No Pairwise Interactions of GmSNAP18, GmSHMT08 and AtPR1 with Suppressed AtPR1 Expression Enhance the Susceptibility of Arabidopsis to Beet Cyst Nematode
Abstract
:1. Introduction
2. Results
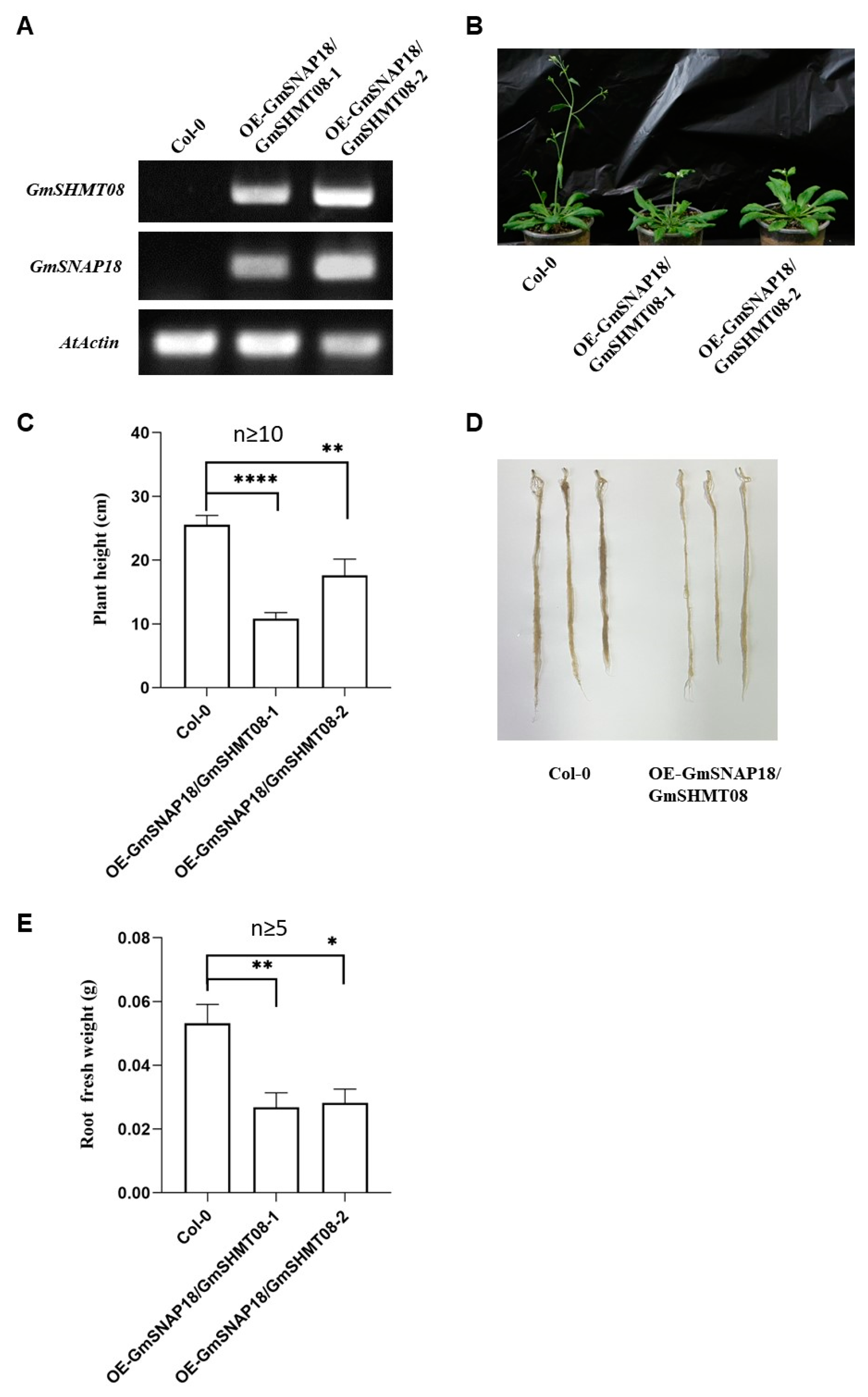
2.1. Simultaneous Overexpression of GmSNAP18 and GmSHMT08 Suppressed the Growth of Arabidopsis
2.2. Simultaneous Overexpression of GmSNAP18 and GmSHMT08 Enhanced Susceptibility of Arabidopsis to BCN
2.3. Simultaneous Overexpression of GmSNAP18 and GmSHMT08 Suppressed the Expression Patterns of AtPR1 on the Salicylic Acid Signaling Pathway in Arabidopsis
2.4. Simultaneous Overexpression of GmSNAP18 and GmSHMT08 Did Not Impact Expression Patterns of AtJAR1 and AtHEL1 on the Jasmonic Acid and Ethylene Signaling Pathways in Arabidopsis
2.5. Subcellular and Interaction Localizations of AtSNAP2, AtSHMT4, and AtPR1
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Nematodes
4.2. Gene Cloning and Plasmid Construction
4.3. Arabidopsis Transformation and Molecular Identification
4.4. Growth Parameter Measurement of Transgenic Arabidopsis
4.5. Phenotyping of Arabidopsis Infected with BCN
4.6. Quantitative Real-Time PCR
4.7. Subcellular Localization and BiFC Assay
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lilley, C.J.; Atkinson, H.J.; Urwin, P.E. Molecular aspects of cyst nematodes. Mol. Plant Pathol. 2005, 6, 577–588. [Google Scholar] [CrossRef]
- Koenning, S.R.; Wrather, J.A. Suppression of soybean yield potential in the continental United States by plant diseases from 2006 to 2009. Plant Health. Prog. 2010, 11, 5. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Jiang, R.; Peng, H.; Liu, S. Soybean cyst nematodes: A destructive threat to soybean production in China. Phytopathol. Res. 2021, 3, 19. [Google Scholar] [CrossRef]
- Brucker, E.; Carlson, S.; Wright, E.; Niblack, T.; Diers, B. Rhg1 alleles from soybean PI 437654 and PI 88788 respond differentially to isolates of Heterodera glycines in the greenhouse. Theor. Appl. Genet. 2005, 111, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.E.; Lee, T.G.; Guo, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 2012, 338, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.E.; Bayless, A.M.; Wang, K.; Guo, X.L.; Song, Q.J.; Jiang, J.M.; Bent, A.F. Distinct copy number, coding sequence, and locus methylation patterns underlie Rhg1-mediated soybean resistance to soybean cyst nematode. Plant Physiol. 2014, 165, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.G.; Kumar, I.; Diers, B.W.; Hudson, M.E. Evolution and selection of Rhg1, a copy-number variant nematode-resistance locus. Mol. Ecol. 2015, 24, 1774–1791. [Google Scholar] [CrossRef]
- Yu, N.; Lee, T.G.; Rosa, D.P.; Hudson, M.; Diers, B.W. Impact of Rhg1 copy number, type, and interaction with Rhg4 on resistance to Heterodera glycines in soybean. Theor. Appl. Genet. 2016, 129, 2403–2412. [Google Scholar] [CrossRef]
- Meksem, K.; Pantazopoulos, P.; Njiti, V.N.; Hyten, L.D.; Arelli, P.R.; Lightfoot, D.A. ‘Forrest’ resistance to the soybean cyst nematode is bigenic: Saturation mapping of the Rhg1 and Rhg4 loci. Theor. Appl. Genet. 2001, 103, 710–717. [Google Scholar] [CrossRef]
- Liu, S.; Kandoth, P.K.; Warren, S.D.; Yeckel, G.; Heinz, R.; Alden, J.; Yang, C.; Jamai, A.; El-Mellouki, T.; Juvale, P.S.; et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 2012, 492, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kandoth, P.K.; Lakhssassi, N.; Kang, J.; Colantonio, V.; Heinz, R.; Yeckel, G.; Zhou, Z.; Bekal, S.; Dapprich, J.; et al. The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat. Commun. 2017, 8, 14822. [Google Scholar] [CrossRef] [PubMed]
- Lakhssassi, N.; Liu, S.; Bekal, S.; Zhou, Z.; Colantonio, V.; Lambert, K.; Barakat, A.; Meksem, K. Characterization of the soluble NSF attachment protein gene family identifies two members involved in additive resistance to a plant pathogen. Sci. Rep. 2017, 7, 45226. [Google Scholar] [CrossRef]
- Shaibu, A.S.; Zhang, S.; Ma, J.; Feng, Y.; Huai, Y.; Qi, J.; Li, J.; Abdelghany, A.M.; Azam, M.; Htway, H.T.P.; et al. The GmSNAP11 contributes to resistance to soybean cyst nematode race 4 in Glycine max. Front. Plant Sci. 2022, 13, 939763. [Google Scholar] [CrossRef]
- Bayless, A.M.; Smith, J.M.; Song, J.; Mcminn, P.H.; Teillet, A.; August, B.K.; Bent, A.F. Disease resistance through impairment of α-SNAP-NSF interaction and vesicular trafficking by soybean Rhg1. Proc. Natl. Acad. Sci. USA 2016, 113, E7375–E7382. [Google Scholar] [CrossRef] [PubMed]
- Bayless, A.M.; Zapotocny, R.W.; Grunwald, D.J.; Amundson, K.K.; Diers, B.W.; Bent, A.F. An atypical N-ethylmaleimide sensitive factor enables the viability of nematode-resistant Rhg1 soybeans. Proc. Natl. Acad. Sci. USA 2018, 115, E4512–E4521. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zielinski, R.E.; Hudson, M.E. t-SNAREs bind the Rhg1 α-SNAP and mediate soybean cyst nematode resistance. Plant J. 2020, 104, 318–331. [Google Scholar] [CrossRef]
- Wang, R.; Deng, M.M.; Yang, C.; Yu, Q.Q.; Zhang, L.; Zhu, Q.; Guo, X.L. A Qa-SNARE complex contributes to soybean cyst nematode resistance via regulation of mitochondria-mediated cell death. J. Exp. Bot. 2021, 72, 7145–7162. [Google Scholar] [CrossRef]
- Korasick, D.A.; Kandoth, P.K.; Tanner, J.J.; Mitchum, M.G.; Beamer, L.J. Impaired folate binding of serine hydroxymethyltransferase 8 from soybean underlies resistance to the soybean cyst nematode. J. Biol. Chem. 2020, 295, 3708–3718. [Google Scholar] [CrossRef]
- Patil, G.B.; Lakhssassi, N.; Wan, J.R.; Song, L.; Zhou, Z.; Klepadlo, M.; Vuong, T.D.; Stec, A.O.; Kahil, S.S.; Colantonio, V.; et al. Whole-genome re-sequencing reveals the impact of the interaction of copy number variants of the rhg1 and Rhg4 genes on broad-based resistance to soybean cyst nematode. Plant Biotechnol. J. 2019, 17, 1595–1611. [Google Scholar] [CrossRef]
- Lakhssassi, N.; Piya, S.; Bekal, S.; Liu, S.; Zhou, Z.; Bergounioux, C.; Miao, L.; Meksem, J.; Lakhssassi, A.; Jones, K.; et al. A pathogenesis related protein GmPR08-Bet VI promotes a molecular interaction between the GmSHMT08 and GmSNAP18 in resistance to Heterodera glycines. Plant Biotechnol. J. 2020, 18, 1810–1829. [Google Scholar] [CrossRef]
- Butler, K.J.; Chen, S.Y.; Smith, J.M.; Wang, X.H.; Bent, A.F. Soybean resistance locus Rhg1 confers resistance to multiple cyst nematodes in diverse plant species. Phytopathology 2019, 109, 2107–2115. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, Y.; Kong, L.; Huang, W.; Peng, D.; Liu, S. Opposite Beet cyst nematode infection phenotypes of transgenic Arabidopsis between overexpressing GmSNAP18 and AtSNAP2 and between overexpressing GmSHMT08 and AtSHMT4. Phytopathology 2022, 112, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, S. Beet cyst nematode HsSNARE1 interacts with both AtSNAP2 and AtPR1 and promotes disease in Arabidopsis. J. Adv. Res. 2023, 47, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Concibido, V.C.; Diers, B.W.; Arelli, P.R. A decade of QTL mapping for cyst nematode resistance in soybean. Crop Sci. 2004, 44, 1121–1131. [Google Scholar] [CrossRef]
- Siddique, S.; Radakovic, Z.S.; De La Torre, C.M.; Chronis, D.; Novák, O.; Ramireddy, E.; Holbein, J.; Matera, C.; Hütten, M.; Gutbrod, P.; et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc. Natl. Acad. Sci. USA 2015, 112, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Sugiyama, S.; Matsuura, H.; Arie, T.; Masuta, C. Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol. 2010, 51, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.F.; Beard, H.; Brewer, E.; Kabir, S.; MacDonald, M.H.; Youssef, R.M. Arabidopsis genes, AtNPR1, AtTGA2 and AtPR-5, confer partial resistance to soybean cyst nematode (Heterodera glycines) when overexpressed in transgenic soybean roots. BMC Plant Biol. 2014, 14, 96. [Google Scholar] [CrossRef]
- Lakhssassi, N.; Piya, S.; Knizia, D.; El Baze, A.; Cullen, M.A.; Meksem, J.; Lakhssassi, A.; Hewezi, T.; Meksem, K. Mutations at the serine hydroxymethyltransferase impact its interaction with a soluble NSF attachment protein and a pathogenesis-related protein in soybean. Vaccines 2020, 8, 349. [Google Scholar] [CrossRef]
- Nuaima, R.H.; Heuer, H. Genetic variation among Heterodera schachtii populations coincided with differences in invasion and propagation in roots of a set of cruciferous plants. Int. J. Mol. Sci. 2023, 24, 6848. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Understanding molecular plant-nematode interactions to develop alternative approaches for nematode control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Schmidt, L.K.; Lohse, M.; Hashimoto, K.; Bock, R.; Kudla, J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008, 56, 505–516. [Google Scholar] [CrossRef]
- Luo, S.; Liu, S.; Kong, L.; Peng, H.; Huang, W.; Jian, H.; Peng, D. Two venom allergen-like proteins, HaVAP1 and HaVAP2, are involved in the parasitism of Heterodera avenae. Mol. Plant Pathol. 2019, 20, 471–484. [Google Scholar] [CrossRef]





| Name | Primer Sequence (5’-3’) |
|---|---|
| Primers for cloning of rhg1-a GmSNAP18 and GmSHMT08 | |
| rhg1-a GmSNAP18 (Glyma.18g022500) | F: ATGGCCGATCAGTTATCGAAGGG |
| R: TCAAGTAATAACCTCATACTCCTCA | |
| GmSHMT08 (Glyma.08g108900) | F: ATGGATCCAGTAAGCGTGTGG |
| R: CTAATCCTTGTACTTCATTTCAG | |
| Primers for plasmid construction | |
| rhg1-a GmSNAP18 | F: TGTGACCTCGAGACTAGTATGGCCGATCAGTTATCGAAGGG |
| R: CCGTCGCACCATACTAGTAGTAATAACCTCATACTCCTCAAG | |
| GmSHMT08 | F: TGTGACCTCGAGACTAGTATGGATCCAGTAAGCGTGTGG |
| R: CCGTCGCACCATACTAGTATCCTTGTACTTCATTTCAG | |
| Primers for identification of transgenic Arabidopsis | |
| rhg1-a GmSNAP18 | F: CAAGCTCGCCAAATCATGGG |
| R: AGCAATGTGCAGCATCGACA | |
| GmSHMT08 | F: ATGGATCCAGTAAGCGTGTGGGGTA |
| R: TGAGCGGCAGAGGTTTTCG | |
| AtActin (At5g09810) | F: GCATGAAGATCAAGGTGGTTGCAC |
| R: ATGGACCTGACTCATCGTACTCACT | |
| Primers for gene expression analyses | |
| AtPR1 (At2g14610) | F: ACGGGGAAAACTTAGCCTGG |
| R: TTGGCACATCCGAGTCTCAC | |
| AtPR5 (At1g75040) | F: AGGCTGCAACTTTGACGC |
| R: AGAAATCTTTGCCGCCATC | |
| AtHEL1 (At3g04720) | F: GATAAGCCGTACGCATGGC |
| R: TCACCCTTAAACACTTGCCG | |
| AtJAR1 (At2g46370) | F: GCTACATTTGCTGTGATTCCG |
| R: GGTATCGATACAACCCTGCG | |
| AtActin | F: GCATGAAGATCAAGGTGGTTGCAC |
| R: ATGGACCTGACTCATCGTACTCACT | |
| Primers for bimolecular fluorescence complementation (BiFC) | |
| pSPYNE(R)173-AtSNAP2 | F: AGGCCTACTAGTGGATCCATGGGGGATCATCTGGTGAG |
| R: TTCGAGCTCCTACCCGGGTCATGTAAGGTCATCCTCCTCTAG | |
| pSPYNE(R)173-AtPR1 | F: AGGCCTACTAGTGGATCCATGAATTTTACTGGCTATTC |
| R: TTCGAGCTCCTACCCGGGTTAGTATGGCTTCTCGTTCACA | |
| pSPYCE(M)-AtPR1 | F: ACTAGTGGATCCATCGATATGAATTTTACTGGCTATTC |
| R: GTATGGGTACATCCCGGGGTATGGCTTCTCGTTCACA | |
| pSPYNE(R)173-AtSHMT4 | F: AGGCCTACTAGTGGATCCATGGAACCAGTCTCTTCATG |
| R: TTCGAGCTCCTACCCGGGCTAATCCTTGTACTTCATCTC | |
| pSPYCE(M)-AtSHMT4 | F: ACTAGTGGATCCATCGATATGGAACCAGTCTCTTCATG |
| R: GTATGGGTACATCCCGGGATCCTTGTACTTCATCTC | |
| Primers for subcellular localization | |
| pYBA1132-AtPR1 | F: TCTAGAACTAGTGGATCCATGAATTTTACTGGCTATTC |
| R: GAGGTCGACGGTATCGATGTATGGCTTCTCGTTCACA | |
| pYBA1132-AtSNAP2 | F: TCTAGAACTAGTGGATCCATGGGGGATCATCTGGTGAG |
| R: GAGGTCGACGGTATCGATTGTAAGGTCATCCTCCTCTAG | |
| pYBA1132-AtSHMT4 | F: TCTAGAACTAGTGGATCCATGGAACCAGTCTCTTCATG |
| R: GAGGTCGACGGTATCGATATCCTTGTACTTCATCTC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, J.; Kong, L.; Huang, W.; Peng, H.; Peng, D.; Meksem, K.; Liu, S. No Pairwise Interactions of GmSNAP18, GmSHMT08 and AtPR1 with Suppressed AtPR1 Expression Enhance the Susceptibility of Arabidopsis to Beet Cyst Nematode. Plants 2023, 12, 4118. https://doi.org/10.3390/plants12244118
Zhang L, Zhao J, Kong L, Huang W, Peng H, Peng D, Meksem K, Liu S. No Pairwise Interactions of GmSNAP18, GmSHMT08 and AtPR1 with Suppressed AtPR1 Expression Enhance the Susceptibility of Arabidopsis to Beet Cyst Nematode. Plants. 2023; 12(24):4118. https://doi.org/10.3390/plants12244118
Chicago/Turabian StyleZhang, Liuping, Jie Zhao, Lingan Kong, Wenkun Huang, Huan Peng, Deliang Peng, Khalid Meksem, and Shiming Liu. 2023. "No Pairwise Interactions of GmSNAP18, GmSHMT08 and AtPR1 with Suppressed AtPR1 Expression Enhance the Susceptibility of Arabidopsis to Beet Cyst Nematode" Plants 12, no. 24: 4118. https://doi.org/10.3390/plants12244118





