Abstract
In plants, ferritin proteins play an important role in iron (Fe) storage which contributes to plant growth and development. However, the biological functions of ferritins in fruit trees are essentially unknown. In this study, three Ferritin genes were isolated from ‘Zhentong No. 3’ peach, which were named PpFer1-PpFer3. The expression levels of these genes were different in distinct tissues/organs. Notably, PpFer1 was the most abundantly expressed Ferritin family gene in all tested tissues of ‘Zhentong No. 3’ peach; its expression levels were significantly enhanced throughout the entire peach seedling under Fe toxicity and H2O2 stress, particularly in the leaves. In addition, over-expression of PpFer1 was effective in rescuing the retarded growth of Arabidopsis fer1-2 knockout mutant, embodied in enhanced fresh weight, primary root length, lateral root numbers, total root length, total leaf chlorophyll, stomatal conductance (Gs), net photosynthetic rate (Pn), transpiration rate, and tissue Fe concentration. This study provides insights into understanding the molecular mechanisms of Fe storage and sequestration in perennial fruit trees.
1. Introduction
Iron (Fe) is one of the abundant mineral elements in plant cells, and it participates in many metabolic pathways and life processes, such as photosynthesis, respiration, hormone synthesis, energy metabolism, and DNA repair [1,2,3,4]. In particular, Fe deficiency in soils causes serious crop yield decrease and quality reduction, whereas excessive Fe may impair plant growth and cause soil pollution [5,6,7]. Therefore, plants need to take advantage of accurate Fe uptake, transport, storage, and utilization strategies to maintain normal growth and development.
In higher plants, two kinds of root Fe absorption strategies have been identified, especially under Fe deficiency stress [7,8,9,10,11]. Strategy I is found in dicotyledons and non-gramineous monocotyledons, in which Fe3+ is reduced to Fe2+ through ferric reduction oxide (FRO), and Fe2+ is absorbed by iron regulated transporters (IRT). Strategy II is observed in gramineous plants, in which Fe3+ is absorbed through a specific type of Fe3+ chelator phytosideophore (PS) pathway that depends on yellow-stripe (YS) or yellow-stripe-like (YSL) transporters [10,11,12].
The mobilization of intracellular Fe2+ is crucial for plant growth and development, especially under Fe-deficient conditions. When Fe2+ enters the cell, it needs to be transported to each organelle for distribution and utilization or stored to form an intracellular Fe2+ pool. However, when Fe2+ in the cytoplasm is excessively stored, it will also cause Fe2+ toxicity which affects plant growth. Previous studies showed that transporter proteins, like natural-resistance-associated macrophage proteins (NRAMPs), permease in chloroplast (PIC), and vacuolar iron transporters (VIT), are involved in the transport and distribution of Fe2+ within plant cells [7,8,9,10,11]. The plastid can act as an Fe2+ pool in the cell, sensing and regulating the concentration of Fe2+ to adapt to changes in external Fe supply [7,11,13]. In seeds of Arabidopsis thaliana, the globoids in the vacuoles are the main storage pool for Fe2+, containing approximately 50% of total Fe2+. Typically, intracellular Fe2+ is favorably stored in vacuoles and may also be chelated into ferritin, which is utilized in various Fe2+-dependent metabolic pathways or physiological processes. In particular, ferritin is a type of 24-protein polymer encoded by nuclear genes, which has a highly conserved structure in eukaryotes, and is crucial for fine-tuning the content of various metal elements required for plant metabolisms [12,14,15]. Notably, four Ferritin family genes (AtFer1-AtFer4) have been identified in Arabidopsis, and AtFer1, AtFer3, and AtFer4 are highly expressed in leaves, whereas AtFer2 is specifically expressed in seeds. Moreover, AtFer1 is induced by excessive Fe and H2O2 stress, AtFer2 is induced by abscisic acid (ABA) treatment, and AtFer3 is induced by excessive Fe stress [14,15]. Arabidopsis Ferritin proteins are located in chloroplasts and form complexes with Fe2+, participating in the regulation of intracellular Fe2+ storage and sequestration, and maintaining plant tolerance to adverse environmental stresses, such as drought [16], water loss [17], and reactive oxygen species (ROS) [13,14,18]. The growth of the Arabidopsis fer1fer3fer4 triple mutant was severely inhibited, and the intracellular Fe2+ content was sharply decreased. The Arabidopsis fer2 mutant was very sensitive to ROS stress, and the seed germination rate was severely affected [13,14,18]. In addition, Ferritin is also involved in regulating the structure of roots. In the Arabidopsis fer1fer3fer4 triple mutant, the destruction of ROS production and equilibration lead to changes in the structure of roots [18]. In recent years, ferritin homologous genes have been reported subsequently in plants, including soybean (Glycine max) [19], cut rose (Rosa chinensis) [17], and cassava (Manihot esculenta) [20]. However, biological functions of Ferritin proteins in fruit trees are essentially unknown.
Peach (Prunus persica L.) is a fruit that is popular worldwide, and its genome has been sequenced [21]. Among trace elements necessary for maintaining fruit tree growth and development, peach trees exhibit the highest demand for Fe, a factor closely tied to fruit quality and fruit yield [1,2,3]. In this study, a Ferritin family gene (PpFer1) was isolated from an elite peach variety, ‘Zhentong No. 3’, and differential responses of PpFer1 to abiotic stresses (including Fe depletion, Fe toxicity, ABA, and H2O2 treatment) and the Fe2+ storage function were further determined. This study contributes to uncovering the molecular mechanisms of Fe storage and sequestration in fruit trees.
2. Materials and Methods
2.1. Plant Material and Growth Condition
The 7-year-old ‘Zhentong No. 3’ peach trees grown at the Zhenjiang Academy of Agricultural Sciences (Zhenjiang, China) were used in this study. Samples of leaves, stems and roots of seedlings, as well as bud-period flowers, full-blooming flowers, leaves, phloem, and fruit from both the young fruit stage (YFS) and mature fruit stage (MFS) of 7-year-old trees were collected and frozen in liquid nitrogen before qRT-PCR analysis. Biological replicates were conducted three times, each consisting of 15 distinct samples.
Tissue-cultured ‘Zhenjiang No. 3’ seedlings were germinated on half-strength MS solid medium (pH 5.8) for 1 month before being transferred to half-strength MS liquid solution in plastic incubators in a growth chamber [4,22]. For Fe depletion treatments, Fe was omitted from the half-strength MS liquid solution. For Fe toxicity treatments, seedlings were grown in half-strength MS liquid solution containing 500 μmol∙L−1 FeCl3 (pH 5.8). For ABA treatments, seedlings were grown in half-strength MS liquid medium supplied with 100 μmol∙L−1 ABA (pH 5.8), as previously described [17]. For oxidative stress treatments, seedlings were grown in half-strength liquid solution supplied with fresh H2O2 to a final concentration of 5% (v/v), as described in [17]. Seedlings were exposed to stress treatment for 48 h before expression analysis. Biological replicates were conducted three times, each involving 15 seedlings.
2.2. Physiological Analysis
The fresh weight of Arabidopsis seedlings was determined using a Thermo Electron Analytical Balance (Waltham, MA, USA). The roots of Arabidopsis seedlings were scanned using an Epson Rhizo scanner (Long Beach, CA, USA), and primary root length, lateral root numbers, and total root length were analyzed with the Epson WinRHIZO software 14.0 (Long Beach, CA, USA). Arabidopsis samples were digested using the HNO3-HClO4 method, and Fe concentration was assayed using ICP-AES systems (Thermo Electron, Waltham, MA, USA). The stomatal conductance (Gs), net photosynthetic rate (Pn), and transpiration rate (Tr) were measured using a portable Li-COR Photosynthetic Apparatus (Lincoln, NE, USA) as previously described [4]. Chlorophyll was extracted using 95% ethanol and quantified using the BioRad SmartSpec 3000 spectrophotometer (Wadsworth, IL, USA), as previously mentioned [4].
2.3. Isolation and Cloning of PpFer Genes from Peach
Taking the amino acid sequences of Arabidopsis AtFer1-4 as reference sequences [7,23], putative PpFer genes were obtained by screening the Peach Genome Database [21]. The genomic DNA sequence and coding sequence (CDS) of PpFer genes were downloaded (Table 1). The amino acid sequences of PpFer proteins were retrieved and verified regarding whether they possessed the Ferritin domain (PF00210) or not using the Pfam and InterProScan 4.8 online servers. Specific prime pairs were designed for CDS cloning of PpFer genes. The total RNA from 1-month-old ‘Zhentong No. 3’ seedlings was extracted using the RNAprep Pure Plant Kit (TianGen, Beijing, China) and synthesized into the first strand cDNA using the PrimeScriptTM RT reagent kit (Takara, Dalian, China). The CDSs of PpFer genes were amplified using the Prime STARTM HS DNA polymerase (Takara, Dalian, China) and further sequenced by Shenggong Bioengineering Co., Ltd. (Shanghai, China).

Table 1.
PpFer gene information.
2.4. Phylogenetic Tree Construction
The alignment of amino acid sequences of Ferritin homologues from peach, Vitis vinifera (VvFer1-4), A. thaliana (AtFer1-4), Arachis hypogaea (AdFer1-4), Camellia oleifera (BnFer1-4), Brassica rapa (BrFer1-3), Cicer arietinum (CaFer1-3), Gossypium hirsutum (GhFer1-3), soybean (GmFer1-4), Hevea brasiliensis (HbFer2-4), M. domestica (MdFer3 and MdFer4), cassava (MeFer1-4), Nicotiana tabacum (NtFer1-2), Ricinus communis (RcFer2-3), S. lycopersicum (StFer1 and StFer2), and Fragaria vesca (FvFer3-4) was carried out using Cluster X 2.0.13 software. A phylogenetic tree of plant Ferritin homologues was constructed using the maximum likelihood method in MEGA 15.0, and a bootstrap test with 1000 replicates was performed to assess the confidence of the tree.
2.5. Quantitative Real Time PCR (qRT-PCR)
Specific primers for PpFer genes were designed using the NCBI/Primer-BLAST on-line server. Primer sequences are listed in Supplemental Table S1. PCR analysis was conducted on the 7500 Real Time PCR System (Applied Biosystems, New York, NY, USA), using the SYBR Premix Ex Taq (TaKaRa, Kyoto, Japan) reaction kit. The peach Ubiquitin gene served as the internal control, as established in previous studies [24,25]. Relative expression levels of PpFer genes were presented after normalization to the internal control Ubiquitin, based on three independent biological repeats, each with three technical replicates.
To investigate the response of PpFer genes under abiotic stress treatments at the transcriptional level, the expression value under control conditions was set as 1. If the relative expression value under Fe depletion was <1, it indicated a decrease in gene expression level (depicted in blue). If the relative expression under Fe depletion value was >1, it signified an increase in gene expression level (depicted in red). The heat map of expression change was generated using the HemI software 18.3 [4,22].
2.6. Generation of Transgenic Arabidopsis Complementing PpFer1 Gene
The recombinant plasmid pBH-PpFer1 was constructed by cloning the CDS of the PpFer1 gene into the pBH vector [4,22]. This process utilized the forward primer of 5′-GACGGATCCATGCTTCTCAAAGGTTCTCC-3′ (BamH I underlined) and reverse primer of 5′-GAGTCTAGATCACGCAGCAATTGCATCAAC-3′ (Xba I underlined). The resulting recombinant plasmid was subcloned into Agrobacterium tumefaciens EHA 105 and subsequently transformed into the Arabidopsis fer1-2 knockout homozygote mutant [26], which had been previously germinated on half-strength MS solid medium over 3 weeks, using the floral dip method. Independent T1 generations of fer1-2/35S::PpFer1 complementation lines were obtained by screening hygromycin-resistant regenerated Arabidopsis seedlings. Genomic DNA was extracted from the T1 generation of fer1-2/35S::PpFer1 lines using the Universal Genomic DNA Extraction Kit (TaKaRa, Dalian, China). The existence of an 846 bp product of PpFer1 was further verified by reverse transcription PCR. T1 generation seedlings of fer1-2/35S::PpFer1 were grown on half-strength MS solid medium for 2 weeks. Total RNA from shoots and roots of T1 transgenic lines was extracted using the RNAprep Pure Plant Kit (TianGen, Beijing, China) and synthesized into the first strand cDNA using the PrimeScriptTM RT reagent kit (Takara, Dalian, China) for the determination of PpFer1 presence. Purified T3 generation seeds of #2 and #11 fer1-2/35S::PpFer1 lines were harvested and sown on half-strength MS solid medium where they were kept for 7 days before physiological analysis. Biological replicates were conducted three times, each involving 30 seedlings.
2.7. Statistical Analysis
Graphs were generated using Origin 12.0 software, and significant differences were analyzed using Student’s t-test in SPSS 13.0 software (SPSS Chicago, IL, USA) or Fisher’s LSD test in the ANOVA software 13.0, with details provided in the legends.
3. Results
3.1. Isolation of Ferritin Genes in Peach
In total, three putative Ferritin genes were identified from the peach genome, which were named PpFer1-PpFer3 (Table 1 and Figure 1). Verification of the protein domain demonstrated that all of the PpFer proteins exhibit the Ferritin domain (PF00210), indicating that all of them are Ferritin transporters (Figure 1). The percentage of amino acid sequence identities among peach Ferritins was 70.82% (Figure 1). The percentage of amino acid sequence identities among peach Ferritins and homologues from 15 other plants was 56.65% (Figure S1).

Figure 1.
Amino acid alignment of Ferritin proteins from peach and Arabidopsis. The color of black, pink, and dark green indicates the identity of 100%, 85%, and the range between 45% and 70%, respectively, at the same amino acid residue.
Phylogenetic tree analysis showed that PpFer1, PpFer2, and PpFer3 were tightly clustered with strawberry FvFer4, grape VvFer4, and apple MdFer4, respectively, implying that PpFer transporters possess relatively close evolutionary distance from Rosaceae homologues (Figure 2).
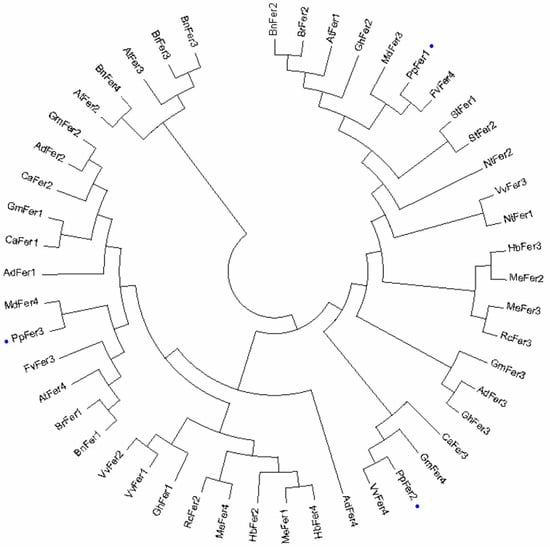
Figure 2.
Phylogenetic tree of plant Ferritin homologues. The alignment of amino acid sequences of Ferritin homologues from peach (PpFer1-3), Vitis vinifera (VvFer1-4), Arabidopsis thaliana (AtFer1-4), Arachis hypogaea (AdFer1-4), Camellia oleifera (BnFer1-4), Brassica rapa (BrFer1-3), Cicer arietinum (CaFer1-3), Gossypium hirsutum (GhFer1-3), Glycine max (GmFer1-4), Hevea brasiliensis (HbFer2-4), M. domestica (MdFer3 and MdFer4), Manihot esculenta (MeFer1-4), Nicotiana tabacum (NtFer1-2), Ricinus communis (RcFer2-3), Solanum lycopersicum (StFer1 and StFer2), and Fragaria vesca (FvFer3-4) was carried out using Cluster X 2.0.13 software. A phylogenetic tree was constructed using the maximum likelihood method in MEGA 15.0. Grape PpFer proteins are labelled with blue dots.
3.2. Expression Profiles of PpFer Genes
Results showed that the expression levels of PpFer genes were quite distinct among different tested tissues, including different tissues of ‘Zhentong No. 3’ seedlings, flowers in both bud period and full blooming stage, and annual leaves, phloem, and fruits from both the young fruit stage and mature fruit stage (Figure 3). Notably, the overall expression of PpFer1 was the most abundant, followed by PpFer3, with PpFer2 being specifically expressed in fruit from the young fruit stage (YFS). In addition, the highest expression level of PpFer1 was observed in leaves from the mature fruit stage (MFS), followed by phloem from the YFS and full-bloom flowers, and the highest expression level of PpFer3 was found in roots of seedlings, followed by phloem, fruit and leaves from the YFS (Figure 3).
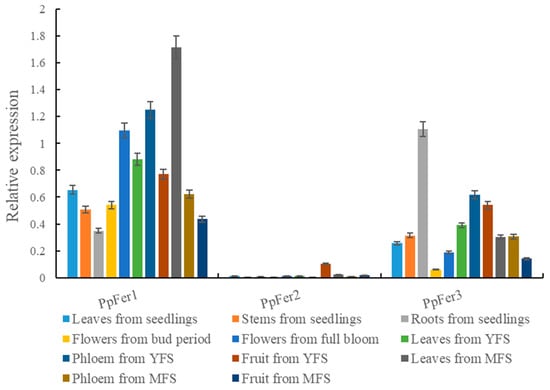
Figure 3.
Tissue-specific expression analysis of PpFer genes. Tissue samples from tissue-cultured seedlings, and young leaves, mature leaves, full blooming flowers, young fruits, and mature fruits from 7-year-old ‘Zhentong No. 3’ trees were collected on specific dates of 2021, and frozen immediately in liquid nitrogen before qRT-PCR analysis. YFS, young fruit stage. MFS, mature fruit stage.
3.3. Differential Response of PpFer Genes under Abiotic Stress Treatment in Tissue-Cultured Seedlings
Further analysis showed that PpFer genes responded differentially to abiotic stresses, including Fe depletion, Fe toxicity, ABA stress, and H2O2 stress, in tissue-cultured peach seedlings (Figure 4). In detail, PpFer1 was quite sensitive to Fe toxicity and H2O2 treatment, and its expression levels were up-regulated throughout the whole plant seedlings. PpFer3 responded to Fe toxicity and ABA treatment, and its expression levels were significantly increased in all tested tissues (leaves, stems, or roots). However, expression of PpFer2 changed little in all tested tissues under every treatment in this study (Figure 4).
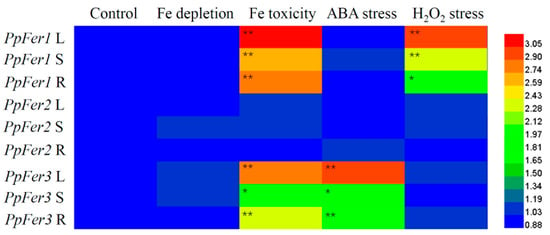
Figure 4.
Response of PpFer genes to abiotic stress treatments in tissue-cultured seedlings. One-month-old tissue-cultured ‘Zhentong No. 3’ seedlings were subjected to iron depletion, Fe toxicity (500 μmol∙L−1 FeCl3, pH 5.8), 100 μmol∙L−1 ABA (pH 5.8), or 5% (v/v) H2O2 (pH 5.8) treatment for 48 h before qRT-PCR analysis. Relative expression levels of PpFer genes were presented after normalization to Ubiquitin (the internal control) from three independent biological replicates. Asterisks indicate statistical differences found between the control and abiotic stress treatment using Student’s t-test in SPSS 13.0 software (* p < 0.05, ** p < 0.01).
3.4. PpFer1 Rescued the Retarded Growth of Arabidopsis fer1-2 Mutant
In Arabidopsis, growth of fer1-2 knockout mutant was hindered, accompanied by chlorosis symptoms [26]. To determine whether grape PpFer1 could restore the normal growth of fer1-2 mutant, PpFer1 was subcloned into the binary expression vector pHB (Figure S2). At least seven putative (#1, #10, #11, #12, #14, #15, and #16) T1 generation fer1-2/35S::PpFer1 complementation lines were verified using reverse transcription PCR for the presence of a 846 kb fragment of PpFer1 (Figure S2). Purified T3 generation of #1 and #10 fer1-2/35S::PpFer1 lines were randomly selected for further physiological analysis.
Compared with control conditions, growth of both wild type and fer1-2 mutant lines was decreased under Fe depletion, Fe toxicity, ABA treatment, or H2O2 treatment, which was embodied in reduced total fresh weight, primary root length, and lateral root numbers (Figure 5 and Figure 6). Compared with the wild type, growth of fer1-2 mutant lines was hindered under control conditions, Fe toxicity, ABA treatment, or H2O2 treatment (Figure 5), accompanied by decreased fresh weight (Figure 6A), primary root length (Figure 6B), and lateral root numbers (Figure 6C). However, no growth difference was observed between fer1-2 mutant and the wild type under Fe depletion treatment (Figure 6).
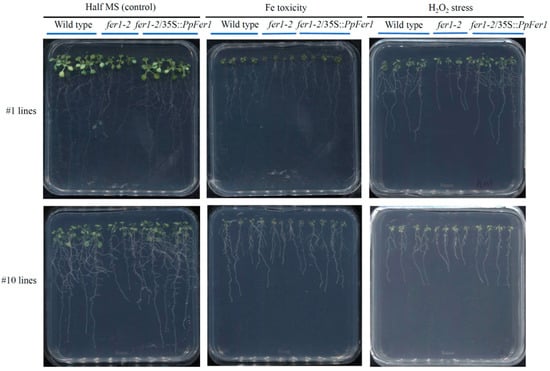
Figure 5.
Phenotype analysis of PpFer1 over-expression transgenic Arabidopsis seedlings. T3 generation seeds of #1 and #10 lines were germinated on half-strength MS solid medium, and then subjected to 100 μmol∙L−1 ABA (pH 5.8) or 5% (v/v) H2O2 (pH 5.8) treatment for 7 days before phenotype analysis.
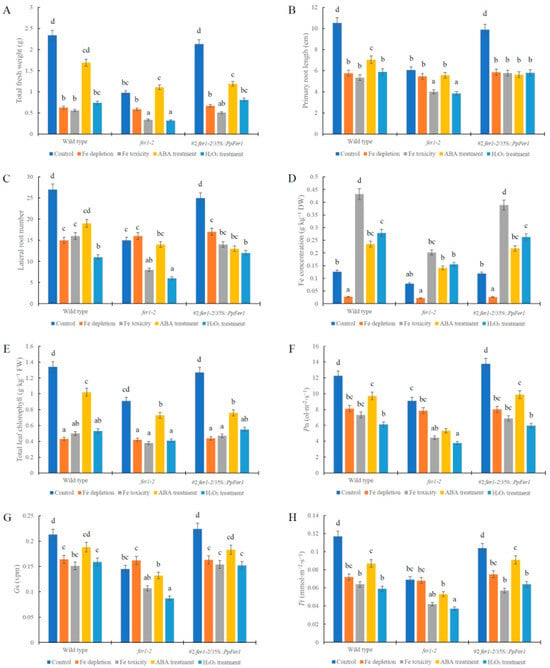
Figure 6.
Physiological analysis of PpFer1 over-expression in transgenic Arabidopsis seedlings. (A) Total fresh weight. (B) Primary root length. (C) Lateral root number. (D) Fe concentration. (E) Total leaf chlorophyll. (F) Pn. (G) Gs. (H) Tr. T3 generation seeds of #1 lines were germinated on half-strength MS solid medium and then subjected to 100 μmol∙L−1 ABA (pH 5.8) or 5% (v/v) H2O2 (pH 5.8) treatment for 7 days before physiological analysis. Data are presented as means ± SE (n = 30). Letters indicate statistical differences between the wild type, fer1-2, and fer1-2/35S::PpFer1 lines under all treatments using Fisher’s LSD test in ANOVA software 13.0.
The #1 and #10 lines were paired with different wild type seedlings. We conducted statistical analysis on both the #1 and #10 lines, and an identical trend was observed. Data from the #1 fer1-2/35S::PpFer1 lines are presented in this study (Figure 6). Notably, growth of #1 fer1-2/35S::PpFer1 lines were significantly strengthened compared with that of Arabidopsis fer1-2 mutant lines under control conditions, Fe toxicity, or H2O2 treatment, as evidenced by increased total fresh weight, primary root length, and lateral root numbers, which were similar to those of the wild type (Figure 5 and Figure 6). These findings indicate that over-expression of PpFer1 successfully restored the retarded growth of Arabidopsis fer1-2 mutant lines under control conditions, Fe toxicity, or H2O2 treatment. However, the growth of the #1 fer1-2/35S::PpFer1 lines remained the same as that of the fer1-2 mutant lines under ABA treatment or Fe depletion conditions.
In addition, the tissue Fe concentration (Figure 6D), total leaf chlorophyll (Figure 6E), Pn (Figure 6F), Gs (Figure 6G), and Tr (Figure 6H) of fer1-2 mutant lines was reduced under control conditions, Fe toxicity, H2O2 treatment, or ABA treatment but changed little under Fe depletion, compared with that of wild type lines (Figure 6). In particular, both the #1 and #10 fer1-2/35S::PpFer1 lines exhibited higher tissue Fe concentration, total leaf chlorophyll, Pn, Gs, and Tr than fer1-2 mutant lines under control conditions, Fe toxicity, and H2O2 treatment, but changed little under Fe depletion or ABA treatment (Figure 6).
4. Discussion
In fruit trees, Fe is one of the most indispensable mineral elements. It directly affects tree growth, flowering, fruit quality formation, and fruit yield [1,3,4,24,25]. Currently, the effective Fe concentration in natural soils does not correspond with normal growth of fruit trees under normal pH values [1,3,7]. However, molecular mechanisms towards Fe uptake, transport, distribution, and storage in fruit trees are essentially unknown. In particular, peach is a dicotyledonous fruit tree that belongs to the Mechanism I Fe absorption category of plants [7,10,11]. In this study, three Ferritin transporters were isolated from peach. These transporters are prone to being closely clustered with Rosaceae homologues, implying that peach Ferritins may possess a close genetic distance and similar biological functions to Rosaceae fruit trees, as a result of long-term evolution. Therefore, studying the biological function of peach Ferritin transporters contributes to revealing the biological function of Ferritin homologues from Rosaceae fruit trees.
In this study, PpFer1 and PpFer3 could be detected in all tested tissues but PpFer3 is specifically expressed in young-stage fruit. Notably, PpFer1 is highly expressed in leaves, which was in line with AtFer1, AtFer3, and AtFer4 in Arabidopsis, PbFer2 in pear [27], and MeFer4 in cassava [20]. PpFer1 is also highly expressed in full-bloom flowers, which is similar to RhFer in cut rose [17], whereas PpFer2 is exclusively expressed in young peach fruit, Arabidopsis AtFer2 is specifically expressed in roots, and tomato SlFer is majorly expressed in root tips. These findings suggest that Ferritin transporters possess extensive expression profiles and some of them are likely to be functional in specific tissues or organs in plants.
Previous studies have demonstrated that AtFer1 and AtFer3 are induced by excessive Fe and H2O2 stress, and AtFer2 is not responsive to iron [23]. Consistently, PpFer1 and PpFer3 were responsive to excessive Fe and H2O2, and their expression was significantly up-regulated throughout the whole seedling. These findings imply that PpFer1 and PpFer3 are likely to be active in regulating the Fe storage capacity in peach cells under Fe toxicity conditions or reactive oxygen species stress, thus maximally maintaining the cytosol Fe concentration in moderation so as to secure the basic life activities depending on Fe. Simultaneously, the Fe concentration was enhanced under Fe toxicity but reduced under H2O2 treatment in all tested Arabidopsis lines, which contributes to securing the basic growth of peach seedlings. In pear, PbFer2 is inhibited by iron deficiency stress [27]. However, PpFer genes did not respond to Fe depletion in this study. We speculate that PpFer genes are prone to being active in storing Fe in peach trees under excessive Fe conditions but not Fe deficiency conditions. PpFer2 was very much less expressed in tested peach tissues/organs and had little response to any abiotic treatment in this study. We hypothesize that this gene is likely to be a pseudogene that may have lost its protein-coding ability due to accumulated mutations or unprocessed segmental duplication over long-term evolution [28]; this idea requires further verification.
Expression of AtFer2 in Arabidopsis [16,23] and RhFer in cut rose [17] was induced by ABA treatment, which was also observed in PpFer3 in this study. Indeed, ABA treatment induced the cellular Fe accumulation in all tested Arabidopsis lines, which was in accord with previous studies in transgenic tomato [29]. Nonetheless, these genes regulated by both Fe and ABA may play a role in the crosstalk between Fe and ABA, and may be an intermediate hub for the cross-linking of iron and ABA signals.
In Arabidopsis, AtFer1 regulates the free Fe levels in plant cells and knockout of AtFer1 accelerated natural senescence of Arabidopsis seedlings with hindered growth status [26]. As the most abundantly expressed Ferritin gene in grape, the maximum expression of PpFer1 was detected in aboveground parts of peach trees and was increased in all tested tissues under excessive Fe and H2O2 stress. Favorably, the over-expression of PpFer1 was effective in restoring the retarded growth of Arabidopsis fer1 knockout mutant. Notably, tissue Fe concentration and photosynthesis performance were significantly strengthened in fer1-2/35S::PpFer1 lines, which may partially explain the rescued growth status. The complementation of PpFer1 may actively mobilize the Fe storage capacity of fer1-2/35S::PpFer1 lines, thereby preventing Fe toxicity or oxidative stress, which helps maintain basic life activities or metabolic processes that rely on a moderate Fe level. Synchronously, total leaf chlorophyll, Pn, Gs, and Tr were increased in fer1-2/35S::PpFer1 lines. Considering that MeFer4 is induced by low temperature, and transgenic lines with MeFer4 over-expression have enhanced cold resistance in cassava [20], we speculate that Ferritin transporters are implicated in enhancing plant resistance to undesired abiotic stresses, including cold, Fe toxicity, and H2O2 stress. These findings again support the proposition that Ferritin transporters control interaction between Fe homeostasis and oxidative stress in plants [13,17,20]. Nonetheless, PpFer1 may be an important Ferritin transporter that is involved in Fe storage in peach, especially under excessive Fe or oxidative stress conditions.
5. Conclusions
Three PpFer genes were isolated from peach; their expression levels were significantly different in distinct tissues/organs. PpFer1 was the most abundantly expressed Ferritin gene in peach; its expression was induced under excessive Fe and H2O2 stress in all tested tissues. Over-expression of PpFer1 rescued the retarded growth of Arabidopsis fir1-2 knockout mutant, which was embodied in strengthened fresh weight, primary root length, total root length, total leaf chlorophyll, Pn, Gs, Tr, and tissue Fe concentration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12244093/s1, Figure S1: Amino acid alignment of known plant Ferritin homologues; Figure S2: Generation of PpFer1 over-expression transgenic Arabidopsis seedlings. (A) Construction of recombinant plasmid pBH-PpFer1. (B) PCR verification of PpFer1 in T1 generation fer1-2/35S::PpFer1 lines. Note: M, standard DL2000 DNA ladder (Takara, Dalian, China); Table S1: Specific primers used for quantitative RT-PCR.
Author Contributions
Conceptualization, Z.S. and J.Z.; methodology, Y.Y., Y.T. and S.S.; validation, M.L., Y.N. and A.D.; investigation, Y.Y., Y.T., M.L. and Y.N.; data curation, Y.T. and A.D.; writing—original draft preparation, Z.S.; writing—review and editing, J.Z. and A.D.; project administration, Z.S.; funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Project of Science and Technology of Shandong Province (2022CXGC010605), the China Agriculture Research System of MOF and MARA (CARS-29-17), the China Scholarship Council Fund (202208370080), Jiangsu Agriculture Science and Technology Innovation Fund (CX(23)3064) and UKRI BBSRC (X008843/1).
Data Availability Statement
The data are contained within the present article and in its Supplementary Materials.
Acknowledgments
The authors are grateful to grateful to Julia M. Davies, Department of Plant Sciences, University of Cambridge for critical reading and valuable suggestions. The authors are grateful to Irene Murgia, Dipartimento di Biologia, Università degli Studi di Milano, for fer1-2 mutant donation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barton, L.L.; Abadia, J. Iron Nutrition in Plants and Rhizospheric Microorganisms; Springer: New York, NY, USA, 2006; pp. 85–101. [Google Scholar]
- Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Couturier, J.; Touraine, B.; Briat, J.F.; Gaymard, F.; Rouhier, N. The iron-sulfur cluster assembly machineries in plants: Current knowledge and open questions. Front. Plant Sci. 2013, 4, 259. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Z.; Lin, S.Z.; Fu, J.Y.; Chen, Y.H.; Zhang, H.X.; Li, J.Z.; Liang, M.X. Heterologous expression of ISU1 gene from Fragaria vesca enhances plant tolerance to Fe depletion in Arabidopsis. Plant Physiol. Biochem. 2022, 184, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tagliavini, M.; Abadía, J.; Rombolà, A.D.; Tsipouridis, C.; Marangoni, B. Agronomic means for the control of iron deficiency chlorosis in deciduous fruit trees. J. Plant Nutr. 2000, 23, 2007–2022. [Google Scholar] [CrossRef]
- Tagliavini, M.; Rombolà, A.D. Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur. J. Agron. 2001, 15, 72–92. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Vert, G. Regulation of iron uptake by IRT1: Endocytosis pulls the trigger. Mol. Plant 2015, 8, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Fourcroy, P.; Tissot, N.; Gaymard, F.; Briat, J.F.; Dubos, C. Facilitated Fe nutrition by phenolic compounds excreted by the Arabidopsis ABCG37/PDR9 transporter requires the IRT1/FRO2 high-affinity root Fe(2+) transport system. Mol. Plant 2016, 9, 485. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef]
- Mondal, S.; Pramanik, K.; Ghosh, S.K.; Pal, P.; Ghosh, P.K.; Ghosh, A.; Maiti, T.K. Molecular insight into arsenic uptake, transport, phytotoxicity, and defense responses in plants: A critical review. Planta 2022, 255, 87. [Google Scholar] [CrossRef]
- Sudarev, V.V.; Dolotova, S.M.; Bukhalovich, S.M.; Bazhenov, S.V.; Ryzhykau, Y.L.; Uversky, V.N.; Bondarev, N.A.; Osipov, S.D.; Mikhailov, A.E.; Kuklina, D.D.; et al. Ferritin self-assembly, structure, function, and biotechnological applications. Int. J. Biol. Macromol. 2023, 224, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Ravet, K.; Touraine, B.; Boucherez, J.; Briat, J.F.; Gaymard, F.; Cellier, F. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009, 57, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Duc, C.; Ravet, K.; Gaymard, F. Ferritins and iron storage in plants. BBA Gen. Subjects 2010, 1800, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Lόpez-Millán, A.F.; Duy, D.; Philippar, K. Chloroplast iron transport proteins-Function and impact on plant physiology. Front. Plant Sci. 2016, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Function of FER2 Gene in Response to Drought Stress in Arabidopsis thaliana. Ph.D. Thesis, Hefei University of Technology, Hefei, China, 2018. (In Chinese). [Google Scholar]
- Liu, J.; Fan, Y.; Zou, J.; Fang, Y.; Wang, L.; Wang, M.; Jiang, X.; Liu, Y.; Gao, J.; Zhang, C. A RhABF2/Ferritin module affects rose (Rosa hybrida) petal dehydration tolerance and senescence by modulating iron levels. Plant J. 2017, 92, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Reyt, G.; Boudouf, S.; Boucherez, J.; Gaymard, F.; Briat, J.F. Iron- and ferritin-dependent reactive oxygen species distribution: Impact on Arabidopsis root system architecture. Mol. Plant 2015, 8, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Soybean ferritin: Implications for iron status of vegetarians. Am. J. Clin. Nutr. 2009, 89, 1680S–1685S. [Google Scholar] [CrossRef]
- Ghislain, M.; Muzhingi, T.; Low, J.W. Zinc and iron fortification in cassava. Nat. Biotechnol. 2019, 37, 130–132. [Google Scholar] [CrossRef]
- Jung, S.; Staton, M.; Lee, T.; Blenda, A.; Svancara, R.; Abbott, A.; Main, D. GDR (Genome Database for Rosaceae): Integrated web database for Rosaceae genomics and genetics data. Nucleic Acids Res. 2008, 36, D1034–D1040. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.M.; Lin, S.Z.; Wang, J.P.; Tang, M.L.; Huang, J.F.; Gao, T.P.; Zhang, H.X.; Song, Z.Z. Heterologous expression of the MiHAK14 homologue from Mangifera indica enhances plant tolerance to K+ deficiency and salinity stress in Arabidopsis. Plant Growth Regul. 2022, 98, 39–49. [Google Scholar] [CrossRef]
- Petit, J.M.; Briat, J.F.; Lobréaux, S. Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem. J. 2001, 359, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Z.; Guo, S.L.; Ma, R.J.; Zhang, B.B.; Guo, S.L.; Yu, M.L.; Korir, N.K. Differential expression of iron–sulfur cluster biosynthesis genes during peach fruit development and ripening, and their response to iron compound spraying. Sci. Hortic. 2016, 207, 73–81. [Google Scholar] [CrossRef]
- Song, Z.Z.; Zhang, B.B.; Zhang, C.H.; Ma, R.J.; Yu, M.L. Differential expression of iron-sulfur cluster biosynthesis genes during peach flowering. Biol. Plantarum 2016, 60, 79–85. [Google Scholar] [CrossRef]
- Murgia, I.; Vazzola, V.; Tarantino, D.; Cellier, F.; Ravet, K.; Briat, J.F.; Soave, C. Knock-out of ferritin AtFer1 causes earlier onset of age-dependent leaf senescence in Arabidopsis. Plant Physiol. Biochem. 2007, 45, 898–907. [Google Scholar] [CrossRef]
- Zhong, C.; Su, J.; Tang, T.T.; Ding, W.; Zhu, L.W.; Jia, B. Cloning and differential expression analysis of Fer2 gene in leaf of ‘Dangshansuli’ pear. J. Nanjing Agric. Univ. 2013, 36, 33–38. (In Chinese) [Google Scholar]
- Cheetham, S.W.; Faulkner, G.J.; Dinger, M.E. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat. Rev. Genet. 2019, 21, 191–201. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Applications of abscisic acid and increasing concentrations of calcium affect the partitioning of mineral nutrients between tomato leaf and fruit tissue. Sci. Hortic. 2019, 5, 49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).