Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. cv. “Yidali”) in Plant Factory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
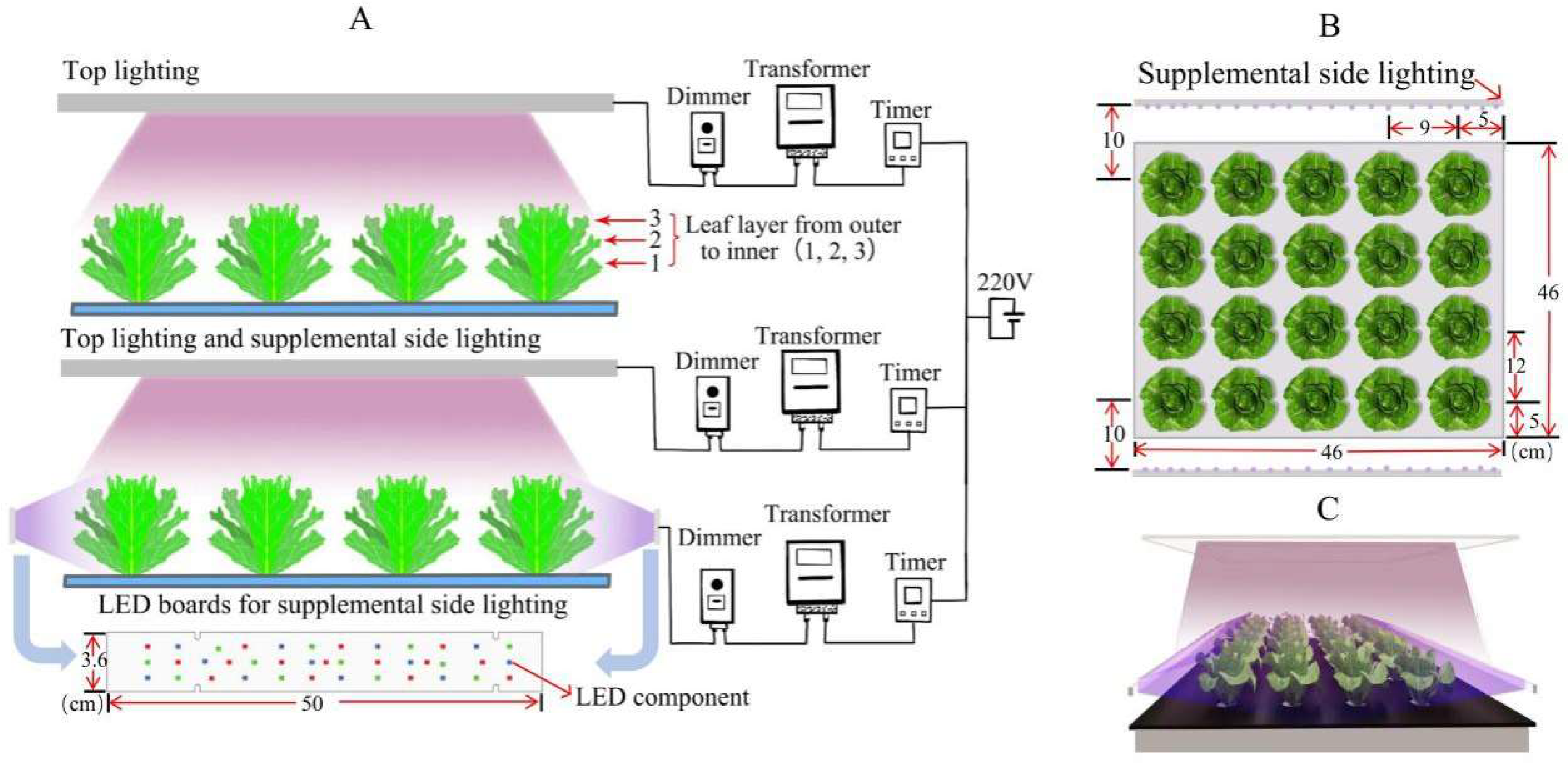
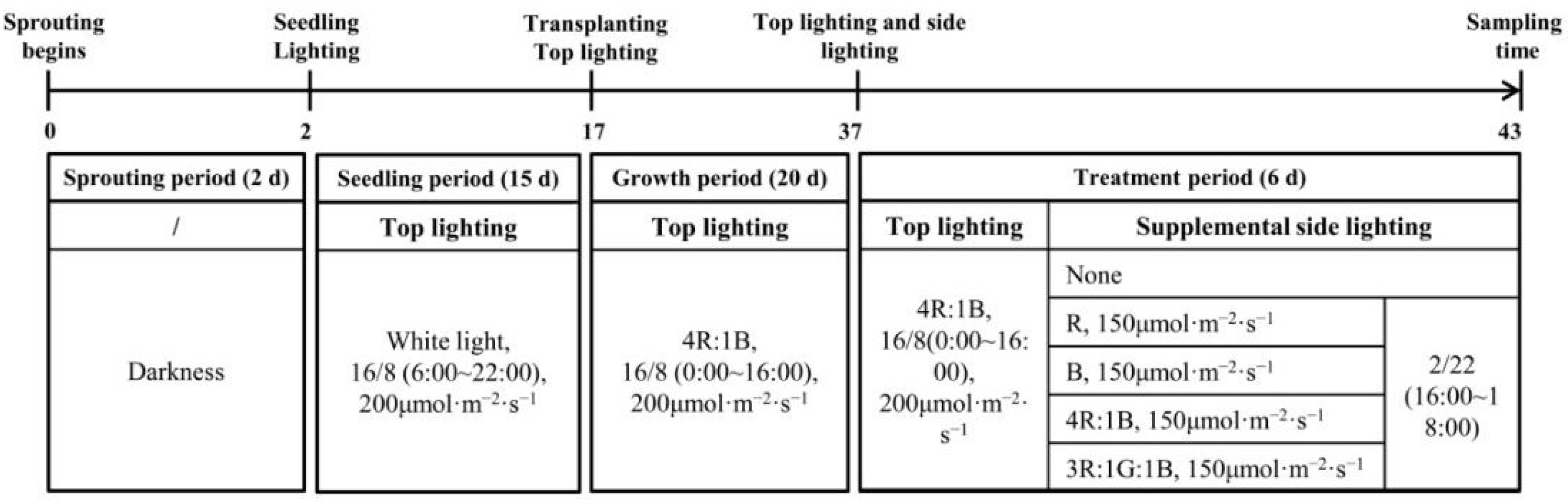
2.2. Lighting Treatments
2.3. Yield, Leafy Morphology, and Chlorophyll Measurements
2.4. Nitrate Measurement
2.5. Soluble Sugar and Starch Measurements
2.6. Flavonoid and Phenolics Measurements
2.7. Statistical Analysis
3. Results
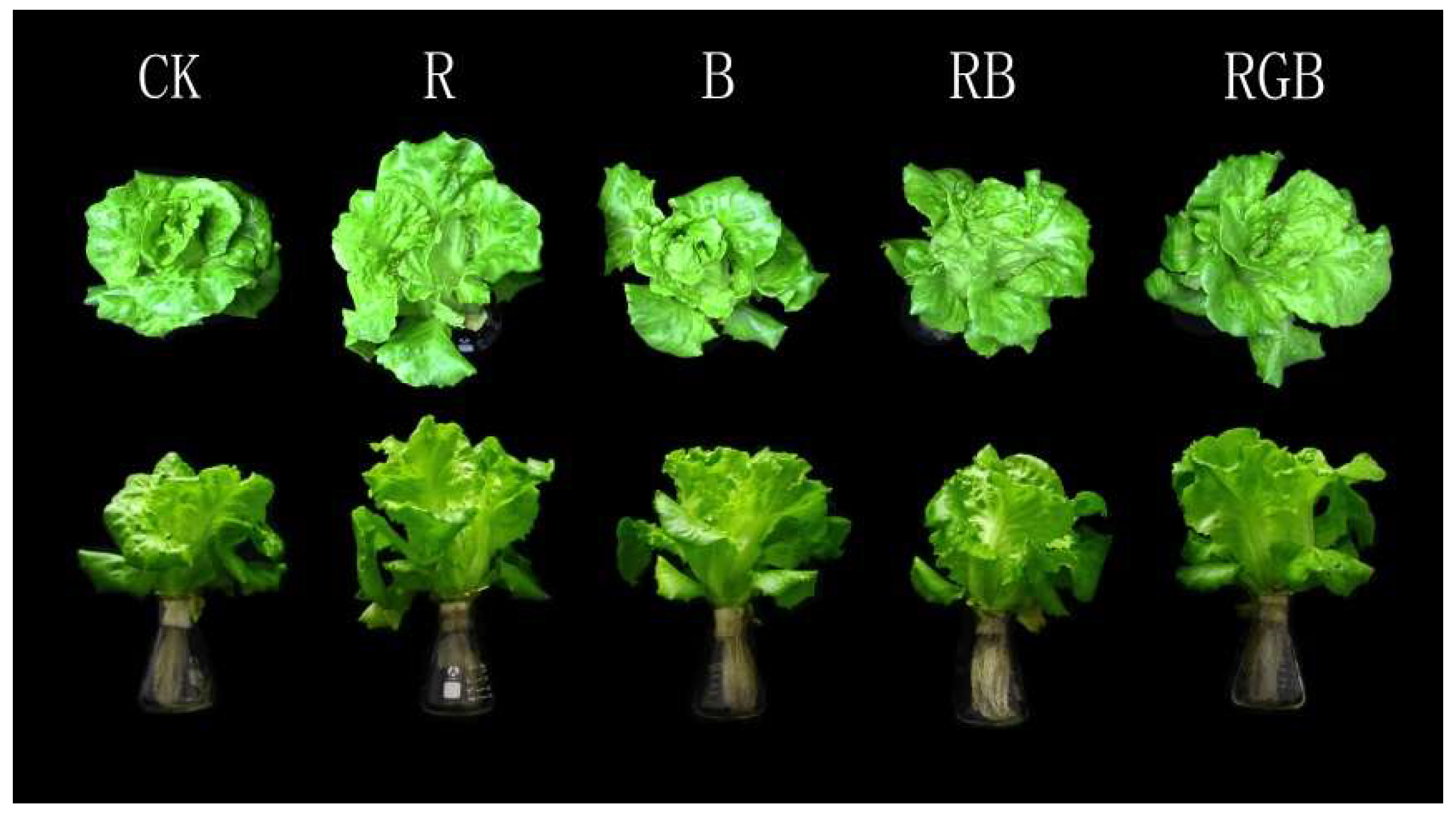
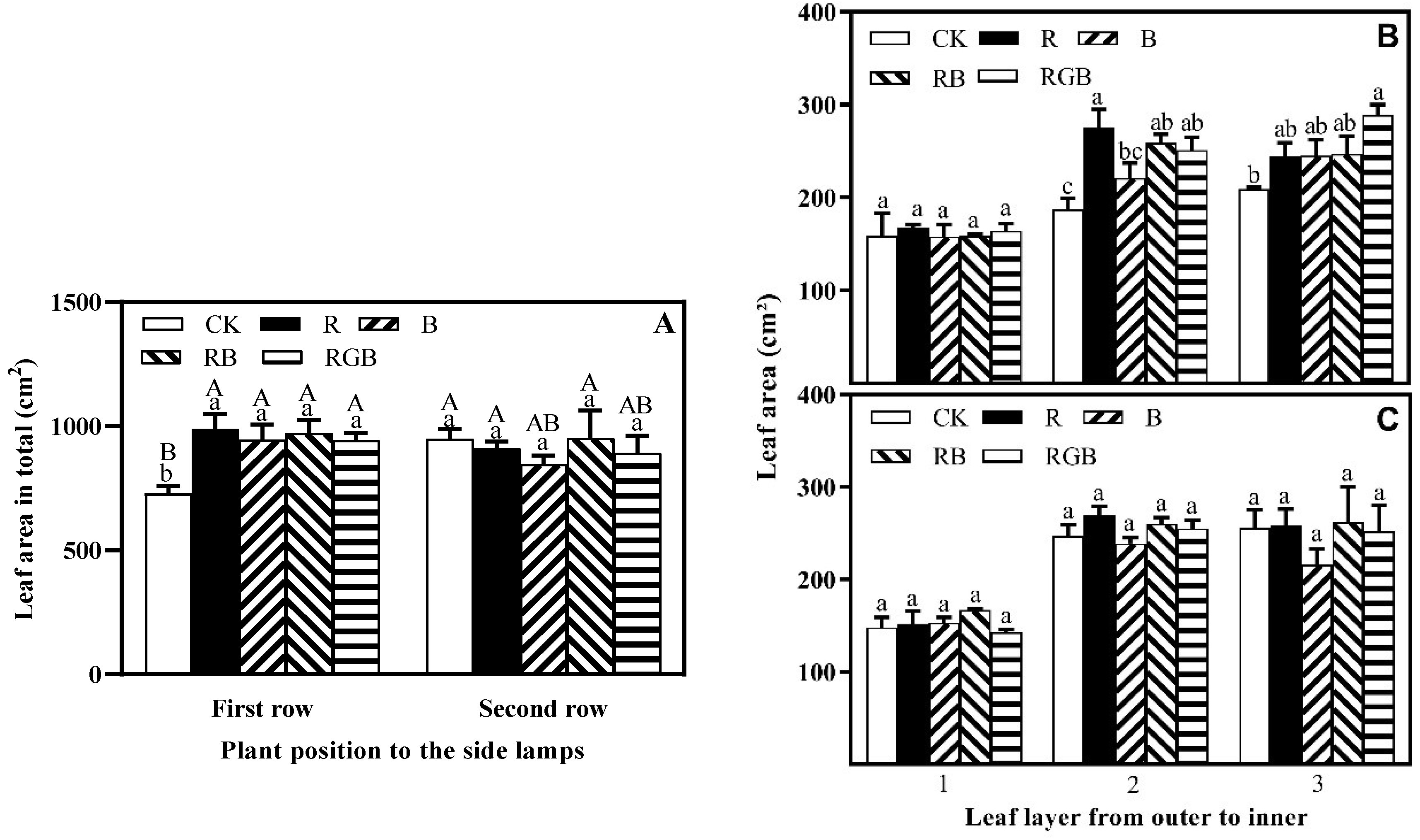
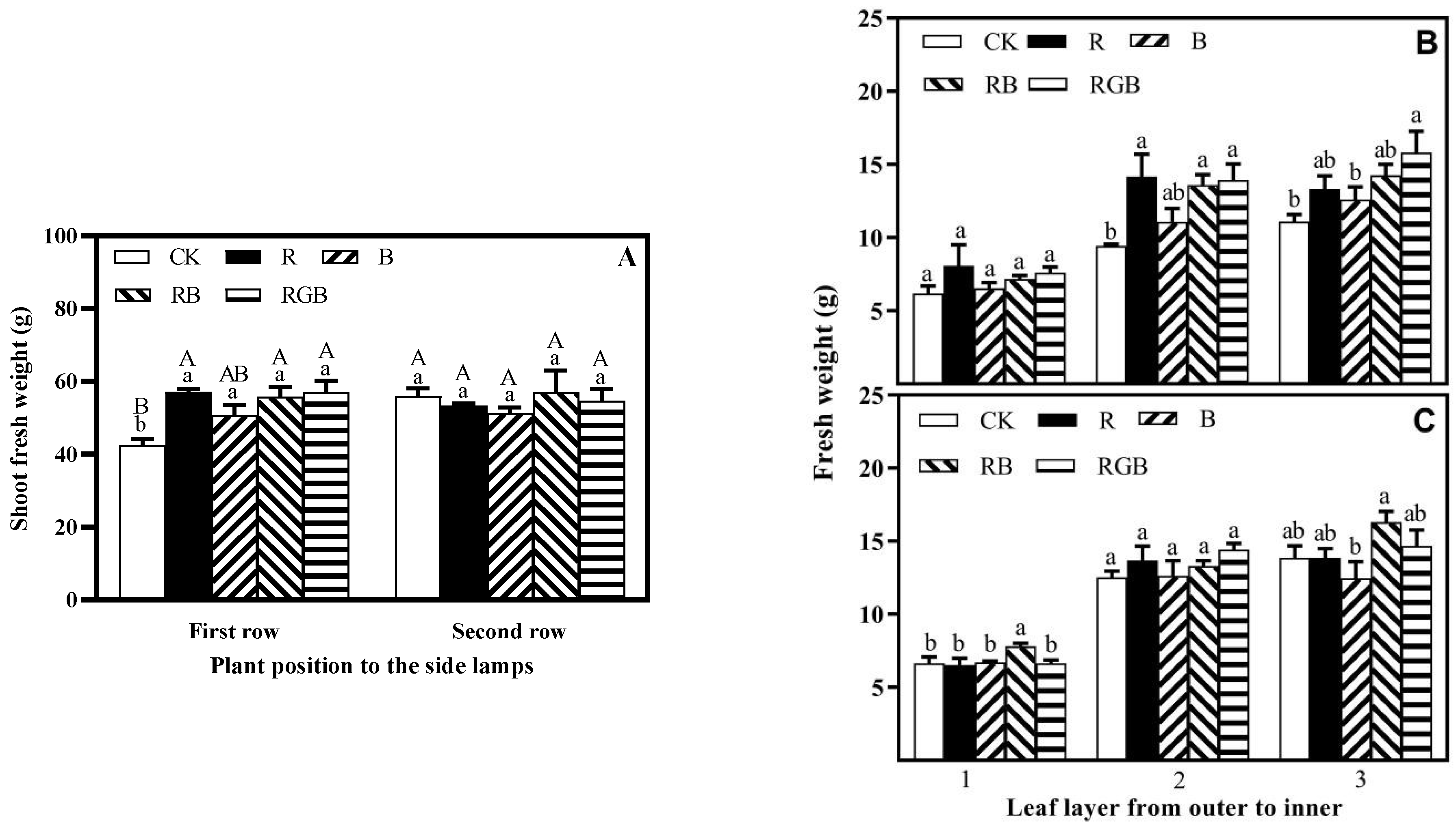
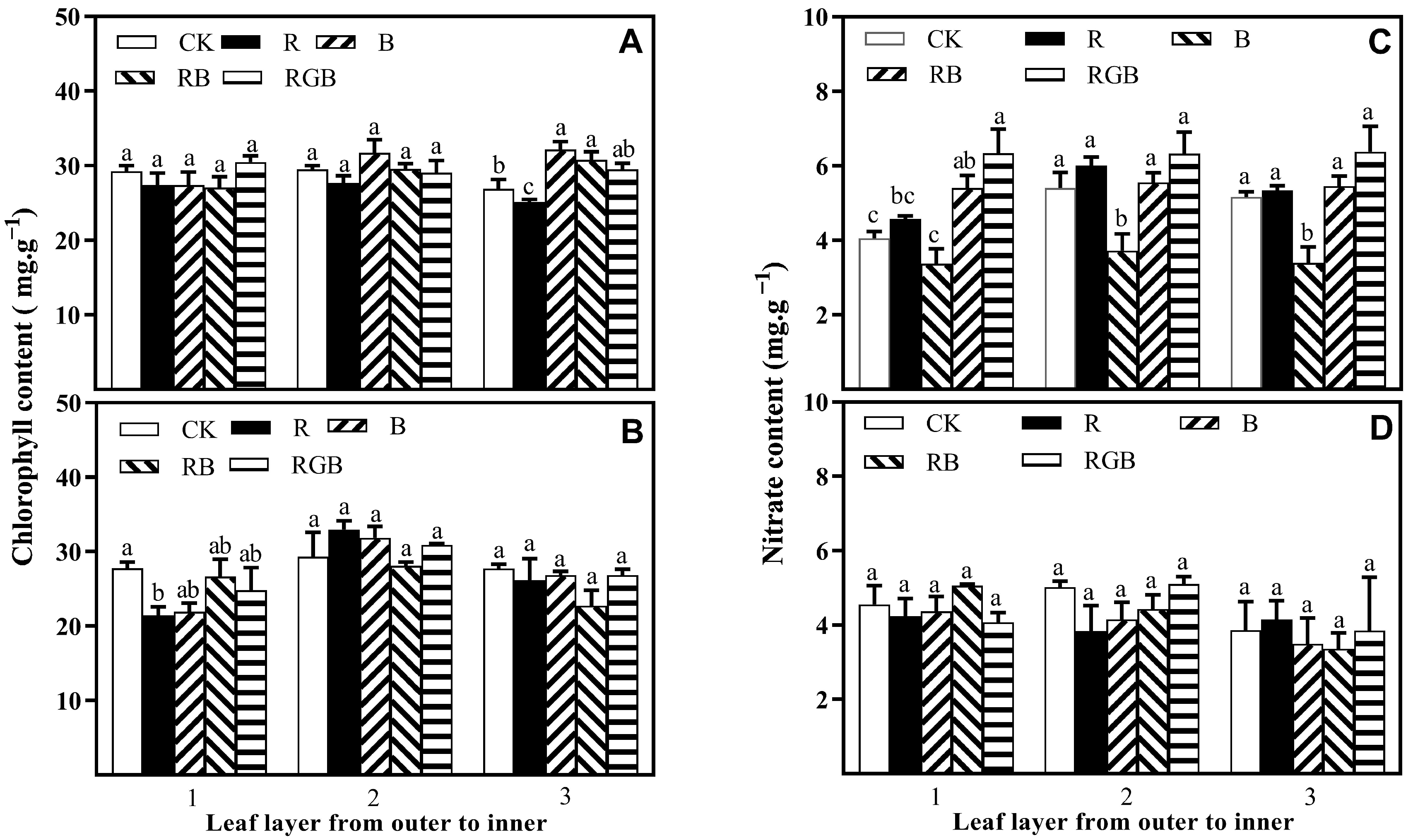
3.1. Growth, Yield, Chlorophyll Content, and Nitrate Content
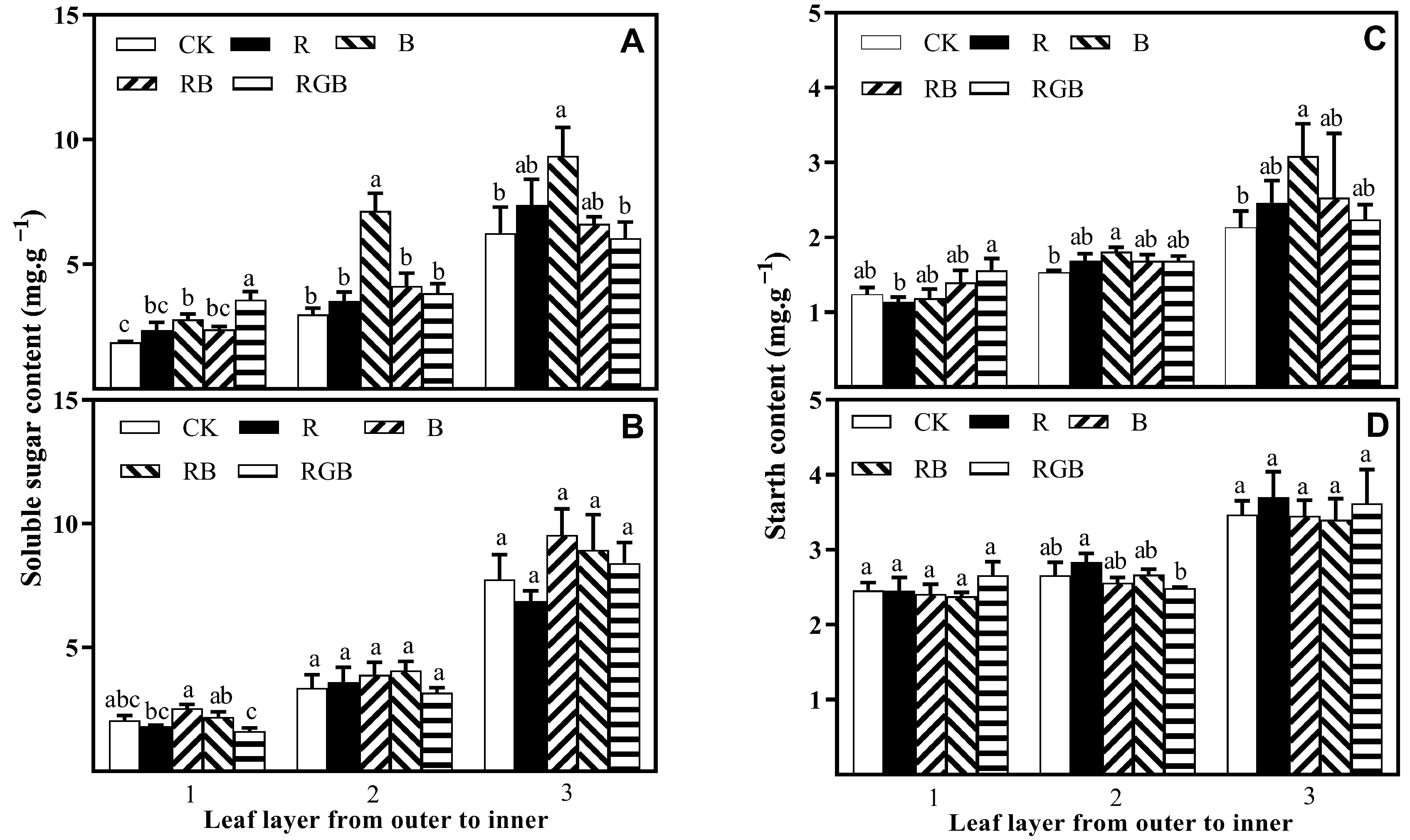
3.2. Sugar and Starch Contents
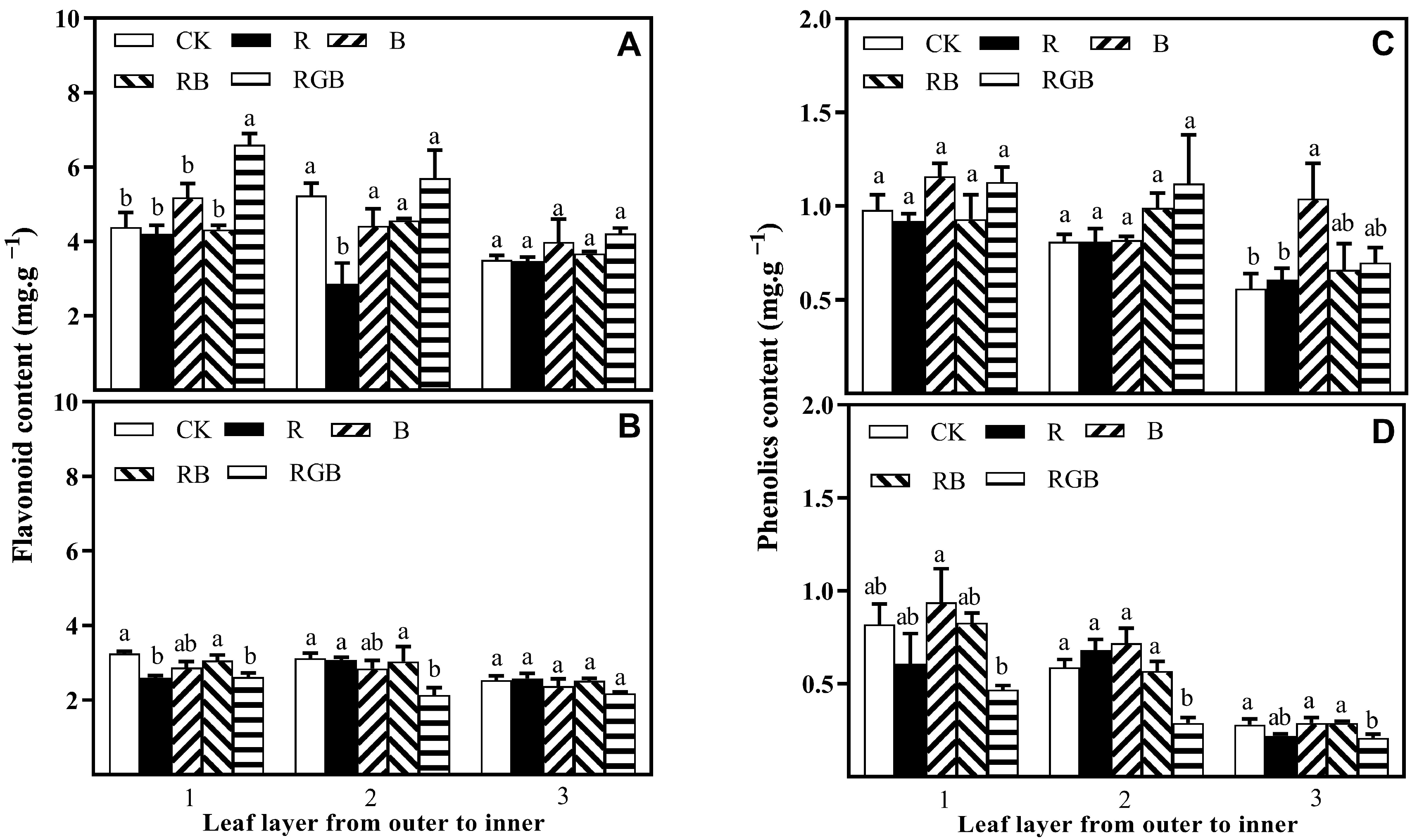
3.3. Flavonoid and Phenolics Contents
4. Discussion
4.1. Side Lighting Increased Lettuce Yield Irrespective of Spectral Qualities
4.2. Side Lighting Affected Lettuce Nutritional Quality, Varying with Spectral Qualities
4.3. Spatial Response Diversity of Lettuce Plants to Different Side Lighting Spectral Qualities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandon, M.; Kozai, T.; Lu, N.; Yamaguchi, T.; Takagaki, M.; Maruo, T.; Yamori, W. Next revolution of agriculture: A review of innovations in plant factories. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 723–740. [Google Scholar]
- Yamori, W.; Zhang, G.; Takagaki, M.; Maruo, T. Feasibility study of rice growth in plant factories. J. Rice Res. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Hayashi, E. Current status of commercial plant factories with LED lighting market in Asia, Europe, and other regions. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer: Singapore, 2016; pp. 295–308. [Google Scholar]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B. 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shen, S.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental upward lighting from underneath to obtain higher marketable lettuce (Lactuca Sativa) leaf fresh weight by retarding senescence of outer leaves. Front. Plant Sci. 2015, 6, 1110. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Zhang, G.; Shen, S.; Supaibulwatana, K.; Watanabe, C.K.A.; Yamori, W. A combination of downward lighting and supplemental upward lighting improves plant growth in a closed plant factory with artificial lighting. HortScience 2017, 52, 831–835. [Google Scholar] [CrossRef]
- Saengtharatip, S.; Lu, N.; Takagaki, M. Supplemental upward LED lighting for growing romaine lettuce (Lactuca Sativa) in a plant factory: Cost performance by light intensity and different light spectra. Acta Hortic. 2018, 623–630. [Google Scholar] [CrossRef]
- Saengtharatip, S.; Joshi, J.; Zhang, G.; Takagaki, M.; Kozai, T.; Yamori, W. Optimal light wavelength for a novel cultivation system with a supplemental upward lighting in plant factory with artificial lighting. Environ. Control. Biol. 2021, 59, 21–27. [Google Scholar] [CrossRef]
- Saito, K.; Goto, E. Evaluation of the enhancement of photosynthetic rate in a komatsuna (Brassica rapa L. var. perviridis) canopy with upward lighting using an optical simulation in a plant factory with artificial light. Front. Plant Sci. 2023, 14, 1111338. [Google Scholar]
- Frantz, J.M.; Joly, R.J.; Mitchell, C.A. Intracanopy lighting influences radiation capture, productivity, and leaf senescence in cowpea canopies. J. Am. Soc. Hortic. Sci. 2000, 125, 694–701. [Google Scholar] [CrossRef]
- Hovi-Pekkanen, T.; Tahvonen, R. Effects of interlighting on yield and external fruit quality in year-round cultivated cucumber. Sci. Hortic. 2008, 116, 152–161. [Google Scholar] [CrossRef]
- Kim, D.; Moon, T.; Kwon, S.; Hwang, I.; Son, J.E. Supplemental inter-lighting with additional far-red to red and blue light increases the growth and yield of greenhouse sweet peppers (Capsicum annuum L.) in winter. Hortic. Environ. Biotechnol. 2023, 64, 83–95. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, D.; Moon, T.; Son, J.E. Evaluation of the light use efficiency and water use efficiency of sweet peppers subjected to supplemental interlighting in greenhouses. Hortic. Environ. Biotechnol. 2023, 64, 605–614. [Google Scholar] [CrossRef]
- Pettersen, R.I.; Torre, S.; Gislerød, H.R. Effects of intracanopy lighting on photosynthetic characteristics in cucumber. Sci. Hortic. 2010, 125, 77–81. [Google Scholar] [CrossRef]
- Schipper, R.; Van Der Meer, M.; De Visser, P.H.B.; Heuvelink, E.; Marcelis, L.F.M. Consequences of intra-canopy and top LED lighting for uniformity of light distribution in a tomato crop. Front. Plant Sci. 2023, 14, 1012529. [Google Scholar] [CrossRef]
- Wenke, L. Definition and application strategy for stereo lighting in plant factory. China Light Light. 2021, 6, 1–3. [Google Scholar]
- Hawley, D.; Graham, T.; Stasiak, M.; Dixon, M. Improving cannabis bud quality and yield with subcanopy lighting. HortScience 2018, 53, 1593–1599. [Google Scholar] [CrossRef]
- Yamori, N.; Matsushima, Y.; Yamori, W. Upward led lighting from the base suppresses senescence of lower leaves and promotes flowering in indoor rose management. HortScience 2021, 56, 716–721. [Google Scholar] [CrossRef]
- Wang, M.; Wei, H.; Jeong, B.R. Lighting direction affects leaf morphology, stomatal characteristics, and physiology of head lettuce (Lactuca sativa L.). Int. J. Mol. Sci. 2021, 22, 3157. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.; Jeong, B.R. Side lighting enhances morphophysiology and runner formation by upregulating photosynthesis in strawberry grown in controlled environment. Agronomy 2021, 12, 24. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Lighting from top and side enhances photosynthesis and plant performance by improving light usage efficiency. Int. J. Mol. Sci. 2022, 23, 2448. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The effect of LED light spectra on the growth, yield and nutritional value of red and green lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shao, M.; Liu, W.; Li, B.; Wang, Q.; Liu, J.; Wen, Y.; Yang, Q. Regulation of ascorbate accumulation and metabolism in lettuce by end-of-production high light irradiation provided by red and blue LEDs. Environ. Exp. Bot. 2021, 189, 104567. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W. Regulation of accumulation and metabolism circadian rhythms of starch and sucrose in two leaf-color lettuces by red: Blue ratios of led continuous light. Environ. Exp. Bot. 2022, 196, 104811. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Li, H.; Sun, H.; Li, M. Prediction of soluble sugar content in cabbage by near infrared spectrometer. Spectrosc. Spectr. Anal. 2018, 38, 3058–3063. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Kozai, T.; Zhang, G. Some aspects of the light environment. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer: Singapore, 2016; pp. 49–55. [Google Scholar]
- Arcel, M.M.; Yousef, A.F.; Shen, Z.H.; Nyimbo, W.J.; Zheng, S.H. Optimizing lettuce yields and quality by incorporating movable downward lighting with a supplemental adjustable sideward lighting system in a plant factory. PeerJ. 2023, 11, e15401. [Google Scholar] [CrossRef]
- Kozai, T.; Kino, S.; Jeong, B.R.; Kinowaki, M.; Ochiai, M.; Hayashi, M.; Mori, K. A sideward lighting system using diffusive optical fibers for production of vigorous micropropagated plantlets. Acta Hortic. 1992, 319, 237–242. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, J.; Hu, J.; Jeong, B.R. Effect of supplementary light intensity on quality of grafted tomato seedlings and expression of two photosynthetic genes and proteins. Agronomy 2019, 9, 339. [Google Scholar] [CrossRef]
- Khan, M.S.; Hameed, W.; Nozoe, M.; Shiina, T. Disruption of the psbA gene by the copy correction mechanism reveals that the expression of plastid-encoded genes is regulated by photosynthesis activity. J. Plant Res. 2007, 120, 421–430. [Google Scholar] [CrossRef]
- Leelavathi, S.; Bhardwaj, A.; Kumar, S.; Dass, A.; Pathak, R.; Pandey, S.S.; Tripathy, B.C.; Padmalatha, K.V.; Dhandapani, G.; Kanakachari, M.; et al. Genome-wide transcriptome and proteome analyses of tobacco psaA and psbA deletion mutants. Plant Mol. Biol. 2011, 76, 407–423. [Google Scholar] [CrossRef]
- Mitchell, C.; Both, A.J.; Bourget, C.; Burr, J.; Kubota, C.; Lopez, R.; Morrow, R.; Runkle, E. LEDs: The future of greenhouse lighting! Chron. Hortic. 2012, 52, 6–12. [Google Scholar]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Bian, Z.; Cheng, R.F.; Yang, Q.C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Wanlai, Z.; Wenke, L.; Qichang, Y. Reducing nitrate content in lettuce by pre-harvest continuous light delivered by red and blue light-emitting diodes. J. Plant Nutr. 2013, 36, 481–490. [Google Scholar] [CrossRef]
- Yamori, W.; Nagai, T.; Makino, A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 2011, 34, 764–777. [Google Scholar] [CrossRef]
- Shao, M.; Liu, W.; Zhou, C.; Wang, Q.; Li, B. Alternation of temporally overlapped red and blue light under continuous irradiation affected yield, antioxidant capacity and nutritional quality of purple-leaf lettuce. Sci. Hortic. 2022, 295, 110864. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Scaife, A.; Schloemer, S. The diurnal pattern of nitrate uptake and reduction by spinach (Spinacia oleracea L.). Ann. Bot. 1994, 73, 337–343. [Google Scholar] [CrossRef]
- Lillo, C. Light regulation of nitrate reductase in green leaves of higher plants. Physiol. Plant. 1994, 90, 616–620. [Google Scholar] [CrossRef]
- Cheng, C.L.; Acedo, G.N.; Cristinsin, M.; Conkling, M.A. Sucrose mimics the light induction of arabidopsis nitrate reductase gene transcription. Proc. Natl. Acad. Sci. USA 1992, 89, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, S.; Rothstein, S.; Oaks, A. Regulation of the accumulation and reduction of nitrate by nitrogen and carbon metabolites in maize seedlings. Plant Physiol. 1997, 114, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Blom-Zandstra, M.; Lampe, J.E. The role of nitrate in the osmoregulation of lettuce (Lactuca sativa L.) grown at different light intensities. J. Exp. Bot. 1985, 36, 1043–1052. [Google Scholar] [CrossRef]
- Deng, J.-M.; Bin, J.-H.; Pan, R.-C. Effects of light quality on the primary nitrogen assimination of rice (Oryza sativa L.) seedlings. Acta Bot. Sin. 2000, 42, 234–238. [Google Scholar]
- Shi, H.; Han, J.; Guan, C.; Yuan, T. Effects of red and blue light proportion on leaf growth, carbon-nitrogen metabolism and quality in tobacc. Acta Agron. Sin. 1999, 25, 215–220. [Google Scholar]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Matsuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supplemental blue light. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Pech, R.; Volna, A.; Hunt, L.; Bartas, M.; Cerven, J.; Pecinka, P.; Spunda, V.; Nezval, J. Regulation of phenolic compound production by light varying in spectral quality and total irradiance. Int. J. Mol. Sci. 2022, 23, 6533. [Google Scholar] [CrossRef] [PubMed]
- Rosati, C.; Cadic, A.; Duron, M.; Ingouff, M.; Simoneau, P. Molecular characterization of the anthocyanidin synthase gene in Forsythia×intermedia reveals organ-specific expression during flower development. Plant Sci. 1999, 149, 73–79. [Google Scholar] [CrossRef]
- Ma, X.; Ma, H.; Chen, Q.; Ma, Y.; Daugulis, A.J.; Liang, J.; Zheng, P. The Influence of monochromatic lights on flavonoid production by the fungus sanghuangporus vaninii: Modeling of kinetic profiles and expression levels of important genes in flavonoid synthesis. Biochem. Eng. J. 2021, 166, 107876. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Miao, C.; Yang, S.; Xu, J.; Wang, H.; Zhang, Y.; Cui, J.; Zhang, H.; Jin, H.; Lu, P.; He, L.; et al. Effects of light intensity on growth and quality of lettuce and spinach cultivars in a plant factory. Plants 2023, 12, 3337. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Saito, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Light environment optimization for lettuce growth in plant factory. IFAC Proc. Vol. 2011, 44, 605–609. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.B.; Roh, Y.H.; Choi, I.L.; Kim, J.; Kim, Y.; Yoon, H.S.; Kang, H.M. Effect of various LED light qualities, including wide red spectrum-LED, on the growth and quality of mini red romaine lettuce (cv. Breen). Plants 2023, 12, 2056. [Google Scholar] [CrossRef]
- Razzak, M.A.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Effects of supplementing green light to red and blue light on the growth and yield of lettuce in plant factories. Sci. Hortic. 2022, 305, 111429. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Wang, Z.; Liu, W.; Ding, Y.; Zhang, Q.; Wang, S. Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. cv. “Yidali”) in Plant Factory. Plants 2023, 12, 4147. https://doi.org/10.3390/plants12244147
Chen R, Wang Z, Liu W, Ding Y, Zhang Q, Wang S. Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. cv. “Yidali”) in Plant Factory. Plants. 2023; 12(24):4147. https://doi.org/10.3390/plants12244147
Chicago/Turabian StyleChen, Ren, Zhenwei Wang, Wenke Liu, Yuteng Ding, Qishuan Zhang, and Shurong Wang. 2023. "Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. cv. “Yidali”) in Plant Factory" Plants 12, no. 24: 4147. https://doi.org/10.3390/plants12244147
APA StyleChen, R., Wang, Z., Liu, W., Ding, Y., Zhang, Q., & Wang, S. (2023). Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. cv. “Yidali”) in Plant Factory. Plants, 12(24), 4147. https://doi.org/10.3390/plants12244147





