; Guo, X.; Mu, X.; Du, J. Organic acid metabolism in Chinese dwarf cherry [Cerasus humilis (Bge. ) Sok.] is controlled by a complex gene regulatory network. Front. Plant Sci. 2022, 13, 982112. [Google Scholar] [CrossRef]
- Cheng, H.; Kong, W.; Tang, T.; Ren, K.; Zhang, K.; Wei, H.; Lin, T. Identification of Key Gene Networks Controlling Soluble Sugar and Organic Acid Metabolism During Oriental Melon Fruit Development by Integrated Analysis of Metabolic and Transcriptomic Analyses. Front. Plant Sci. 2022, 13, 830517. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tian, C.; Ji, S.; Ni, F.; Fan, X.; Yang, Y.; Sun, C.; Gong, H.; Zhang, A. Integrative analyses of metabolome and transcriptome reveals metabolomic variations and candidate genes involved in sweet cherry (Prunus avium L. ) fruit quality during development and ripening. PLoS ONE 2021, 16, e260004. [Google Scholar] [CrossRef]
- Bulley, S.M.; Rassam, M.; Hoser, D.; Otto, W.; Schünemann, N.; Wright, M.; Macrae, E.; Gleave, A.; Laing, W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 2009, 60, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Zhang, Z.; Wu, Y.; Chen, Y.; Li, K.; Deng, H.; Zhang, J.; Zhang, X.; Wang, J.; Liu, Z.; et al. A comprehensive metabolic map reveals major quality regulations in red-flesh kiwifruit (Actinidia chinensis). New Phytol. 2023, 238, 2064–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, F.; Liang, D.; Li, J.; Wang, Y. Ascorbate Biosynthesis during Early Fruit Development Is the Main Reason for Its Accumulation in Kiwi. PLoS ONE 2010, 5, e14281. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Wang, P.; Ma, F. Ascorbic Acid Accumulation and Expression of Genes Involved in Its Biosynthesis and Recycling in Developing Apple Fruit. J. Am. Soc. Hortic. Sci. 2011, 136, 231–238. [Google Scholar] [CrossRef]
- Imai, T.; Ban, Y.; Terakami, S.; Yamamoto, T.; Moriguchi, T. L-Ascorbate biosynthesis in peach: Cloning of six L-galactose pathway-related genes and their expression during peach fruit development. Physiol. Plant. 2010, 136, 139–149. [Google Scholar] [CrossRef]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Muñoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef]
- Bulley, S.; Wright, M.; Rommens, C.; Yan, H.; Rassam, M.; Lin-Wang, K.; Andre, C.; Brewster, D.; Karunairetnam, S.; Allan, A.C.; et al. Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol. J. 2012, 10, 390–397. [Google Scholar] [CrossRef]
- Qian, W.; Yu, C.; Qin, H.; Liu, X.; Zhang, A.; Johansen, I.E.; Wang, D. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J. 2007, 49, 399–413. [Google Scholar] [CrossRef]
- Giovannoni, S.; Foster, R.; Rappe, M.; Epstein, S. New Cultivation Strategies Bring More Microbial Plankton Species into the Laboratory. Oceanography 2007, 20, 62–69. [Google Scholar] [CrossRef]
- Gatzek, S.; Wheeler, G.L.; Smirnoff, N. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J. 2002, 30, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.; Renz, F.S.A.; Kossmann, J. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J. 1999, 19, 131–141. [Google Scholar] [CrossRef]
- Linster, C.L.; Gomez, T.A.; Christensen, K.C.; Adler, L.N.; Young, B.D.; Brenner, C.; Clarke, S.G. Arabidopsis VTC2 Encodes a GDP-l-Galactose Phosphorylase, the Last Unknown Enzyme in the Smirnoff-Wheeler Pathway to Ascorbic Acid in Plants. J. Biol. Chem. 2007, 282, 18879–18885. [Google Scholar] [CrossRef] [PubMed]
- Laing, W.A.; Wright, M.A.; Cooney, J.; Bulley, S.M. The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. USA 2007, 104, 9534–9539. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, M.; An, H. Expression of GDP-L-galactose pyrophosphatase and its relationship with ascorbate accumulation in Rosa roxburghii. Acta Hortic. Sin. 2014, 41, 1175–1182. [Google Scholar]
- Mellidou, I.; Chagné, D.; Laing, W.A.; Keulemans, J.; Davey, M.W. Allelic Variation in Paralogs of GDP-l-Galactose Phosphorylase Is a Major Determinant of Vitamin C Concentrations in Apple Fruit. Plant Physiol. 2012, 160, 1613–1629. [Google Scholar] [CrossRef]
- Liu, X.; Wu, R.; Bulley, S.; Zhong, C.; Li, D. Kiwifruit MYBS1-like and GBF3 transcription factors influence L-ascorbic acid biosynthesis by activating transcription of GDP-L-galactose phosphorylase 3. New Phytol. 2022, 234, 1782–1800. [Google Scholar] [CrossRef]
- Cruz-Rus, E.; Botella, M.A.; Valpuesta, V.; Gomez-Jimenez, M.C. Analysis of genes involved in l-ascorbic acid biosynthesis during growth and ripening of grape berries. J. Plant Physiol. 2010, 167, 739–748. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, J.; Liang, D.; Guo, C.; Ma, F. The relationship between GalUR expression and ascorbate accumulation in Kiwifruit. Acta Hortic. Sin. 2011, 38, 1641–1649. [Google Scholar]
- Li, M.J.; Ma, F.W.; Zhang, M.; Pu, F. Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala). Plant Sci. 2008, 174, 606–612. [Google Scholar] [CrossRef]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality controland deduplication using fastp. iMeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Kim, D.; Ben, L.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Yang, L.; Smyth, G.K.; Wei, S. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Amit, I. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2013, 29, 644–652. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.; Han, L.; He, Q. clusterProfiler: An R package for Statistical Analysis and Visualization of Functional Profiles for Genes and Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Feng, X.; Jiang, J.; Yan, P.; Li, Z.; Luo, W.; Chen, Y.; Ye, W. Transcriptome Analysis Reveals Fruit Quality Formation in Actinidia eriantha Benth. Plants 2023, 12, 4079. https://doi.org/10.3390/plants12244079
Wang P, Feng X, Jiang J, Yan P, Li Z, Luo W, Chen Y, Ye W. Transcriptome Analysis Reveals Fruit Quality Formation in Actinidia eriantha Benth. Plants. 2023; 12(24):4079. https://doi.org/10.3390/plants12244079
Chicago/Turabian StyleWang, Peiyu, Xin Feng, Jinlan Jiang, Peipei Yan, Zunwen Li, Weihong Luo, Yiting Chen, and Wei Ye. 2023. "Transcriptome Analysis Reveals Fruit Quality Formation in Actinidia eriantha Benth" Plants 12, no. 24: 4079. https://doi.org/10.3390/plants12244079
APA StyleWang, P., Feng, X., Jiang, J., Yan, P., Li, Z., Luo, W., Chen, Y., & Ye, W. (2023). Transcriptome Analysis Reveals Fruit Quality Formation in Actinidia eriantha Benth. Plants, 12(24), 4079. https://doi.org/10.3390/plants12244079




